Abstract
We summarize the chest CT manifestations and prognoses of children with Mycoplasma pneumoniae pneumonia combined with necrotizing pneumonia. We retrospectively analyzed the chest CT manifestations and prognoses of 155 cases of necrotizing pneumonia in children due to Mycoplasma pneumoniae infection and compared the differences in clinical features and laboratory indices between the group with unilateral monolobar necrosis of the lungs (Group A) and the group with unilateral multilobar and bilateral necrosis (Group B). The chest CT findings of the children in both groups revealed that the area of lung necrosis was confined to the unilateral monolobe in 124 children. The necrotic condition of the lungs included only hypodense shadows in 80 children (51.61%) and cystic cavities in the necrotic areas in 75 children (48.39%). Bronchoscopic manifestations: Endobronchitis was present in 135 children, ulcerative necrosis of the bronchi in 47, and occlusive bronchitis in four. A total of 101 children were followed up. A small percentage of patients have residual manifestations such as lobar atelectasis and bronchial wall changes. The number of days of fever and cases of respiratory distress were significantly greater in group B than in group A. Chest CT reveals pulmonary necrosis in children due to Mycoplasma pneumoniae infection: the area of pulmonary necrosis is mostly unilateral and unilobar, the lower lungs are predominant, and areas of reduced enhancement can be seen on enhanced CT. CT manifestations after clinical treatment may be approximately normal or leave a striped shadow, lung atelectasis, or pleural thickening.
Similar content being viewed by others
Introduction
Mycoplasma pneumoniae (MP) infection is usually considered a self-limiting disease, with clinical manifestations that are in most cases mild, similar to the respiratory symptoms caused by other pathogen infections, and lack specific manifestations1. Mycoplasma pneumoniae pneumonia (MPP) is often not detected in time because of the lack of reliable immediate diagnosis1. MP is one of the major pathogens that causes respiratory infections in humans. MP has now become one of the most common pathogens of community-acquired pneumonia in children, accounting for approximately 10–40% of community-acquired pneumonia cases2. Some studies have shown that the positive detection rate of MP in Chinese children with community-acquired pneumonia reaches 10–30%3. The main clinical manifestations of children with MPP are fever and persistent dry cough. In addition to respiratory symptoms, MP infections can lead to a variety of serious intrapulmonary and extrapulmonary injuries, such as necrotizing pneumonia (NP), pulmonary embolism, central nervous system inflammation, acute myocardial injury, and thrombosis1. NP in children is characterized by liquefaction necrosis of lung tissue and is a serious complication of community-acquired pneumonia in children, accounting for approximately 9.0% of community-acquired pneumonia in children4. In recent years, with the gradual increase in the incidence of MPP, the number of NPs caused by MP infections has also increased annually5,6. Children with NP progress rapidly and are associated with a greater risk of complications, such as sepsis, pneumothorax, and respiratory failure7,8. Therefore, understanding the clinical features, imaging manifestations and laboratory tests of children with MPP combined with NP is important for clinicians to recognize NP at an early stage to improve the prognosis of these children. There are fewer relevant studies describing the CT manifestations of children with MP infection leading to NP. In this study, the clinical data of 155 children with MPP combined with NP were included, and their characteristics were analyzed to provide a reference for clinicians.
Materials and methods
Study population
In this study, we used 155 children with MPP combined with NP hospitalized at the Children’s Hospital Affiliated with Zhengzhou University from January 2023 to May 2024 as the study population. They were divided into group A (124 patients) and group B (31 patients) according to the size of the area of distribution of lung necrosis.
Inclusion and exclusion criteria
The diagnostic criteria for MPP are as follows9,10: (1) fever, cough, shortness of breath and abnormal breath sounds; (2) typical chest imaging changes such as interstitial infiltrates, segmental and lobar solid changes, and hilar lymph node enlargement; and (3) positive for MP-DNA or RNA. A diagnosis is made when the above conditions are met.
Assays for MP-DNA or RNA: The test specimens were derived from pharyngeal swabs and sent for testing within 12 h, and the DNA and RNA of MP were detected by fluorescent PCR, and detected by Mycoplasma pneumoniae Nucleic Acid and Resistance Mutation Site Detection Kit (Merle, Jiangsu, China).
The inclusion criteria were as follows: (1) age greater than 1 year and less than 18 years; (2) met the diagnostic criteria for MPP; and (3) multiple air-containing cystic cavities or thin-walled cavities without fluid‒air flattening in the area of solid lung lesions on chest CT10,11. Enhanced CT of the chest may reveal areas of hypodense necrosis.
The exclusion criteria were as follows: (1) children with cavitary lung disease, such as pulmonary tuberculosis or pulmonary abscess; (2) children with preexisting diseases of the blood system, cardiovascular system, bronchial asthma, recurrent respiratory infections, immune deficiency, etc.; and (3) patients whose clinical information was incomplete.
Criteria for grouping
The criteria for Group A patients were as follows: the area of lung necrosis was confined to a single lobe of the lung. The criteria for group B were as follows: areas of lung necrosis distributed in unilateral multilobar or bilateral areas of the lung.
Data collection
We collected clinical data related to two groups of children with MPP combined with NP through the medical record system, including (1) general information, age, sex, and days of hospitalization; (2) clinical manifestations such as chest pain, dyspnea, days of fever, and the occurrence of intrapulmonary complications such as pleural effusion, mixed infection, and pulmonary embolism; (3) imaging manifestations such as chest CT and bronchoscopic features; and (4) laboratory test indicators.
CT examination method
CT examination was performed via a Philips BrillianceiCT 64-row spiral CT scanner with a scanning range from the lung apex to the lung base, a scanning interval of 8 mm and a layer thickness of 2 mm. During the scan, the child lies flat on the scanning bed; smaller children need to be accompanied or sedated by a parent and repositioned according to the radiographer’s request for a more satisfactory image.
CT-enhanced scans were performed by injecting nonionic contrast agent (Omnipaque, iodine concentration 300 mg/mL) via a forearm vein at a dose of 2.0–2.5 mL/kg and a flow rate of 0.5–2.5 mL/s, and arterial-phase images were acquired 30 s after injection.
Image analysis
Two pediatric diagnostic imaging physicians with more than 10 years of experience read the films independently and negotiated a consensus conclusion in case of disagreement. The observations included the following: (1) lung necrosis (low-density shadow only, cystic cavity), areas of distribution of lung necrosis (unilateral unilobar, unilateral multilobar and bilateral distribution), areas of distribution of lung solidity (unilateral unilobar, unilateral multilobar and bilateral distribution), pulmonary atelectasis, and pulmonary embolism; and (2) other extrapulmonary lesions: whether the pleura is thickened, whether there is pleural effusion, and whether there is fluid pneumothorax.
Bronchoscopy
Bronchoscopy and alveolar lavage were performed in 145 children with MPP combined with NP.
Observations: bronchial wall alterations: presence of endobronchitis, sputum embolism, ulcerative necrosis, stenosis, occlusive bronchitis, and plastic bronchitis.
Statistical analyses
We used SPSS software (version 21.0) for the statistical analysis. Quantitative information that conformed to a normal distribution was expressed as the mean ± standard deviation and was compared between groups by an independent samples t test. Quantitative information that did not conform to a normal distribution was expressed as the median P50 (P25, P75) and compared via the Mann‒Whitney U test. Qualitative information is expressed as a percentage (%), and p < 0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics and clinical presentation
There were 124 children in group A. The male to female ratio was 1:1, the median age was 7 years, and the number of days of hospitalization was 13 (10, 15) days. In group B, there were 31 children, 18 males and 13 females, with a median age of 6 years and 14 (10, 20) days of hospitalization. There was no statistically significant difference in the age or sex of the children between the two groups. The differences in the number of days of hospitalization and the number of cases of chest pain between the children in Group B and those in Group A were not statistically significant. The difference between the two groups of children in terms of mixed infections was not statistically significant. Mixed infections were present in 61 of the 155 children with MPP in this study. Of these, 17 were co-infected with Streptococcus pneumoniae, 15 with adenovirus, 10 with respiratory syncytial virus, 10 with EBV, and the remainder with other pathogens. The number of days of fever and cases of respiratory distress were significantly greater in group B than in group A Tables 1 and 2.
Chest CT, fiberoptic bronchoscopic manifestations and laboratory tests
The differences in necrotic lung conditions, pulmonary atelectasis, bronchoscopic changes, pleural effusion, fluid pneumothorax and pleural thickening were not statistically significant between the two groups. The differences in white blood cell count, C-reactive protein, D-dimer, procalcitonin, the erythrocyte sedimentation rate, lactate dehydrogenase, and ferritin between the two groups of children were not statistically significant (Table 1).
Treatment and short-term prognosis
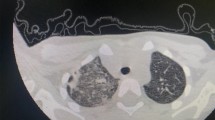
In the present study children with MPP combined with NP, we routinely treated them with macrolide antibacterial drugs and after a course of 5–7 days, those who had poor efficacy were shifted to second line drugs such as tetracyclines and levofloxacin after requesting the pharmacy department and obtaining informed consent from the parents of the children. Based on chest imaging, Bronchoscopy and alveolar lavage was considered for children with severe disease and large pulmonary solid lesions who did not show significant improvement. NP due to MP infection was mostly treated with fiberoptic bronchoscopy, which was performed in 145 out of 155 children. The number of fiberoptic bronchoscopies performed in this study was 2 (1, 3), with a maximum of 5 fiberoptic bronchoscopies. Airway support, such as oxygen and mechanical ventilation, was given to 46 children, and closed chest drainage was performed in 13 children. Among these, lobectomy was performed in one child. There was no statistically significant difference in airway support or fiberoptic bronchoscopy treatment between the two groups. More children in Group B than in Group A underwent closed chest drainage. The difference was statistically significant. Additionally, 101 of 155 children with MPP combined with NP were followed up after discharge. The follow-up time ranged from 1 month to 1 year. Pleural thickening was present in 44 patients, lobular atelectasis in 11 patients, striated shadows in 7 patients, bronchial stenosis in 15 patients, and bronchial dilatation in 14 patients (Fig. 1) (Table 2).
Discussion
Current research suggests that the age of children infected with MP is concentrated in the preschool years, but infections in school-aged children may progress severely2. In the present study, both groups of children were 7 (5,9) years old and were preschool and school aged. Therefore, the age of children with MPP combined with NP is also concentrated in preschool-aged and school-aged children. We hypothesize that this may be related to the fact that bronchial development in preschool-aged and school-aged children is still imperfect and less resistant to pathogenic infections.
In this study, the number of days of fever was significantly greater in children in group B than in those in group A. Fever duration is a significant predictor in children with NP due to MP infection12. The pathogenesis of MPP combined with NP is related not only to the direct injurious effects of MP and toxins on lung tissue but also to the secondary injurious effects caused by the large amount of inflammatory factors released by the body’s immune-inflammatory response. It has also been hypothesized that the prolonged duration of fever may be related to further destruction of the solid areas of the lungs due to necrosis in children13. By combining the results of this study with the relevant literature, we hypothesize that the more extensive the lung necrosis is, the stronger the host immune-inflammatory response, which in turn is reflected in the prolonged duration of fever in the clinical manifestations.
In our study, the percentage of children in group A who experienced dyspnea was significantly lower than that in group B. Previous studies have shown that as necrotizing pneumonia progresses, the clinical manifestations of severe pneumonia, such as persistent high fever and respiratory distress, occur14. We consider that as the disease progresses more severely, the area of lung necrosis is more extensive, more lobes are involved, ventilation is more severely affected, and the child is more likely to develop dyspnea and other manifestations.
Summarizing and generalizing the chest CT manifestations and disease prognosis of children with NP due to MP infection will help clinicians deepen their understanding of this disease so that they can take appropriate therapeutic measures more immediately to improve the prognosis of these children and reduce the occurrence of complications. We summarized the chest imaging manifestations of 155 children and reported that the chest CT manifestations of this disease were dominated by the presence of necrosis in a single lobe of the lung, which accounted for 65.87% of the total cases, whereas areas of hypodensity were observed on enhanced CT (Fig. 2). Lung necrosis predominantly involves the lower lobes of the lungs, which is consistent with the findings of previous studies15. We believe that this finding is related to the anatomical structure of the lungs. As the disease progresses, a thin-walled cystic cavity shadow may appear in the area of necrosis. In the present study, 48.39% of the children presented with cystic cavities in the necrotic areas of the lungs. Previous studies have shown that the mechanism of lung parenchymal necrosis is an inflammatory reaction leading to thrombotic occlusion of pulmonary artery branches and alveolar capillaries, which in turn leads to ischemia and necrosis of the lung parenchyma, which manifests as an area of reduced enhancement on enhanced CT, which is in accordance with the findings of the present study15. Children with NP usually have severe and rapidly progressing disease, often accompanied by a variety of symptoms, such as pulmonary atelectasis, bronchoscopic changes, pleural effusion, fluid pneumothorax, and pleural thickening. In the present study, bronchoscopic changes occurred in 92.26% of the children. Most of the cases involved endobronchitis as well as bronchial sputum embolism caused by the MP infection itself. However, four of these children still developed occlusive bronchiolitis. Occlusive bronchiolitis obliterans (BO) is characterized by peripheral inflammation and fibrosis involving the small airways, leading to narrowing or complete obstruction of the airways16. The clinical presentation of patients with BO is irreversible airway obstruction. Although the occurrence of BO is very rare, it needs to be taken seriously by clinicians because of its irreversibility and the severity of the condition in most children, which manifests as severe bronchial obstruction and hypoxemia17,18. In a study by Wang et al.19 most NPs caused by refractory Mycoplasma pneumoniae were accompanied by pleural effusion. In the present study, 40.65% of the children presented with pleural effusion, which is broadly in line with the findings of previous studies. Imaging of children with MPP combined with NP is generally complex and varied, and current diagnostic criteria for children with NP are still based on chest CT features20. Therefore, understanding the chest CT manifestations of a child will help clinicians better recognize and diagnose them.
CT manifestations of lung necrosis and solid lesions. The lower lobes of lungs showed large and patchy hyperdense shadows with blurred edges and bronchi fill with air and partial liquefaction and necrosis of the solid tissue in some of the shadows. (a) Arrows point to areas of necrotizing pneumonia. (b) Arrows point to areas of pulmonary solid lesions.
Fiberoptic bronchoscopy and alveolar lavage techniques are important tools in the diagnosis and management of children with NP. Pei et al. reported that the bronchoscopic alveolar lavage technique helps reduce local pathogenic microorganisms, promotes the excretion of inflammatory mediators from the lungs, removes sputum and mucus plugs at an early stage, reduces the incidence of luminal stenosis and occlusion, improves the lung ventilation function of children and promotes pulmonary reexpansion21. In the present study, 145 children were treated with bronchoscopic alveolar lavage, while the median number of bronchoscopic alveolar lavages was two. Previous studies have proposed that children with NP are often accompanied by severe bronchopathy and usually require 2 or more lavages, which is consistent with our findings22. In our study, more children in group B than children in group A underwent closed chest drainage, and the difference was statistically significant. Among the children with MPP combined with NP, 13 were treated with closed thoracic drainage, 8 of which were accompanied by pleural effusion. We hypothesize that children with large areas of pulmonary necrosis develop more severe pleural effusions, which often lead to respiratory distress and usually require closed thoracic drainage therapy to relieve compression. In this study, only one child with MPP combined with an NP underwent lobectomy, but there was no follow-up for this patient. Currently, with the increased understanding of the NP, in recent retrospective studies, the percentage of children with the NP undergoing lobectomy has decreased23. Notably, the Guidelines for the Management of Childhood Mycoplasma Pneumonia (2023 Edition) also state that the majority of children with NP have favorable outcomes and do not require surgical resection of the lobes of the lungs10. However, there are still some children with MPP combined with NP who have poor outcomes and prolonged disease after anti-infective treatment and still undergo lobectomy. There is a lack of comparative studies on the efficacy of surgical treatment in children with MPP combined with NP, and prospective studies with large samples are needed to provide further evidence-based support. Recovery after treatment in children with MPP combined with NP is related to the degree and extent of lung necrosis. Previous studies have indicated that although children with NP have a rapid and severe onset and a prolonged course of illness relative to common pneumonia, the majority of children with NP recover better than adults with NP do11,24. Overall, most of the children in the two groups in this study improved clinically after active clinical treatment, and only some of them had residual abnormalities on lung imaging. NP caused by MP infection does not result in any residual cystic cavity in the lesion on chest CT review after treatment because of the small extent of necrosis and the cystic cavity, and a fibrous streak shadow remains in the necrotic area in only some cases. A small percentage of patients also have residual manifestations such as lobar atelectasis and bronchial wall changes such as bronchiectasis and bronchial stenosis.
This study has several limitations. First, the study had a small sample size and enrolled patients from a single center; thus, the results need to be further validated in larger samples and multicenter studies. Second, this was a retrospective study, and there may have been some bias in the selection of cases. Finally, fewer patients were followed up. In summary, chest CT in children with NP due to MP infection is characterized by necrosis of one or both lobes of the lungs and, in this study, mainly a single lobe. Areas of hypodensity are observed on enhanced CT. In severe cases, a thin-walled cystic cavity may develop in the necrotic area. Children tend to experience rapid progression, long disease duration, and slow absorption of lesions, often resulting in symptoms such as striated shadows, pleural thickening, lung atelectasis, and bronchial wall changes. Therefore, summarizing and generalizing the imaging characteristics of children with MPP combined with NP will help clinicians take corresponding measures in a more timely manner and improve the prognosis of these children.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MP:
-
Mycoplasma pneumoniae
- MPP:
-
Mycoplasma pneumoniae pneumonia
- NP:
-
Necrotizing pneumonia
References
Wang, X. et al. Mycoplasma pneumoniae triggers pneumonia epidemic in autumn and winter in Beijing: A multicentre, population-based epidemiological study between 2015 and 2020. Emerg. Microbes. Infect. 11, 1508–1517. https://doi.org/10.1080/22221751.2022.2078228 (2022).
Xu, M. et al. Molecular epidemiology of Mycoplasma pneumoniae pneumonia in children, Wuhan, 2020–2022. BMC Microbiol. 24, 23. https://doi.org/10.1186/s12866-024-03180-0 (2024).
Zhu, Y. G., Tang, X. D., Lu, Y. T., Zhang, J. & Qu, J. M. Contemporary situation of community-acquired pneumonia in China: A systematic review. J. Transl. Int. Med. 6, 26–31. https://doi.org/10.2478/jtim-2018-0006 (2018).
Masters, I. B., Isles, A. F. & Grimwood, K. Necrotizing pneumonia: An emerging problem in children?. Pneumonia 9, 11. https://doi.org/10.1186/s41479-017-0035-0 (2017).
Zheng, B., Zhao, J. & Cao, L. The clinical characteristics and risk factors for necrotizing pneumonia caused by Mycoplasma pneumoniae in children. BMC Infect. Dis. 20, 391. https://doi.org/10.1186/s12879-020-05110-7 (2020).
Teresinha Mocelin, H. et al. Necrotizing pneumonia in children: A review. Paediatr. Respir. Rev. https://doi.org/10.1016/j.prrv.2024.02.003 (2024).
Chen, Y., Li, L., Wang, C., Zhang, Y. & Zhou, Y. Necrotizing Pneumonia in Children: Early Recognition and Management. J. Clin. Med. https://doi.org/10.3390/jcm12062256 (2023).
Krenke, K. et al. Necrotizing pneumonia and its complications in children. Adv. Exp. Med. Biol. 857, 9–17. https://doi.org/10.1007/5584_2014_99 (2015).
Wang, Y. et al. Respiratory microbiota imbalance in children with Mycoplasma pneumoniae pneumonia. Emerg. Microbes. Infect. 12, 2202272. https://doi.org/10.1080/22221751.2023.2202272 (2023).
(NHSC), N. H. C. Guidelines for the diagnosis and treatment of Mycoplasma pneumoniae in children (2023 edition). Infect. Dis. Inform. 36, 291–297 (2023).
Yang, B. et al. Differences of clinical features and prognosis between Mycoplasma pneumoniae necrotizing pneumonia and non-Mycoplasma pneumoniae necrotizing pneumonia in children. BMC Infect. Dis. 21, 797. https://doi.org/10.1186/s12879-021-06469-x (2021).
Luo, Y. & Wang, Y. Risk prediction model for necrotizing pneumonia in children with Mycoplasma pneumoniae pneumonia. J Inflamm Res 16, 2079–2087. https://doi.org/10.2147/jir.S413161 (2023).
Qian, J. et al. Analysis of clinical features and risk factors of necrotizing pneumonia in children. Beijing Da Xue Xue Bao Yi Xue Ban 54, 541–547. https://doi.org/10.19723/j.issn.1671-167X.2022.03.021 (2022).
Fei, W. & Luo, J. Progress in the diagnosis and treatment of necrotizing pneumonia in children. J. Clin. Pediatr. 36, 306–310 (2018).
Wang, R., Xi, Y., Guo, B., Zhu, J. & Han, Q. Imaging characteristics of necrotizing pneumonia caused by Mycoplasma pneumoniae infection in children and evaluation of blood CG-reactive protein, DG dimer. J. Pract. Radiol. 35, 952–955 (2019).
Flanagan, F., Casey, A., Reyes-Múgica, M. & Kurland, G. Post-infectious bronchiolitis obliterans in children. Paediatr. Respir. Rev. 42, 69–78. https://doi.org/10.1016/j.prrv.2022.01.007 (2022).
Kavaliunaite, E. & Aurora, P. Diagnosing and managing bronchiolitis obliterans in children. Expert. Rev. Respir. Med. 13, 481–488. https://doi.org/10.1080/17476348.2019.1586537 (2019).
Teper, A., Colom, A. J., Schubert, R. & Jerkic, P. S. Update in postinfectious bronchiolitis obliterans. Pediatr. Pulmonol. 59, 2338–2348. https://doi.org/10.1002/ppul.26570 (2024).
Wang, X. et al. Necrotizing pneumonia caused by refractory Mycoplasma pneumonia pneumonia in children. World J. Pediatr. 14, 344–349. https://doi.org/10.1007/s12519-018-0162-6 (2018).
Zhou, Y., Hu, M., Ye, B., Chen, Z. & Zhang, Y. Early prediction of necrotizing pneumonia from mycoplasma pneumoniae pneumonia with large pulmonary lesions in children. Sci. Rep. 10, 19061. https://doi.org/10.1038/s41598-020-76083-5 (2020).
Pei, M. et al. Effect of bronchoalveolar lavage on the clinical efficacy, inflammatory factors, and immune function in the treatment of refractory pneumonia in children. Transl. Pediatr. 10, 921–928. https://doi.org/10.21037/tp-21-89 (2021).
Zhang, W. & Wang, Y. Clinical analysis of 41 cases of necrotizing pneumonia in children. Sichuan Med. 43, 284–288 (2022).
Luo, Y. & Wang, Y. Clinical characteristics of necrotizing pneumonia caused by different pathogens. Infect. Drug. Resist. 16, 3777–3786. https://doi.org/10.2147/idr.S419294 (2023).
Blanco-Iglesias, E. et al. Retrospective study in children with necrotizing pneumonia: Nine years of intensive care experience. Pediatr. Infect. Dis. J. 39, 571–575. https://doi.org/10.1097/inf.0000000000002633 (2020).
Funding
Provincial-Ministerial Joint Project of Medical Science and Technology Tackling Program in Henan Province (SB201903028). The Henan Provincial Science and Technology Research Project (LHGJ20240545).
Author information
Authors and Affiliations
Contributions
Jiapu Hou conceptualized the study, drafted the original manuscript, and reviewed and revised it. Ruiyang Sun provided medical records and participated in the revision of the manuscript. Xue Zhang provided medical records and participated in the revision of the manuscript. Wanyu Jia provided medical record information and reviewed and revised the manuscript. Peng Li provided medical records and participated in the revision of the manuscript. Chunlan Song conceptualized the study and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be responsible for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Henan Children’s Hospital (2022-K-L046) and individual consent for this retrospective analysis was waived.
Consent to publish
Informed consent was waived due to its retrospective nature. (Children’s Hospital Affiliated to Zhengzhou University).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, J., Sun, R., Zhang, X. et al. Chest CT characterization of children with necrotizing pneumonia due to Mycoplasma pneumoniae infection. Sci Rep 15, 4283 (2025). https://doi.org/10.1038/s41598-025-88418-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88418-1