Abstract
The study aimed to assess the association of five insulin resistance surrogates, namely HOMA-β (Homeostasis Model Assessment of Beta-cell Function), QUICKI (Quantitative Insulin Sensitivity Check Index), IGR (Insulin Glucose Ratio), e-IS (Estimated Insulin Sensitivity), and Bennett ISI(Bennett’s insulin sensitivity index) with all-cause and cardiovascular mortality in respondents with metabolic syndrome(MetS). The prospective cohort of 6662 participants aged 18 years and older with metabolic syndrome was extracted from the National Health and Nutrition Examination Survey(NHANES 1999–2016). The multivariate Cox proportional hazards regression model, Kaplan-Meier survival curve, and log-rank tests were applied to determine the association between the five indices and all-cause mortality and cardiovascular mortality in the MetS population. Restricted cubic splines, a two-piece segmented Cox proportional hazards model, and threshold effect analyses were performed to evaluate the nonlinear relationship. Sensitivity analyses were then conducted by removing individuals with CKD, CHF, CAD, stroke, or cancer, respectively. All five insulin resistance (IR) surrogates displayed a negative association with all-cause and cardiovascular mortality in participants with metabolic syndrome. Restricted cubic spline curves showed QUICKI, IGR, and e-IS had a nonlinear relationship with statistical significance. MetS population at the highest quartile of HOMA-β or IGR exhibited lower all-cause and cardiovascular event probabilities compared with those at the lowest quartile, and e-IS had a similar correlation with cardiovascular events. Threshold effect analyses showed that there were inflection points of IGR for all-cause and cardiovascular mortality. As IGR gradually approached inflection points, the two types of mortality risks descended by 15%[HR 0.85(0.80,0.91)] and 19%[HR 0.81(0.71,0.92)], respectively. Sensitivity analysis indicated most results were robust, but Bennett ISI did not exhibit significant outcomes in participants without CKD. Our study provides evidence that HOMA-β, QUICKI, IGR, e-IS, and Bennett ISI displayed a reverse correlation with all-cause mortality and cardiovascular mortality in participants with metabolic syndrome. The five IR surrogates should be given more attention during the follow-up of MetS population.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS), a metabolic condition known as syndrome X, was defined comparatively completely for the first time by the World Health Organization (WHO) in 19981,2. This condition is characterized by a cluster of abnormalities such as central obesity, dyslipidemia, rising fasting blood glucose and blood pressure, and each metabolic disorder3. The occurrence of metabolic syndrome is closely related to insulin resistance, diabetes, atherosclerotic disorders containing endothelial dysfunction, ischemic stroke, as well as other life-threatening diseases and premature death4. There is no unified diagnostic criteria for metabolic syndrome among populations of different nations, and the prevalence rate varies among studies from different countries5. However, according to a meta-analysis including 28 million patients, global burden of metabolic syndrome(global prevalence:12.5–31.4%), ethnic-specific central obesity(45.1%), systolic blood pressure ≥ 130mmHg and/or diastolic blood pressure ≥ 85mmHg(42.6%), fasting plasma glucose ≥ 5.6mmol/L(24.5%) were aggravated in the recent years, highlighting the urgent and significant global health issue that MetS has caused6.
Patients with metabolic syndrome present a decreased insulin efficiency in accelerating glucose uptake and utilization, promoting glycogen synthesis. The low efficiency of insulin induces excessive insulin secretion produced by β-cells; the number of β cells starts to reduce and blood glucose elevates. Meanwhile, insulin resistance-mediated increase in free fatty acids is considered a key mechanism in the pathogenesis of metabolic syndrome7. Lifestyles such as Western dietary habits are one of the strong determinants for insulin resistance and MetS, and mounting evidence shows genetic factors also share a proportion of the impact8. Hyperinsulinemic-euglycemic clamp (HEC) test has been regarded as the gold standard of insulin resistance, but it is invasive and time-consuming, and an accumulating body of reliable alternative surrogates to be developed. The triglyceride-glucose (TyG) index is recognized as an effective biochemical substitute to differentiate insulin resistance and has been widely researched9,10. Some studies suggested TyG and its derivative indicators were significantly associated with all-cause and cardiovascular mortality in the MetS population, general population, patients with coronary heart disease, or with diabetes11,12. Meanwhile, we noticed there are other novel surrogates encompassing HOMA-β, QUICKI, IGR, e-IS, and Bennett ISI13,14,15,16. And the relationship between the five novel insulin resistance surrogates and all-cause and cardiovascular mortality has not been thoroughly studied.
Hence, the aim of this research intended to evaluate the association between five selected insulin resistance indicators and all-cause mortality and cardiovascular mortality in MetS population.
Materials and methods
Study design and population
The National Health and Nutrition Examination Survey (NHANES) is a multistage, stratified, population-based study program conducted every two years, holds significant importance in the field of public health. It evaluates the health and nutritional status of adults and children in the United States by selecting a representative sample across the country. The data is collected through a rigorous process that includes demography, diet, physical examinations and laboratory tests. The program, approved by the National Center for Health Statistics (NCHS) Ethical Review Board, ensures informed consent from all participants, further emphasizing its credibility and relevance.
The data employed in this study, including the information of participants in nine cycle years from 1999 to 2016, were downloaded from NHANES database. Participants with metabolic syndrome (MetS) aged 18 and above were selected to enroll in the current study. According to the new International Diabetes Federation1,2, metabolic syndrome is defined as having central obesity measured by expanding waist circumference(WC) > 102 cm in men and > 88 cm in women, and meeting two or more diagnostic criteria as follows: (1) Circulating triglycerides(TG) level ≥ 150 mg/dL (1.69 mmol/L); (2) High-density lipoprotein cholesterol (HDL-C) < 40 mg/dL in males and < 50 mg/dL in females; (3) Fasting blood glucose concentration ≥ 100 mg/dL (5.6 mmol/L) or previous diagnosis of diabetes; (4) Systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg, or taking medicines for arterial hypertension. All participants including in the study fasted for more than 8 h and less than 24 h before collecting blood samples, and blood pressure was measured three times by the investigators17,18.
Definition of insulin resistance(IR) indices
We selected five insulin resistance surrogates calculated based on previous literature14,19,20,21,22. The calculation method for these five indicators is as follows: (1) HOMA-β = 20×fasting insulin (µU/ml)/[fasting glucose (mmol/L) − 3.5]; (2) QUICKI index = 1/[log(fasting insulin(µU/mL)) + log(fasting plasma glucose(mg/dL)]; (3) IGR(U/mol) = Serum insulin (µU/mL)/Serum glucose (mmol/L); (4) e-IS = EXP[4.64725 − 0.02032 (waist, cm) − 0.09779×HbA1c(%) − 0.00235×TG(mg/dl)]; (5) Bennett ISI = 1/(ln(FBG)(mmol/L)×ln(FINS)(µU/ml)). All respondents included in this study were divided into four quartiles(Q1, Q2, Q3, Q4) according to insulin resistance indices.
Mortality data
We downloaded the NHANES Public-Use Linked Mortality Files provided by NCHS, and mortality data were updated to December 31, 2019. The study endpoints contained all-cause mortality and cardiovascular mortality. The cause of death was determined by the International Classification of Diseases 10th revision (ICD-10) coding. All-cause mortality encompasses diseases of the heart (054–068), malignant neoplasms(019–043), chronic lower respiratory diseases (082–086), cerebrovascular diseases (070), diabetes mellitus (046), and other reasons. Cardiovascular mortality refers to diseases of the heart (054–068), and all other causes of death were categorized into non-cardiovascular mortality. Participants with missing death-related information were assumed to be alive in the current study.
Baseline information collection and covariates
Interview and examination information and laboratory data included in the study were collected from the NHANES website. Socio-demographic covariates contained age, gender, race/ethnicity, education level, marital status, and poverty-to-income ratio (PIR). Race/Ethnicity was classified as Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other races. Education level was divided into three categories: below high school, high school graduate or GED, and some college or above. PIR as an index of family income was classified into ≤ 1.5, 1.5–4.0, and >4.0. Alcohol use status was categorized into never drinking, 1–5 drinks/day, > 5–10 drinks/day, > 10 drinks/day. The smoking status was categorized as never smoking(smoked less than 100 cigarettes in his/her entire life), former smoking (smoked more than 100 cigarettes but quit before the survey), and current smoking (smoked more than 100 cigarettes and not quit during the survey). Examination data encompassed blood pressure, height, weight, waist circumference, and BMI(kg/m2), which can calculated by dividing weight by the square of height. Laboratory data included total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), HbA1c, fasting blood glucose and insulin, oral glucose tolerance test, serum creatinine, urine albumin, and urine creatinine. The urinary albumin to creatinine ratio(mg/g) was calculated by dividing urine albumin by urine creatinine, and the estimated glomerular filtration rate (eGFR) was calculated by CKD-EPI equations. Questionnaire information involved a medical history of congestive heart failure(CHF), coronary artery disease (CAD), Chronic kidney disease (CKD), cancer, and diabetes. CHF was determined by self-reported positive answer to “Ever told had congestive heart failure?”. CAD was defined by any affirmative answer to “Ever told you had coronary heart disease?”, “Ever told you had angina/angina pectoris?”, or “Ever told you had heart attack?”. And CKD was diagnosed as: (1) eGFR<60 ml/min/1.73m2;(2) urinary albumin to creatinine ratio(UACR) ≥ 30 mg/g; (3) previous diagnosis of CKD(answered “yes” to the question “Ever told you have a weak kidney?”). Diabetes was defined as FBG ≥ 7.0mmol/L, HbA1c ≥ 6.5%, two-hour glucose concentration(OGTT) ≥ 11.1mmol/L, or previous diagnosis of diabetes. Further information on the above index can be accessible at https://www.cdc.gov/nchs/nhanes.
Statistical analysis
Since the proportion of missing data for all variables is less than 20%, and multiple imputation by chained equations (MICE) were conducted to impute the missing values utilizing R package “mice” for further analysis. Based on the need to present all participants’ baseline indicators, the respondents were categorized into the surviving group and the deceased group according to the participants’ survival status. Continuous variables that conform to normal distribution were displayed as mean ± standard deviation (SD), and other variables were presented as median and quartiles, while the t-test or Wilcoxon rank-sum test was applied to detect statistical significance of discrepancy according to variable conditions. Categorical variables were shown as frequencies and proportions, and the chi-square test was used to determine whether differences existed between the two groups. To ascertain the association between selected insulin resistance indices and all-cause and cardiovascular mortality, we classified all individuals into four quartiles by arranging the IR indicators in ascending order. Then, Cox proportional hazards regression models were established to evaluate potential associations. The proportional hazards assumption was tested by Schoenfeld residuals. Moreover, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to assess the linear association. Three models were utilized to control potential confounding factors: Model 1 was unadjusted. Model 2 was adjusted for age, gender, and race/ethnicity. Model 3 was adjusted for age, gender, race/ethnicity, educational level, marital status, smoking status, alcohol use, BMI, TC, HDL, LDL-C, SCr, eGFR, and UACR. Model 4 was further adjusted for CKD, CHF, CAD, stroke, and Cancer. Restricted cubic spline(RCS) regression with 3–5 knots was utilized to evaluate the nonlinear relationship between respondents and surviving status. After determining the nonlinear relationship, the threshold effect was further analyzed. The inflection point was detected by recursive algorithms and the maximum likelihood ratio method, and a two-piece segmented Cox proportional hazards model was adjusted for confounding factors in Model 4 to analyze threshold effects. Likelihood ratio tests were performed to confirm the nonlinear relationship further. Based on insulin resistance surrogate quartiles, we conducted the Kaplan-Meier (K-M) survival analyses and log-rank tests to explore the survival condition of four quartiles. Sensitivity analysis was performed by conducting the same data analysis process in participants with no clinical manifestation of CHF, CAD, CKD, stroke, or cancer. R software(R version 4.3.2) and IBM SPSS Statistics 27 were applied for all data analysis.
Results
Baseline characteristics of study participants
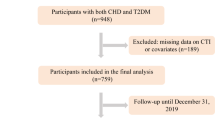
After excluding participants aged <18 years old, participants without blood sample data, with metabolic syndrome, and without incomplete insulin resistance indices, 6662 individuals were finally included in this analysis. And all enrolled participants were categorized into two groups based on their survival status(Table 1). The findings after a follow-up period of 122 months per person and a total of 817,690 person-months, revealed significant differences between the two groups. Individuals who did not survive had notably higher levels of LDL, FBG, e-IS, Bennett ISI, TyG, HOMA-IS, HOMA-β, and all other selected insulin resistance surrogates, except for the QUICKI index, and the p-value of QUICKI is close to the statistically significant value. These individuals were also older, had a higher proportion of women, a smaller proportion of higher education attainment, and significantly more comorbidities such as CAD, CHF, and diabetes.
Association of five selected insulin resistance indicators with all-cause and cardiovascular mortality
Respondents were classified into four quartiles based on each insulin resistance indicator. Cox regression models were applied to evaluate the association between the indicators and survival status (Table 2). Models 1–4 were set to adjust potential confounding factors. All insulin resistance indicators were significantly associated with all-cause, and cardiovascular mortality has statistical significance in respondents with metabolic syndrome. The above results remain the same as those of the unadjusted model after adjustment. All five indices showed a significant negative correlation, whether all-cause or cardiovascular mortality. The RCS regression was applied to detect the nonlinear relationship of the five surrogates with survival status. As is shown in Fig. 1, the fully adjusted spline plots displayed an L-shaped relationship between QUICKI, IGR, e-IS, and all-cause or cardiovascular mortality, with statistical significance confirming a nonlinear relation. Moreover, as the increasing of three surrogates, the risk of death gradually declined. After adjusting all the covariates in Model 4, threshold effect analyses unfolded the inflection points of IGR for all-cause mortality and cardiovascular mortality were 2.34 and 2.34, respectively. As IGR gradually approached inflection points, the two types of mortality risks descended by 15%[HR 0.85(0.80,0.91)] and 19%[HR 0.81(0.71,0.92)], respectively (Table 3). Nevertheless, when IGR exceeded thresholds, there was no noticeable increase in mortality risks. Then, the Kaplan-Meier survival curve and log-rank tests indicated that the MetS population at Q4 of HOMA-β and IGR showed significantly lower death occurrence probability. At the same time, e-IS appeared to have an analogous correlation only with cardiovascular mortality(Fig. 2).
Sensitivity analysis
Our sensitivity analysis, as presented in Supplementary Tables 1–5, underscored the robustness of the above results. Even after the exclusion of participants with CHF, CAD, stroke, cancer, or CKD, HOMA-β, QUICKI, IGR, and eIS maintained their robust performance. However, Bennett ISI did not show a significant association with all-cause and cardiovascular mortality in participants without CKD.
Discussion
In this retrospective study, we investigated the association between five newly identified indicators of insulin resistance and the risk of all-cause or cardiovascular mortality among 6,662 individuals with metabolic syndrome. The study observed that MetS popuationwho passed away during the follow-up period were predominantly female, had a lower education level and higher PIR indicating a potentially lower socioeconomic status, lacked a partner, exhibited poor blood glucose management, had deteriorated renal function, and showed pronounced insulin resistance. Interestingly, all participants had a BMI greater than 20 kg/m2, which seems to suggest that people with a lean physique are less likely to develop metabolic syndrome.The five surrogate indicators displayed a negative association with both all-cause and cardiovascular mortality rates. The QUICKI, e-IS, and IGR notably demonstrated significant L-shaped or U-shaped risk associations. Furthermore, within the MetS cohort, the subgroups with the highest levels of HOMA-β and IGR experienced a higher incidence of mortality events when compared to those with the lowest levels.
Metabolic syndrome is frequently marked by insulin resistance, which typically emerges 10 to 20 years prior to the development of diabetes and is recognized as an independent risk factor for diabetes23. There is a significant demand for the development of alternative, non-invasive indicators to quantitatively assess insulin resistance, including HOMA-IR, HOMA-β, IGR, e-IS, and TyG, as alternatives to the traditional hyperinsulinemic-euglycemic clamp test24,25. These surrogates of insulin resistance have been linked to an increased risk of cardiovascular disease, hypertension, diabetes, and chronic renal failure. TyG, in particular, has been associated with a higher incidence of CAD, myocardial infarction, and both all-cause and cardiovascular mortality in individualswith CAD or diabetes. Meanwhile, elevated levels of HOMA-IR have been correlated with CAD within the diabetic population26,27,28,29.
Our study focused on less commonly examined indicators. HOMA-β, the reciprocal of HOMA-IR, is an established indicator of pancreatic β cell function and exhibits a positive correlation30. Kiyohara and colleagues identified a link between low HOMA-β and an increased risk of poor neurocognitive function in patients with ischemic stroke31. Additionally, Zhou and colleagues found a negative association between HOMA-β and all-cause mortality in non-diabetic patients with ischemic stroke32. Our research tried to explore the relationship between HOMA-β and all-cause or cardiovascular mortality in adults with metabolic syndrome, revealing a negative correlation with a non-significant nonlinear pattern. QUICKI, which has been extensively validated against the standard hyperinsulinemic-euglycemic clamp method, is utilized to evaluate insulin sensitivity and resistance in diabetic or prediabetic patients33. Our findings indicate that QUICKI has a negative relationship with all-cause and cardiovascular mortality in the MetS population, aligning with our previous understanding of insulin sensitivity. The e-IS index, developed and validated by Cree-Green et al., is a relatively applied measure that correlates with the glucose-to-insulin ratio, which is the reciprocal of IGR. And e-IS performed excellently in identifying insulin resistance with a good sensitivity (100%) and specificity (71%) compared to the HEC13. Similar to other IR surrogates, e-IS showed a negative correlation with two types of mortality risks. Concurrently, there was a significant 20% reduction in the risk of all-cause mortality as e-IS levels approached 4.22 [HR 0.80(0.74,0.87)]. The performance of e-IS in the threshold effect analysis mirrored that of IGR, a similarity that is easily explained. The Bennett ISI, an index initially based on fasting insulin to assess the insulin sensitivity, has not gained as much popularity as HOMA-IR and HOMA-β34. In our study, although a correlation was detected, the sensitivity analysis was not robust, indicating a need for more reliable evidence to substantiate the effectiveness of this index. It is noteworthy that the conclusions drawn from the sensitivity analysis were in line with the majority of our findings.
Threshold effect analysis displayed that IGR and e-IS had an L-shaped association. However, only one segment of the curve had statistical significance when applying a two-piece segmented Cox proportional hazards model, which may indicate there was a threshold to distinguish between insulin resistance and non-resistance or whether it can have an impact on mortality rate. In the present study, the threshold of IGR for all-cause and cardiovascular mortality was 2.34, and IGR for all-cause mortality was 4.22. However, this study was only based on a single cohort and needs more research to validate the outcome.
Multiple interpretation of intricate correlation mechanisms between insulin resistance and CVD may possibly be involved in people with MetS. Previous studies have shown that IR can accelerate atherosclerosis and promote the formation of arterial plaques accompanied by hyperglycemia, dyslipidemia, hypertension, etc. During the process, disrupted IR/PI3K/PDK/Akt signaling pathway, downstream activation of mTOR and inhibition of FoxO and GSK3 in vascular endothelial cells, smooth cells and macrophages play a vital role35,36. Insulin may trigger the increasing production of vasoconstrictor factors including endothelin in the state of IR, thereby precipitating pathological vascular stiffening and vasoconstriction37,38. Meanwhile, hyperglycemia induces ROS generation and NF-kB activation, leading to aggravated oxidative stress, systemic inflammation, dysfunctional vasodilation and vascular damage with increased advanced glycation end products (AGEs), adhesion molecules, matricellular proteins, and cytokines etc38,39.
To our knowledge, our study is pioneering in exploring the relationship between HOMA-β, QUICKI, IGR, e-IS, Bennett ISI, and long-term prognosis of MetS population. Our research benefits from a lengthy mean follow-up time of 122 months per person, following reports on other insulin resistance indices such as HOMA-IR, TyG, TyG-WC40. Furthermore, the sample was derived from a nationally representative, population-based cohort with a substantial sample size, enhancing the generalizability of our findings. Additionally, we conducted threshold effect analyses on the five indices, revealing significant risk change in IGR that could inform the development of future clinical prognostic models. However, our study has some limitations. Firstly, NHANES is a cross-sectional study conducted every two years in which information such as personal medical history of CVD and hypertension was obtained through interviews and questionnaires. Hence, there were potential risks of recall bias, reporting bias and interviewer bias leading to inaccurate results. Secondly, because the subjects surveyed in each round of NHANES were different from those previously studied, so we can not explore how temporal changes in the five IR proxies influence the outcomes of MetS population. Thirdly, despite adjusting for numerous covariates to ensure the accuracy of our results, there may be unaccounted potential confounding factors that could introduce bias. Finally, sensitivity analyses suggest that the results for Bennett ISI were not as robust, warranting caution it interpreting its findings. And these findings for Bennett ISI may need to be further verified through data from other sources.
Conclusions
In conclusion, our study has identified a negative correlation between the five insulin resistance surrogates—HOMA-β, QUICKI, IGR, e-IS, and Bennett ISI and the risk of all-cause and cardiovascular mortality among participants with metabolic syndrome. Specifically, a statistically significant L-shaped association was observed between QUICKI, IGR, and e-IS with both all-cause and cardiovascular mortality. While these findings are promising, sensitivity analyses suggest that interpreting Bennett ISI results requires caution. This caution is warranted due to the less robust nature of the data for Bennett ISI, which may be influenced by the limited scope of the available data for further verification.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used Grammarly in order to correct spelling and polish grammar. After using this tool/service, the author(s) reviewed and edited the content as needed and take full responsibility for the content of the publication.
Restricted spline curves showed the nonlinear association of the five insulin resistance indices and all-cause(A-E) and cardiovascular mortality(F-J) in population with metabolic syndrome after adjusted for age, gender, race/ethnicity, educational level, marital status, smoking status, alcohol use, BMI, TC, HDL-C, LDL-C, SCr, eGFR, UACR, CKD, CHF, CAD, stroke, and Cancer.(A) the association between HOMA-β and all-cause mortality; (B) the association between QUICKI and all-cause mortality; (C) the association between IGR and all-cause mortality; (D) the association between eIS and all-cause mortality; (E) the association between Bennett ISI and all-cause mortality; (F) the association between HOMA-β and cardiovascular mortality; (F) the association between QUICKI and cardiovascular mortality; (G) the association between IGR and cardiovascular mortality; (H) the association between e-IS and cardiovascular mortality; (I) the association between Bennett ISI and cardiovascular mortality. HR hazard ratio, CI confidence interval, HOMA-β homeostasis model assessment of β-cell function, QUICKI quantitative insulin sensitivity check index, IGR fasting insulin to glucose ratio, e-IS estimate of insulin sensitivity, Bennett ISI Bennett’s insulin sensitivity index, eGFR estimated glomerular filtration rate, UACR urinary albumin to creatinine ratio, CHF congestive heart failure, CAD coronary artery disease, CKD Chronic kidney disease.
Kaplan-Meier survival analyses and log-rank tests for all-cause and cardiovascular mortality of four groups classified by the ascending rank of five insulin resistance indicators including HOMA-β, QUICKI, IGR, e-IS, and Bennett ISI. HOMA-β homeostasis model assessment of β-cell function, QUICKI quantitative insulin sensitivity check index, IGR fasting insulin to glucose ratio, e-IS estimate of insulin sensitivity, Bennett ISI Bennett’s insulin sensitivity index.
Data availability
All data analyzed in this article can be found in the NHANES public website(https://www.cdc.gov/nchs/nhanes/index.htm).
References
Weihe, P. & Weihrauch-Blüher, S. Metabolic syndrome in children and adolescents: diagnostic criteria, Therapeutic options and perspectives. Curr. Obes. Rep. 8 (4), 472–479. https://doi.org/10.1007/s13679-019-00357-x (2019).
AlbertiKG et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120 (16), 1640–1645. https://doi.org/10.1161/circulationaha.109.192644 (2009).
Saklayen, M. G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 20 (2), 12. https://doi.org/10.1007/s11906-018-0812-z (2018).
Grundy, S. M. et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J. Am. Coll. Cardiol. 44 (3), 720–732. https://doi.org/10.1016/j.jacc.2004.07.001 (2004).
O’Neill, S. & O’Driscoll, L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 16 (1), 1–12. https://doi.org/10.1111/obr.12229 (2015).
Noubiap, J. J. et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 188, 109924. https://doi.org/10.1016/j.diabres.2022.109924 (2022).
Rochlani, Y. et al. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 11 (8), 215–225. https://doi.org/10.1177/1753944717711379 (2017).
Brown, A. E. & Walker, M. Genetics of insulin resistance and the metabolic syndrome. Curr. Cardiol. Rep. 18 (8), 75. https://doi.org/10.1007/s11886-016-0755-4 (2016).
Hong, S., Han, K. & Park, C. Y. The insulin resistance by triglyceride glucose index and risk for dementia: population-based study. Alzheimers Res. Ther. 13 (1), 9. https://doi.org/10.1186/s13195-020-00758-4 (2021).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc. Diabetol. 22 (1), 34. https://doi.org/10.1186/s12933-023-01762-2 (2023).
Yao, Y. et al. The association between TyG and all-cause/non-cardiovascular mortality in general patients with type 2 diabetes mellitus is modified by age: results from the cohort study of NHANES 1999–2018. Cardiovasc. Diabetol. 23 (1), 43. https://doi.org/10.1186/s12933-024-02120-6 (2024).
Liu, X. et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc. Diabetol. 21 (1), 124. https://doi.org/10.1186/s12933-022-01546-0 (2022).
Cree-Green, M. et al. Using simple clinical measures to predict insulin resistance or hyperglycemia in girls with polycystic ovarian syndrome. Pediatr. Diabetes. 19 (8), 1370–1378. https://doi.org/10.1111/pedi.12778 (2018).
Hou, X. Z. et al. Association between different insulin resistance surrogates and all-cause mortality in patients with coronary heart disease and hypertension: NHANES longitudinal cohort study. Cardiovasc. Diabetol. 23 (1), 86. https://doi.org/10.1186/s12933-024-02173-7 (2024).
Ellerbrock, J. et al. Role of Beta cell function and insulin resistance in the development of gestational diabetes Mellitus. Nutrients 14 (12). https://doi.org/10.3390/nu14122444 (2022).
Sánchez-Villanueva, R. et al. Repeated analysis of estimated insulin resistance using the HOMAIR index in nondiabetic patients on peritoneal dialysis and its relationship with cardiovascular disease and mortality. Nefrologia 33 (1), 85–92. https://doi.org/10.3265/Nefrologia.pre2012.Nov.11430 (2013).
Zipf, G. et al. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. 1 (56), 1–37 (2013).
Alizargar, J. et al. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc. Diabetol. 19 (1), 8. https://doi.org/10.1186/s12933-019-0982-2 (2020).
Zhao, Q. et al. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc. Diabetol. 20 (1), 190. https://doi.org/10.1186/s12933-021-01383-7 (2021).
Xue, Y. et al. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front. Endocrinol. (Lausanne). 13, 951689. https://doi.org/10.3389/fendo.2022.951689 (2022).
Baghbani-Oskouei, A. et al. Impact of 3-year changes in fasting insulin and insulin resistance indices on incident hypertension: Tehran lipid and glucose study. Nutr. Metab. (Lond). 16, 76. https://doi.org/10.1186/s12986-019-0402-3 (2019).
Han, Y. et al. The Association of Surrogates of Insulin Resistance with hyperuricemia among middle-aged and older individuals: a Population-based Nationwide Cohort Study. Nutrients 15 (14). https://doi.org/10.3390/nu15143139 (2023).
Borai, A. et al. Selection of the appropriate method for the assessment of insulin resistance. BMC Med. Res. Methodol. 11, 158. https://doi.org/10.1186/1471-2288-11-158 (2011).
Roberts, C. K., Hevener, A. L. & Barnard, R. J. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr. Physiol. 3 (1), 1–58. https://doi.org/10.1002/cphy.c110062 (2013).
Zhang, Y. et al. Evaluation of insulin sensitivity by hyperinsulinemic-euglycemic clamps using stable isotope-labeled glucose. Cell. Discov. 4, 17. https://doi.org/10.1038/s41421-018-0016-3 (2018).
Tao, L. C. et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc. Diabetol. 21 (1), 68. https://doi.org/10.1186/s12933-022-01511-x (2022).
Wang, T. et al. Association between Insulin Resistance and Cardiovascular Disease Risk varies according to glucose tolerance status: a nationwide prospective cohort study. Diabetes Care. 45 (8), 1863–1872. https://doi.org/10.2337/dc22-0202 (2022).
Mahdavi-Roshan, M. et al. Evaluating the use of novel atherogenicity indices and insulin resistance surrogate markers in predicting the risk of coronary artery disease: a case–control investigation with comparison to traditional biomarkers. Lipids Health Dis. 21 (1), 126. https://doi.org/10.1186/s12944-022-01732-9 (2022).
Ren, X. et al. Association between triglyceride-glucose index and chronic kidney disease: a cohort study and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 33 (6), 1121–1128. https://doi.org/10.1016/j.numecd.2023.03.026 (2023).
WA, E. L. et al. The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients 11 (1). https://doi.org/10.3390/nu11010044 (2018).
Kiyohara, T. et al. β-Cell function and clinical outcome in nondiabetic patients with Acute ischemic stroke. Stroke 52 (8), 2621–2628. https://doi.org/10.1161/strokeaha.120.031392 (2021).
Zhou, M. et al. Association between β-cell function estimated by HOMA-β and prognosis of non-diabetic patients with ischaemic stroke. Eur. J. Neurol. 25 (3), 549–555. https://doi.org/10.1111/ene.13546 (2018).
Muniyappa, R. et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 294 (1), E15–26. https://doi.org/10.1152/ajpendo.00645.2007 (2008).
Khamseh, M. E. et al. Insulin Resistance/Sensitivity measures as screening indicators of metabolic-Associated fatty liver disease and liver fibrosis. Dig. Dis. Sci. 69 (4), 1430–1443. https://doi.org/10.1007/s10620-024-08309-9 (2024).
Huang, P. L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2 (5–6), 231–237. https://doi.org/10.1242/dmm.001180 (2009).
Bornfeldt, K. E. & Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell. Metab. 14 (5), 575–585. https://doi.org/10.1016/j.cmet.2011.07.015 (2011).
Muniyappa, R. et al. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am. J. Physiol. Endocrinol. Metab. 319 (3), E629–e46. https://doi.org/10.1152/ajpendo.00247.2020 (2020).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766. https://doi.org/10.1016/j.metabol.2021.154766 (2021).
Beverly, J. K., Budoff, M. J. & Atherosclerosis Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes. 12 (2), 102–104. https://doi.org/10.1111/1753-0407.12970 (2020).
Wei, X. et al. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc. Diabetol. 23 (1), 134. https://doi.org/10.1186/s12933-024-02215-0 (2024).
Funding
This work was supported by the Peking Union Medical College Hospital Young Talent Development Program (UBJ10262).
Author information
Authors and Affiliations
Contributions
Jinhao Liao and Hongbo Yang conceptualized the entire research. Jinhao Liao wrote the main manuscript text and prepared tables and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liao, J., Wang, L., Duan, L. et al. Insulin resistance surrogates are associated with all-cause mortality and cardiovascular mortality in population with metabolic syndrome: a retrospective cohort study of NHANES. Sci Rep 15, 4706 (2025). https://doi.org/10.1038/s41598-025-88296-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88296-7
Keywords
This article is cited by
-
Association between estimated glucose disposal rate and future cardiovascular disease risk across glucose metabolism status: a prospective cohort study
Diabetology & Metabolic Syndrome (2025)