Abstract
With the rapid advancement of proteomics, numerous scholars have investigated the intricate relationships between plasma proteins and various diseases. Therefore, this study aims to elucidate the relationship between BDH1 and type 2 diabetes using Mendelian randomization (MR) and to identify novel targets for the prevention and treatment of type 2 diabetes through proteomics. This study primarily employed the Mendelian Randomization (MR) method, leveraging genetic data from numerous large-scale, publicly accessible genome-wide association studies (GWAS). Within this framework, we adopted a two-step, two-sample MR approach to evaluate the relationships between BDH1, plasma proteins, and type 2 diabetes. Finally, we conducted bidirectional MR analyses along with various sensitivity analyses to ensure the robustness and reliability of the study findings. The inverse variance weighted (IVW) analysis demonstrated that for each 1 standard deviation (SD) increase in BDH1, the risk of type 2 diabetes decreased by 3% (OR: 0.97; 95% CI: 0.95, 0.99). Concurrently, the proteomics-based MR analysis identified 37 plasma proteins associated with type 2 diabetes and 27 plasma proteins associated with BDH1. Notably, NBN, ARG1, and CCL11 were found to mediate the protective effect of BDH1 on type 2 diabetes. Our research findings uncovered the potential protective effect of BDH1 on type 2 diabetes and identified several plasma proteins associated with the disease. These results open new avenues for enhanced exploration of the prevention and treatment of type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes is a chronic metabolic disorder characterized by relative or absolute insulin deficiency, holding a pivotal position among human diseases1. Over the decades, while the precise pathogenesis of type 2 diabetes remains elusive, its onset is undoubtedly influenced by both environmental and genetic factors2. According to the 10th edition of the International Diabetes Federation(IDF) Diabetes Atlas released in 2021, an estimated 537 million adults aged 20–79 worldwide were living with diabetes, constituting one-tenth of the global adult population. Projections suggest that by 2030, this figure will rise to 643 million, and by 2045, it will climb further to 783 million. Concurrently, as the global population is projected to grow by 20% during the same period, the diabetes prevalence is estimated to rise by 46%3. Therefore, it has been dubbed an emerging epidemic by some scholars4. Type 2 diabetes undoubtedly imposes a significant burden on both the healthcare system and society at large5.
Many scholars have extensively studied type 2 diabetes from diverse perspectives to enhance prevention and treatment strategies. Population-based observational studies indicate that obesity, lack of exercise, smoking, and an unhealthy diet are major modifiable risk factors contributing to the development of type 2 diabetes6,7,8. From a genetic perspective, various genes including adrenomedullin(ADM), proprotein convertase subtilisin/kexin type 9(PCSK9), 3-hydroxy-3-methylglutaryl-CoA reductase(HMGCR), and 3-hydroxybutyrate dehydrogenase 1(BDH1) have been closely associated with type 2 diabetes9. BDH1 is a crucial rate-limiting enzyme in ketone metabolism, directly facilitating the breakdown of β-hydroxybutyrate. Previous studies have elucidated that BDH1 inhibits the glucagon response in the liver and pancreas through the promotion of β-hydroxybutyrate production10. A previous in vitro experiment conducted by our team demonstrated that BDH1 can ameliorate diabetic nephropathy and atherosclerosis associated with type 2 diabetes by regulating NFE2 like bZIP transcription factor 2(NRF2)11. These studies collectively underscore the pivotal role of BDH1 in the prevention and treatment of type 2 diabetes. Unfortunately, current research on their association remains limited, and the specific pathways involved are still unclear. With advancements in high-throughput plasma protein detection technology, an increasing number of studies are focusing on the association between plasma proteomics and diseases, aiming to elucidate the molecular pathological basis of these conditions12.
Mendelian randomization (MR) was initially considered an ideal substitute for randomized controlled trials, utilizing genetic tools as reliable proxies for exposure and disease. According to principles of Mendelian genetics, genetic information is randomly allocated at conception, preceding the onset of disease, thereby minimizing confounding biases from various factors13. This study aims to employ MR to investigate the potential association between BDH1 and type 2 diabetes, explore the reciprocal relationship between BDH1, plasma proteins, and type 2 diabetes through plasma proteomics, and assess the potential mediating role of plasma proteins, thereby offering novel insights into the prevention and treatment of type 2 diabetes.
Method
Study design
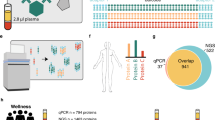
The objective of this study was to assess the causal relationship between BDH1 gene expression levels and type 2 diabetes, while also investigating the association between 1,001 plasma proteins and type 2 diabetes through proteomics and exploring their potential mediating effects. All Mendelian randomization analyses are grounded in three key assumptions: genetic variants must be closely associated with the relevant exposure, not influenced by any confounding factors, and capable of affecting outcomes solely through the relevant exposure14. Figure 1 illustrates the comprehensive design of this Mendelian randomization study.
Study flow chart. BDH1, plasma proteins and source of genetic data for type 2 diabetes; causal association of BDH1 with type 2 diabetes was first assessed by bidirectional two-sample MR, reverse MR was used to ensure the feasibility of mediation analyses, and subsequent two-step MR was used to assess the mediating effect of plasma proteins.
Data sources
All data utilized in this study originate from large-scale genomic studies conducted in European populations and are exclusively sourced from publicly available aggregate datasets (refer to Table S1).
The expression quantitative trait locus (eQTL) data for BDH1 were derived from the first phase of the eQTLGen consortium’s study. The eQTLGen consortium aims to leverage blood-based gene expression data from 31,684 individuals to perform cis- and trans-eQTL analyses, followed by replication validation using relevant single-cell RNA-seq data, to identify potential drivers of various traits15. Genetic data on type 2 diabetes originate from a comprehensive genome-wide association studies(GWAS) study, encompassing 38,841 patients with type 2 diabetes and 451,248 individuals of European ancestry as controls16. Genetic data for plasma proteins are derived from a recent GWAS study, in which researchers employed Olink proteomics data from 54,000 samples in the UK Biobank to pinpoint genetic loci linked to the levels of 1001 diverse plasma proteins17. Furthermore, it is important to note that there is virtually no sample overlap among these three eQTL or GWAS studies, which substantially enhances the reliability of the findings.
Instrument selection
In evaluating the association between BDH1 and type 2 diabetes, a stringent significance threshold of 5 × 10− 8 was applied to select genetic instruments strongly correlated with BDH1. Subsequently, relatively independent SNPs were chosen based on the European 1000 Genomes panel, with criteria of r2 < 0.001 and a distance threshold of 10,000 kb. To assess the mediating role of plasma protein levels, a significance threshold of 1 × 10− 5 was used to select genetic instruments associated with various plasma protein levels. Independent SNPs were then identified using the European 1000 Genomes panel, with an r2 threshold of < 0.001 and a distance criterion of 10,000 kb. Furthermore, the statistical robustness of all SNPs was evaluated using the F-statistic, with the exclusion of SNPs having an F-statistic less than 10 to minimize bias from weak instrumental variables18. Ultimately, the SNPs identified through the aforementioned process can serve as genetic instruments for BDH1 expression, plasma protein levels, and type 2 diabetes, thereby maximizing their potential utility19,20.
Statistical analysis
The primary methodology employed in this study is the Inverse Variance Weighted (IVW) method to evaluate the association between BDH1 and type 2 diabetes. Furthermore, to ensure the robustness of the IVW analysis results, four sensitivity analysis techniques were employed: Weighted Median, Simple Mode, Weighted Mode, and MR-Egger test. The primary methodology of this study involves an initial assessment of the association between BDH1 and type 2 diabetes, calculation of the overall effect size, and exploration of potential reverse associations through reverse MR. Subsequently, the study assessed the association between BDH1 and 1001 plasma proteins, as well as the relationship between 1001 plasma proteins and type 2 diabetes. Cochran’s Q test evaluated the heterogeneity of genetic instruments (P < 0.05), and the MR-Egger intercept test assessed horizontal pleiotropy (P < 0.05). The False Discovery Rate (FDR) was employed for multiple testing corrections, with IVW-FDR < 0.05 serving as the significance threshold. The FDR is widely recognized as a robust statistical method for controlling the false positive rate in the context of multiple hypothesis testing. In comparison to the Bonferroni correction, FDR offers a more rigorous statistical framework for conducting multiple hypothesis tests21. Moreover, given that mediation analysis necessitates the absence of bidirectional associations between exposure and outcome, reverse MR was utilized to investigate the reverse relationship between type 2 diabetes and BDH122. Lastly, the study decomposed the total effect of BDH1 on type 2 diabetes into direct and indirect effects. The coefficient product method was employed to estimate the indirect effect — specifically, the influence of BDH1 on type 2 diabetes through intermediate pathways(1001 plasma proteins). Dividing the indirect effect by the total effect determined the proportion of mediation for each mediator23.
All statistical analyses were conducted using the TwoSampleMR package (version 0.5.6) within the R software environment (version 4.3.3). Significance was evaluated using stringent two-tailed statistical testing criteria.
Result
Instrument selection
Tables S2, S3, and S5 provide comprehensive details on all SNPs utilized in this study. In summary, 38 SNPs were employed to examine the relationship between BDH1 and type 2 diabetes, while 4 SNPs were used to investigate the association between BDH1 and plasma proteins linked to type 2 diabetes. Additionally, owing to the variability in SNPs across different plasma proteins, a minimum of 4 SNPs were selected to assess the association between 1,001 plasma proteins and type 2 diabetes. All chosen genetic instruments exhibited F statistics surpassing 10, underscoring the study’s mitigation of biases stemming from feeble instrumental variables.
Effect of BDH1 on type 2 diabetes
Tables S2 and Fig. 2A present the evaluation results regarding the bidirectional association between BDH1 and type 2 diabetes. The results of the IVW analysis demonstrate that each 1-standard deviation increase in BDH1 is associated with a 3% reduction in the risk of type 2 diabetes (OR: 0.97; 95% CI: 0.95, 0.99). Despite the lack of significance in MR Egger, Weighted Median, Simple Mode, and Weighted Mode sensitivity analyses, all these methods consistently revealed directional findings aligned with IVW analysis. Furthermore, Cochran’s Q test and MR-Egger intercept regression detected no evidence of heterogeneity or horizontal pleiotropy. Moreover, bidirectional causal analysis using MR revealed no evidence of reverse causation between type 2 diabetes and BDH1, providing adequate justification for subsequent mediation analysis(Fig. 2B).
Forest plot for bidirectional MR analysis of direct causality between BDH1 and type 2 diabetes. (A) MR for assessing the association of BDH1 with type 2 diabetes mellitus; (B) Reverse MR for assessing the association between type 2 diabetes and BDH1. BDH1, 3-hydroxybutyrate dehydrogenase 1; MR, Mendelian randomization.
Proteome-wide MR analysis identified 37 plasma proteins for type 2 diabetes
Table S3 and Fig. 3A illustrate that IVW analysis detected 156 plasma proteins significantly linked with type 2 diabetes (P < 0.05). Even after FDR correction, 55 plasma proteins maintained consistent associations with type 2 diabetes (IVW-FDR < 0.05). Of these, 26 plasma proteins exhibited a negative correlation with the risk of type 2 diabetes, while the remaining 29 showed a positive correlation. Predictions of genetic risk for type 2 diabetes ranged from 0.79 for keratin 18(KRT18) to 1.47 for RAB37, member RAS oncogene family(RAB37). Although Cochran’s Q test detected heterogeneity in some results, sensitivity analyses including MR Egger, Weighted Median, Simple Mode, and Weighted Mode consistently confirmed the trends observed in IVW, indicating negligible heterogeneity. Additionally, MR-Egger intercept regression identified horizontal pleiotropy in the study results of 18 plasma proteins, including abhydrolase domain containing 14B(ABHD14B) and adenosine deaminase 2(ADA2) (see Table S4).
Proteome-wide MR analysis identified 27 plasma proteins for BDH1
Table S5 and Fig. 3A illustrate that IVW analysis detected 27 plasma proteins significantly associated with BDH1 (P < 0.05). Among these, BDH1 exhibited negative correlations with 17 plasma proteins and positive correlations with 10 plasma proteins. Despite Cochran’s Q test detecting heterogeneity in some results, sensitivity analyses including MR Egger, Weighted Median, Simple Mode, and Weighted Mode consistently corroborated the trends observed in IVW, suggesting minimal heterogeneity. Furthermore, MR-Egger intercept regression found no evidence of horizontal pleiotropy (see Table S6).
NBN mediates the protective effects of BDH1 on type 2 diabetes
Table S7 and Fig. 3B illustrate the potential mediating effects of nibrin(NBN), arginase 1(ARG1), and C-C motif chemokine ligand 11(CCL11) in the protective mechanism of BDH1 against type 2 diabetes, with mediation proportions of 62.2%, 22.7%, and 17.6%, respectively. Regrettably, no additional plasma proteins exhibited potential mediating effects.
Discussion
This MR study utilized various analytical methods to explore the interrelations among BDH1, plasma proteins, and type 2 diabetes. The study revealed that for every 1 standard deviation increase in BDH1, the risk of developing type 2 diabetes decreases. Moreover, the study identified 37 plasma proteins associated with type 2 diabetes and 27 plasma proteins associated with BDH1. Subsequent mediation analysis suggested that NBN, ARG1, and CCL11 may mediate BDH1’s protective effect against type 2 diabetes.
First and foremost, BDH1 exerts a protective effect against type 2 diabetes. BDH1 is an enzyme crucial for cellular metabolism, lipid metabolism, and redox reactions, facilitating the interconversion of acetoacetic acid and β-hydroxybutyrate with NAD+/NADH24,24. Previous studies have indicated a close association between BDH1 and conditions such as pregnancy and cardiovascular diseases26. Brahma et al.27 discovered that diabetes inhibits cardiac ketone oxidation through gene expression regulation, promoting cardiac metabolic reprogramming and reducing the expression of cardiac BDH1 and succinyl-CoA transferase. Moreover, in vitro experiments have demonstrated that BDH1 can mitigate inflammation by modulating the NRF2 pathway, a relationship indisputably linked to type 2 diabetes28,29.
Additionally, we identified 37 plasma proteins that are associated with type 2 diabetes through proteomic analysis. Keratin 18(KRT18), a cytokeratin fragment released by damaged liver cells, is commonly regarded as a biomarker for liver injury30. However, its association with type 2 diabetes remains inconclusive due to conflicting results in existing studies. Observational studies in American and Japanese populations found no significant difference in KRT18 levels between patients with type 2 diabetes and non-diabetic individuals, but they did find associations between KRT18 and glycemic markers such as glycated hemoglobin31,32. Conversely, a study conducted on the Chinese population reported elevated levels of KRT18 in patients with type 2 diabetes30. These conflicting findings may be attributed to sampling errors and small sample sizes. Nonetheless, it highlights the necessity for further investigation into the relationship between KRT18 and type 2 diabetes.
ARG1, a pivotal hydrolytic enzyme in the urea cycle, is primarily responsible for converting L-arginine to L-ornithine and urea33. Overexpression of ARG1 results in decreased arginine levels, reducing substrate availability for the NOS III pathway and promoting endothelial dysfunction development34. An observational study conducted in Germany identified significantly elevated levels of ARG1 in obese patients35. A randomized controlled trial indicated a positive correlation between ARG1 activity and hyperglycemia, implying that heightened activity might impair NO production in patients with type 2 diabetes, a phenomenon insulin could potentially alleviate by moderating ARG1 activity36. Another population-based study pinpointed SNPs rs2781666 and rs2781665 in ARG1 as significant contributors to heightened risk of type 2 diabetes development37. Our study corroborates these findings from previous research, thereby validating the precision of our sample selection. Furthermore, we hypothesize that ARG1 could be a downstream factor influenced by BDH1, although research on the specific mechanisms linking ARG1 and BDH1 remains limited and necessitates further exploration. Furthermore, we identified a potential link between NBN and type 2 diabetes, though current research primarily concentrates on various cancers like breast cancer38, mandating further fundamental research to elucidate these specific associations. CCL11, a chemokine implicated in immune regulation and inflammatory responses, exhibits chemotactic activity toward eosinophils and is thought to be associated with conditions such as atopic dermatitis, allergic rhinitis, asthma, and parasitic infections39. A population-based study has suggested a potential association between CCL11 and type 1 diabetes; however, no research currently exists linking it to type 2 diabetes or related glucose metabolism processes40. Thus, this presents a promising avenue for future investigation.
This study boasts several notable strengths. Firstly, genetic data concerning BDH1, type 2 diabetes, and plasma proteins were obtained from extensive population-based GWAS studies. These studies were meticulously structured to minimize sample overlap, thereby mitigating potential confounding effects arising from shared samples across datasets. Secondly, all selected genetic instruments exhibited F statistics exceeding 10, signifying robust instrument strength. This rigorous selection criterion effectively minimized biases from weaker instruments and bolstered the credibility of the instrumental variables utilized in the analysis. Lastly, Cochran’s Q test and MR-Egger intercept regression were utilized to further assess the reliability and robustness of the study findings. Notably, while the results from four sensitivity analyses, including MR-Egger, Weighted Median, Simple Mode, and Weighted Mode, were non-significant, the findings from Cochran’s Q test and MR-Egger intercept regression, consistent with the inherent assumptions of MR, indicate no evidence of heterogeneity or horizontal pleiotropy between the exposure and outcome. In this context, the IVW analysis offers the most robust explanation of the relationship between BDH1 and type 2 diabetes41.
While this study offers a comprehensive analysis, it is imperative to acknowledge several limitations that may impact the interpretation of our findings. Firstly, MR itself is subject to inherent limitations, including phenotypic heterogeneity and issues related to developmental compensation, which could potentially affect the accuracy and generalizability of our research outcomes42. Secondly, our analyses were based on aggregate-level statistics, which constrained our capacity to perform detailed stratified analyses or conduct in-depth individual-level investigations. Additionally, this limitation prevented us from confirming the potential overlap of samples across the three distinct GWAS studies. Thirdly, given that our study predominantly includes participants of European descent, caution is warranted when extrapolating these results to other populations, such as Asians. Future studies are essential to validate our findings across diverse ethnic groups. Fourthly, the genetic data for BDH1 (N = 31,684, 11,000,000 SNPs) and type 2 diabetes (N = 490,089, 21,311,942 SNPs) were obtained from publicly available summary statistics across two distinct studies. The sample sizes vary substantially, with BDH1 having a comparatively smaller sample size, which may introduce potential bias. Fortunately, the number of SNPs examined in the two studies is relatively similar, which helps mitigate the bias introduced by the disparity in sample sizes to some extent. Lastly, despite identifying potential causal relationships between BDH1, plasma proteins, and type 2 diabetes, our understanding of the underlying mechanisms remains incomplete, underscoring the necessity for further fundamental research to elucidate these intricate pathways. Furthermore, various factors, such as environmental and lifestyle factors, may influence the onset and progression of type 2 diabetes. Consequently, future research should also explore the mediating effects of these factors.
Conclusions
In conclusion, this comprehensive protein-proteomics-based Mendelian randomization study elucidates that BDH1 exerts a protective effect against type 2 diabetes. Additionally, the study identifies 37 plasma proteins closely linked to type 2 diabetes, providing promising insights for advancing strategies to prevent and treat this prevalent metabolic disorder.
Data availability
The datasets supporting the conclusions of this article are included within the article.
References
Zhang, Y. Y., Gui, J., Chen, B. X. & Wan, Q. Correlation of renal function indicators and vascular damage in T2DM patients with normal renal function. Front. Endocrinol. 14, 1292397. https://doi.org/10.3389/fendo.2023.1292397 (2023).
Kato, M. & Natarajan, R. Diabetic nephropathy–emerging epigenetic mechanisms. Nat. Rev. Nephrol. 10 (9), 517–530. https://doi.org/10.1038/nrneph.2014.116 (2014).
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 (2022).
Yuan, S. et al. Plasma proteins and onset of type 2 diabetes and diabetic complications: Proteome-wide mendelian randomization and colocalization analyses. Cell. Rep. Med. 4 (9). https://doi.org/10.1016/j.xcrm.2023.101174 (2023).
Rowley, W. R. et al. : Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 2017;20(1):6–12. (2030). https://doi.org/10.1089/pop.2015.0181
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444 (7121), 840–846. https://doi.org/10.1038/nature05482 (2006).
Luo, M., Yu, C., Del Pozo Cruz, B., Chen, L. & Ding, D. Accelerometer-measured intensity-specific physical activity, genetic risk and incident type 2 diabetes: a prospective cohort study. Br. J. Sports Med. 57 (19), 1257–1264. https://doi.org/10.1136/bjsports-2022-106653 (2023).
Forouhi, N. G., Misra, A., Mohan, V., Taylor, R. & Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 361, k2234. https://doi.org/10.1136/bmj.k2234 (2018).
Flannick, J. et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature 570 (7759), 71–76. https://doi.org/10.1038/s41586-019-1231-2 (2019).
Pan, A. et al. The mitochondrial β-oxidation enzyme HADHA restrains hepatic glucagon response by promoting β-hydroxybutyrate production. Nat. Commun. 13 (1), 386. https://doi.org/10.1038/s41467-022-28044-x (2022).
Lin, J. et al. D-β-Hydroxybutyrate dehydrogenase mitigates Diabetes-Induced atherosclerosis through the activation of Nrf2. Thromb. Haemost. 123 (10), 1003–1015. https://doi.org/10.1055/s-0043-1770985 (2023).
Elhadad, M. A. et al. Deciphering the plasma proteome of type 2 diabetes. Diabetes 69 (12), 2766–2778. https://doi.org/10.2337/db20-0296 (2020).
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27 (8), 1133–1163. https://doi.org/10.1002/sim.3034 (2008).
Levin, M. G. & Burgess, S. Mendelian randomization as a Tool for Cardiovascular Research: a review. JAMA Cardiol. 9 (1), 79–89. https://doi.org/10.1001/jamacardio.2023.4115 (2024).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53 (9), 1300–1310. https://doi.org/10.1038/s41588-021-00913-z (2021).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53 (10), 1415–1424. https://doi.org/10.1038/s41588-021-00931-x (2021).
Suhre, K. Genetic associations with ratios between protein levels detect new pQTLs and reveal protein-protein interactions. Cell. Genomics. 4 (3), 100506. https://doi.org/10.1016/j.xgen.2024.100506 (2024).
Papadimitriou, N. et al. Physical activity and risks of breast and colorectal cancer: a mendelian randomisation analysis. Nat. Commun. 11 (1), 597. https://doi.org/10.1038/s41467-020-14389-8 (2020).
Zeng, W., Jiang, S., Cun, D., Huang, F. & Jiang, Z. Tracing links between micronutrients and type 2 diabetes risk: the singular role of selenium. Front. Endocrinol. 15, 1422796. https://doi.org/10.3389/fendo.2024.1422796 (2024).
Zeng, W. et al. Causal associations between human gut microbiota and osteomyelitis: a mendelian randomization study. Front. Cell. Infect. Microbiol. 14, 1338989. https://doi.org/10.3389/fcimb.2024.1338989 (2024).
Me, G., Sr, R. & Mr, S. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 67 (8). https://doi.org/10.1016/j.jclinepi.2014.03.012 (2014).
Sanderson, E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect. Med. 11 (2), a038984. https://doi.org/10.1101/cshperspect.a038984 (2021).
VanderWeele, T. J. Mediation analysis: a practitioner’s guide. Annu. Rev. Public. Health. 37, 17–32. https://doi.org/10.1146/annurev-publhealth-032315-021402 (2016).
Zhang, H. et al. MTA2 triggered R-loop trans-regulates BDH1-mediated β-hydroxybutyrylation and potentiates propagation of hepatocellular carcinoma stem cells. Signal. Transduct. Target. Ther. 6 (1), 135. https://doi.org/10.1038/s41392-021-00464-z (2021).
Huang, J. et al. BDH1-mediated LRRC31 regulation dependent on histone lysine β-hydroxybutyrylation to promote lung adenocarcinoma progression. MedComm 4 (6), e449. https://doi.org/10.1002/mco2.449 (2023).
Abdul Kadir, A., Clarke, K. & Evans, R. D. Cardiac ketone body metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (6), 165739. https://doi.org/10.1016/j.bbadis.2020.165739 (2020).
Brahma, M. K. et al. Increased glucose availability attenuates myocardial ketone body utilization. J. Am. Heart Assoc. 9 (15), e013039. https://doi.org/10.1161/JAHA.119.013039 (2020).
Xu, B. T. et al. Bdh1 overexpression ameliorates hepatic injury by activation of Nrf2 in a MAFLD mouse model. Cell. Death Discov. 8 (1), 49. https://doi.org/10.1038/s41420-022-00840-w (2022).
Cai, W., Chong, K., Huang, Y., Huang, C. & Yin, L. Empagliflozin improves mitochondrial dysfunction in diabetic cardiomyopathy by modulating ketone body metabolism and oxidative stress. Redox Biol. 69, 103010. https://doi.org/10.1016/j.redox.2023.103010 (2024).
Chang, Y. H. et al. Elevated serum cytokeratin-18 concentration in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Ann. Clin. Biochem. 56 (1), 141–147. https://doi.org/10.1177/0004563218796259 (2019).
Cusi, K. et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 60 (1), 167–174. https://doi.org/10.1016/j.jhep.2013.07.042 (2014).
Miyasato, M. et al. The cytokeratin-18 fragment level as a biomarker of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Clin. Chim. Acta Int. J. Clin. Chem. 433, 184–189. https://doi.org/10.1016/j.cca.2014.03.018 (2014).
M(II)-coordination. Polymers (M = zn and cd) constructed from 1,2-bis[4-(pyridin-3-yl)phenoxy]ethane and 1,4-benzenedicarboxylic acid. J. Mol. Struct. 1084, 1–8. https://doi.org/10.1016/j.molstruc.2014.12.014 (2015).
Regulation of MAP kinase-. Mediated endothelial dysfunction in hyperglycemia via arginase I and eNOS dysregulation. Biochim. Biophys. Acta BBA - Mol. Cell. Res. 1866 (9), 1398–1411. https://doi.org/10.1016/j.bbamcr.2019.05.004 (2019).
Jung, C., Figulla, H. R., Lichtenauer, M., Franz, M. & Pernow, J. Increased levels of circulating arginase I in overweight compared to normal weight adolescents. Pediatr. Diabetes. 15 (1), 51–56. https://doi.org/10.1111/pedi.12054 (2014).
Kashyap, S. R., Lara, A., Zhang, R., Park, Y. M. & DeFronzo, R. A. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care. 31 (1), 134–139. https://doi.org/10.2337/dc07-1198 (2008).
Fawad Ali Shah, S. et al. ARG1 single nucleotide polymorphisms rs2781666 and rs2781665 confer risk of type 2 diabetes mellitus. EXCLI J. 17, 847–855. https://doi.org/10.17179/excli2018-1178 (2018).
Zhong, A., Cheng, C. S., Lu, R. Q. & Guo, L. Suppression of NBS1 upregulates CyclinB to Induce Olaparib Sensitivity in Ovarian Cancer. Technol. Cancer Res. Treat. 23, 15330338231212085. https://doi.org/10.1177/15330338231212085 (2024).
Hs, C. et al. A single nucleotide polymorphism on the promoter of eotaxin1 associates with its mRNA expression and asthma phenotypes. J. Immunol. Baltim. Md. 1950. 174 (3). https://doi.org/10.4049/jimmunol.174.3.1525 (2005).
Z, J. & M, N. Expression of CC chemokines CCL2, CCL5, and CCL11 is associated with duration of disease and complications in type-1 diabetes: a study on Iranian diabetic patients. Clin. Lab. 59, 9–10. https://doi.org/10.7754/clin.lab.2012.120810 (2013).
Sg, S. B. Interpreting findings from mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32 (5). https://doi.org/10.1007/s10654-017-0255-x (2017).
Haycock, P. C. et al. Best (but oft-forgotten) practices: the design, analysis, and interpretation of mendelian randomization studies. Am. J. Clin. Nutr. 103 (4), 965–978. https://doi.org/10.3945/ajcn.115.118216 (2016).
Acknowledgements
We are grateful to the authors and participants of all GWASs for the summary statistics used.
Funding
The Ministry of Science and Technology of China provided funding for this study through grant 2016YFC0901200.
Author information
Authors and Affiliations
Contributions
YYL and YYZ made equal contributions to this work performing the statistical analysis, interpreting the results, writing the paper, and they share the first authorship. QW proposed the ideas. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study is based on publicly available summarized data. Ethical approval and informed consent had been obtained in all original studies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, YY., Zhang, YY. & Wan, Q. Interconnections between BDH1-plasma protein-type 2 diabetes Mellitus: a mediated mendelian randomization analysis using plasma proteomics. Sci Rep 15, 3342 (2025). https://doi.org/10.1038/s41598-025-88196-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88196-w