Abstract
Transseptal puncture (TSP) is widely used in catheter-based cardiac interventions to gain left atrial (LA) access, but its workflow has remained largely unchanged and is still a source of serious complications. Pulsed field ablation (PFA) for pulmonary vein isolation (PVI) has been shown to be at least comparable with radiofrequency ablation (RFA) in terms of safety and efficacy. However, PFA catheter delivery to the LA typically requires a standard TSP and an over the wire sheath exchange which can limit workflow and lengthen procedure time—a shorter procedure time being a proposed advantage of PFA over RFA. This study aimed to evaluate a simplified workflow for direct TSP using the PFA sheath (Faradrive, Boston Scientific). We retrospectively analyzed 166 patients undergoing PVI with PFA in our center. TSP was performed by combining a 16.8F PFA sheath (Faradrive, Boston Scientific) and a RF-guidewire (Versacross, Boston Scientific) as a direct approach without the need over an over-the-wire exchange. The patient population had a mean age of 63.8 ± 11.3 years and was 72.3% male (n = 119/166). TSP was achieved in all patients (n = 166, 100%) without complication. The mean time from groin puncture to LA ablation catheter delivery was 16.2 ± 5.5 min with a mean fluoroscopy time of 15.7 ± 12.7 min. Dilator and sheath crossed the septum with no significant resistance in all cases (n = 168, 100%). The RF-guidewire allowed catheters to be tracked back up to the superior vena cava without exchange in cases where another dropdown was desired to locate a preferred puncture site. The stiffness of the wire provided adequate support to advance all sheaths to the left side regardless of outer diameter. This is the first case series on the use of a RF-guidewire combined with the PFA sheath for TSP. This study proved that an over a RF-powered guidewire TSP directly with 16.8F PFA sheath is feasible, reproducible, and safe. This very simplified workflow eliminates the need for both a rigid metal needle and an over the wire sheath exchange reducing procedure time and complexity, fluoroscopy time and potential related risks.
Similar content being viewed by others
Introduction
Transseptal puncture (TSP) to access the left atrium (LA) for pulmonary vein isolation (PVI) is conventionally performed using a metal Brockenbrough needle to puncture the fossa ovalis1. TSP remains associated with serious complications, potentially life threatening, most commonly related to pericardial effusion and cardiac tamponade2. The increasing use of transesophageal or intracardiac echocardiography has improved safety by improving the positioning of the needle engaging the fossa ovalis3,4. Despite this the risk of cardiac perforation and tamponade persists in normal sized or moderately enlarged left atria. Other reports have highlighted risks of shaving of plastic particles from the sheath and dilator, coring of cardiac tissue, and possibly related silent strokes5,6. More recent catheter-based procedures such as pulsed field ablation (PFA) still requires an over the wire sheath exchange due to the size, diameter, shape, or structure of sheaths7. Sheath exchange carries the risk of potential air or thrombus embolism or the loss of LA access1,5,8. Alternative techniques, such as the use of radiofrequency-powered needles or guidewires, have emerged in recent years, showing improvement in safety, efficacy, and efficiency2,6,9. Simplified workflows aiming at eliminating the usual transseptal sheath exchange for LA access have also been reported10,11.
To improve the efficacy and safety while reducing fluoroscopy of the PFA AF ablation procedure, a simplified TSP strategy was developed that combines a RF powered pigtail wire with the 16.8F PFA catheter and dilator for a single and direct access to the left atrium. The present study aimed to demonstrate the value of this simplified strategy for patients undergoing AF ablation via the PFA system.
Methods
TSP was attempted in 166 patients using an RF powered flexible wire (VersaCross, Baylis Medical Company) through the PFA sheath (Faradrive, Boston Scientifc). Based on a previously described simplified workflow, we retrospectively assessed the feasibility and safety of a direct and exchangeless TSP9. Data was gathered from the Mater Private Hospital (MPH) AF Registry. Institutional review board ethics approval was sought and attained -MMUH/MPH IRB Ethics Approval Reference 1/378/2283. The analysis was conducted according to the Declaration of Helsinki guidelines. Written informed consent was obtained from all patients to record their data in our registry and use the data for research and publication.
Study population
We performed a retrospective analysis of patients undergoing AF ablation via PFA at our institution. Our inclusion criteria were patients above the age of 18 years with an AF diagnosis undergoing catheter ablation from April 2022 to November 2023. Direct TSP using a radiofrequency wire delivered via the PFA sheath was performed in 166 patients. Data variables collected included baseline demographics, medical history, complications, procedure time, fluoroscopy time, LA dwell time and to from femoral access to TSP.
Transeptal puncture characteristics
All procedures were performed under general anesthesia. Transoesphageal (TOE) was used in all patients to verify the absence of intracardiac thrombus and to guide TSP. Dual ultrasound guided right femoral vein puncture was performed with placement of a 7 and 8 Fr sheath via modified Seldinger technique. A steerable decapolar catheter or standard quadripolar catheter was passed in the 7 Fr sheath and positioned in the coronary sinus. A long 0.035” J shape wire was passed in the 8 Fr sheath up to the superior vena cava (SVC). The 8 Fr sheath was then removed, a skin incision was made, and the PFA sheath (16.8 Fr outer diameter, 13 Fr inner diameter, Faradrive, Boston Scientific) was advanced over the wire. The 0.035” was removed and a 180 cm long, 0.035” pigtail RF wire (Versacross, Baylis) was backloaded in the PFA sheath dilator and connected to a dedicated RF generator (RFP-100A RF Puncture generator) (Fig. 1). The pigtail RF wire is connected to the generator with a connector cable which is clipped to the proximal tip of the wire. Because there is no fixed connector, it enables the guidewire to be used as a standard exchange length guidewire. An indifferent (dispersive) patch electrode is placed on the patients lower back to facilitate delivery of RF energy.
The pigtail radiofrequency wire loaded into the pulsed field ablation sheath. (a) Diagram denoting the radiofrequency (RF) pigtail wire pre-loaded within the pulsed field ablation (PFA) sheath and dilator pre transeptal puncture with the transesophageal (TOE) probe and coronary sinus (CS) catheters in site. (b) Video of the transeptal puncture technique under fluoroscopy.
The assembly of sheath, dilator, and flexible RF powered guidewire was pulled down with clockwise rotation towards a 5 o’clock position under fluoroscopic and TOE guidance to engage the middle part of the fossa ovalis (Figs. 2 & 3). Then the radio-opaque distal of Versacross wire was positioned and supported at the edge of the dilator to ensure a perpendicular contact with the interatrial septum. Once proper positioning and tenting of the septum was confirmed with TOE, RF energy was delivered at a fixed frequency in a range of 450 to 480 kHz in a monopolar mode for a one second pulse resulting in the perforation of the septum with minimal pressure applied (Figs. 2 & 3). The guidewire is then advanced into the left atrium where it resumes its pigtail configuration, confirming LA access on TOE, and subsequently supporting progression of the dilator and Faradrive sheath across the septum. The TOE probe was removed following confirmation of LA access post TSP in all cases.
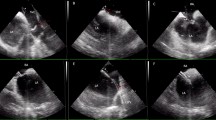
Advancement of the radiofrequency Pigtail wire into the left atrium. Transesophageal views: (A) Radiofrequency wire reshaping to its pigtail form inside the left atrium. (B) Catheter sheath crossing through the septum and into the left atrium. (C) Catheter sheath in the left atrium with the dilator and radiofrequency wire withdrawn.
All patients prescribed direct oral anticoagulation (DOAC) were instructed to hold their DOAC 12 to 24 hours prior to TSP. All patients received a weight adjusted bolus of 130 IU/kg of unfractionated heparin immediately after TSP with a target activated clotting time (ACT) of 250-350s. Additional heparin was administered as required to ensure an ACT > 300 secs throughout the procedure. After cautious removal of the dilator and guidewire, the sheath was aspirated and flushed. The PFA catheter, flushed and readied, equipped with the initial 0.035” J shape guidewire was advanced to the LA through the 16.8 Fr PFA sheath and AF ablation was performed (Faradrive, Boston Scientific).
Post ablation follow-up
After the ablation procedure, a transthoracic echocardiogram was performed immediately post procedure to ensure there was no evidence of pericardial effusion. Patients were observed overnight and discharged on anti-arrhythmic agents at the operator’s discretion together with oral anticoagulation.
Patients were observed at a routine outpatient clinic appointment 1-3 months post procedure.
Statistical analysis
Descriptive statistics summarized patient’s characteristics, procedural data, safety and follow up data.
Continuous variables were expressed as mean ± SD and categorical data as frequencies and percentages.
Results
Patient characteristics
We prospectively enrolled 166 patients undergoing AF catheter ablation via PFA at our institution. 153 Patient characteristics are shown in Table 1. At the time of ablation all patients were receiving oral 154 anticoagulation. Direct TSP was performed in all patients. There were no patients with prior atrial septal 155 defect repair or occluder devices.
Procedural characteristics
We performed 166 TSP via the outlined direct-puncture method. Successful TSP was achieved in all 162 patients at first attempt (Table 2). The median time from groin puncture to LA ablation catheter delivery 163 was 16.2 ± 5.5 min, fluoroscopic time 15.7 ± 12.7 min, procedure time 39.2 ± 12.9 min and LA dwell 164 time was 25.5 ± 4.6 min. The mean radiation dose was 45.9 ± 50.5 mGy.
Complications
We observed no major complications, no failure to cross or aborted TSPs (Table 3). All patients had a 175 transesophageal echocardiogram during TSP and a transthoracic echocardiogram post procedure none 176 of which showed a pericardial effusion.
Discussion
Our study is the largest series utilizing the RF powered flexible pigtail wire direct TSP technique since the initial experience in 2005 and follow up study in 201512,13,14,15. We describe a direct TSP technique using the commercially available PFA sheath and dilator (Faradrive, Boston Scientific). Our results demonstrate this direct TSP technique is: (1) effective; (2) safe and (3) has multiple advantages such as a simplified workflow.
Firstly, we demonstrated that this technique is safe as we did not observe any peri-procedural or post procedural complications. All transeptal punctures were performed under transesophageal echocardiogram guidance with a transthoracic echocardiogram performed post procedure in all cases which did not demonstrate a pericardial effusion. The use of RF needle has been shown to have comparable complication rates to a conventional approach and has been associated with a lower rate of arterial thromboembolism and pericardial effusion16,17. The blunt, round and pigtail shape of the RF wire make repositioning and rewiring much easier which may account for the reduced complication rates observed. Instead of the usual workflow involved in re-wiring (removing the needle, retracting the sheath and dilator to the SVC, then rewiring to position the needle again for a better drop-down position) the RF wire can simply be pushed forward and back up to the SVC. This eliminates additional flushing steps, minimizing effort and time required to attain a more favorable positioning in the fossa ovalis. The combination of a stiff guide wire with a pigtail tip, a bigger sheath and a stiff tapered dilator provides the ideal support required for a safe crossing of the interatrial septum in all circumstances, eliminating the need for predilating with a smaller sheath and for exchanging sheath over the wire. Additional, the PFA sheath tapering is longer and therefore less steep allowing for less forceful dilatation. While this simplified workflow helped improve the efficiency of the procedure by eliminating steps from a conventional approach and potentially avoided the loss of position in the LA, it may also provide clinical benefit due to a reduction in sheath exchanges. Previous studies have shown catheter exchanges over transseptal sheaths to be associated with a higher risk of silent cerebral embolism18. Furthermore, the transparent sheath facilitates detection of air on exchange of catheters via the PFA sheath into the LA which may reduce the risk of air embolization. Additionally, the use of the PFA catheter with a conventional TSP showed pericardial tamponade rates of 0.36-0.97%, stroke rates 0.12%-0.39% and vascular complication rates 2.2%-3.28% which is comparable to those with RF catheter ablation7,19. Overall, our results show that this direct TSP technique using the PFA catheter in combination with an RF wire is a safe and effective technique for gaining access to the LA in AF catheter ablation.
Secondly, our data showed this technique was effective with 166 TSP cases achieved without issue on the first attempt. This confirms and supports the idea that a direct and exchange less TSP is feasible and safe when utilizing larger calibre sheaths, as required for other ablation energy techniques such as balloon cryoablation. The design and characteristics of the PFA sheath and dilator with the robustness and stiffness of this flexible RF wire, make it safe and easy to use. In the initial series the RF sheath, dilator and wire were highlighted as less supportive and torque responsive with an overall different tactile feel and feedback to the St Jude Medical SL0 sheath, dilator and BRK needle. This was an initial obstacle to routine use of the RF needle system necessitating a switch to the Torflex Superstrong dilator12. As is routinely the case in procedures requiring TSP, a proportion of thick, fibrotic, or aneurismal septa were observed. However, these situations were never a challenge in this series. The dilator and sheath always crossed the septum with no significant resistance. Electrocautery’s effectiveness in cases such as this has been reported several times20,21,22,23.
Thirdly this technique showed procedural and fluoroscopy times much lower than those previously reported7,19,24. Both use of an RF pigtail wire as well as a direct TSP technique have been shown to have lower transeptal times than with a conventional approach2,6,15,25. The combination of a direct TSP technique using an RF pigtail wire may account for the significant reduction in procedure time observed in our study. Another potential explanation is the PFA technique learning curve as the procedure and fluoroscopy times reported here are more in keeping with those of more recent studies involving PFA19. Interestingly previous studies have reported longer procedure and ablation times with single TSP techniques compared to double TSP techniques26. The authors of the study hypothesize the need to exchange mapping and ablation catheters through the access site may account for this finding. In our cohort of patients mapping catheters were not used as most patients were undergoing their first ablation procedure which in all cases involved empirical anatomical ablation rather than mapping-based substrate ablation. In our European center, 3D electroanatomic mapping is not routinely used in patients with paroxysmal AF undergoing their first AF ablation.
Limitations of this study are that it is a single center experience observational study, the technique was performed by two very experienced electrophysiologists who perform a very high volume of AF ablation. As such the results shown above may not be directly applicable to other centers. It is a single arm study for feasibility and therefore we do not have a direct comparator group to the conventional TSP approach.
Conclusion
This series proves that a direc transseptal approach for PFA using a 16.8F PFA sheath and an RF powered guidewire is feasible, reproducible, and safe. This very simplified workflow eliminates steps and related risks of air/thrombus embolism and displacement. It may also improve safety and reduce procedure time, fluoroscopy time and TSP time.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Manolis, A. S. Transseptal access to the LeD atrium: Tips and tricks to keep it safe derived from single operator experience and review of the literature. Curr. Cardiol. Rev. 13(4), 305–318 (2017).
Andrade, J. G. et al. Randomized trial of conven_onal versus radiofrequency needle transseptal puncture for cryoballoon abla_on: the CRYO-LATS trial. J. Interv. Cardiac. Electrophysiol. 65(2), 481–9 (2022).
Frangieh, A. H. et al. Intracardiac versus transesophageal echocardiography for leD atrial appendage occlusion with watchman. Catheter Cardiovasc. Interv. 90(2), 331–338 (2017).
Isath, A. et al. Does the use of intracardiac echocardiography during atrial fibrilla_on catheter abla_on improve outcomes and cost? A na_onwide 14-year analysis from 2001 to 2014. J. Interv. Card Electrophysiol. 61(3), 461–8 (2021).
Hsu, J. C. et al. Randomized trial of conven_onal transseptal needle versus radiofrequency energy needle puncture for leD atrial access (the TRAVERSE-LA study). J. Am. Heart Assoc. 2(5), e000428 (2013).
Dewland, T. A. et al. Randomized comparison of a radiofrequency wire versus a radiofrequency needle system for transseptal puncture. JACC Clin. Electrophysiol. 9(5), 611–9 (2023).
Reddy, V. Y. et al. Pulsed field or conven_onal thermal abla_on for paroxysmal atrial fibrilla_on. N. Engl. J. Med. 389(18), 1660–71 (2023).
Kueffer, T. et al. Elimina_ng transseptal sheath exchange for pulsed field abla_on procedures using a direct over-the-needle transseptal access with the Faradrive sheath. EP Europace 25(4), 1500–2 (2023).
Jauvert, G., Grimard, C., Alonso, C. & Lazarus, A. Use of a radiofrequency guidewire to simplify workflow for leD atrium access: a case series. J. Interv. Card Electrophysiol. 59(3), 551–556 (2020).
Du, Z. et al. Single transseptal puncture technique and contact force catheter: A simplified abla_on strategy for paroxysmal atrial fibrilla_on. Exp. Ther. Med. 20(3), 2611–2616 (2020).
Silva Cunha, P. et al. A simplified single transseptal puncture approach using high-density 3D voltage mapping for atrial fibrilla_on abla_on: acute complica_ons and long-term results. Front. Cardiovasc. Med. 10, 1309900 (2023).
Sakata, Y. & Feldman, T. Transcatheter crea_on of atrial septal perfora_on using a radiofrequency transseptal system: novel approach as an alterna_ve to transseptal needle puncture. Catheter Cardiovasc. Interv. 64(3), 327–332 (2005).
Veldtman, G. R. et al. Radiofrequency perfora_on and conven_onal needle percutaneous transseptal leD heart access: pathological features. Catheter Cardiovasc. Interv. 65(4), 556–563 (2005).
Sherman, W., Lee, P., Hartley, A. & Love, B. Transatrial septal catheteriza_on using a new radiofrequency probe. Catheter Cardiovasc. Interv. 66(1), 14–17 (2005).
Jauvert, G., Grimard, C., Lazarus, A. & Alonso, C. Comparison of a radiofrequency powered flexible needle with a classic rigid Brockenbrough needle for transseptal punctures in terms of safety and efficacy. Heart Lung Circ. 24(2), 173–178 (2015).
Tokuda, M. et al. Radiofrequency needle for transseptal puncture is associated with lower incidence of thromboembolism during catheter abla_on of atrial fibrilla_on: propensity score-matched analysis. Heart Vessels 33(10), 1238–1244 (2018).
Winkle, R. A., Mead, R. H., Engel, G. & Patrawala, R. A. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrilla_on abla_on. Heart Rhythm 8(9), 1411–1415 (2011).
Deneke, T. et al. Exchanging catheters over a single transseptal sheath during LeD Atrial Abla_on is associated with a higher risk for silent cerebral events. Indian Pacing Electrophysiol. J. 14(5), 240–249 (2014).
Turagam, M. K. et al. Safety and Effec_veness of Pulsed Field Abla_on to Treat Atrial Fibrilla_on: One-Year Outcomes From the MANIFEST-PF Registry. Circulation 148(1), 35–46 (2023).
Knecht, S. et al. Radiofrequency puncture of the fossa ovalis for resistant transseptal access. Circ. Arrhythm. Electrophysiol. 1(3), 169–174 (2008).
Capulzini, L. et al. Feasibility, safety, and outcome of a challenging transseptal puncture facilitated by radiofrequency energy delivery: a prospec_ve single-centre study. Europace 12(5), 662–667 (2010).
Elayi, C. S., Gurley, J. C., Di Sessa, T. G. & Kakavand, B. Surgical electrocautery facilitated transseptal puncture in children. Pacing Clin. Electrophysiol. 34(7), 827–831 (2011).
Abed, H. S., Alasady, M., Lau, D. H., Lim, H. S. & Sanders, P. Approach to the difficult transseptal: diathermy facilitated leD atrial access. Heart Lung Circ. 21(2), 108–112 (2012).
Schmidt, B. et al. EUropean real-world outcomes with Pulsed field abla_On in pa_ents with symptoma_c atRIAl fibrilla_on: lessons from the mul_-centre EU-PORIA registry. Europace https://doi.org/10.1093/europace/euad185 (2023).
Sarrazin, J. F. et al. Prospec_ve comparison between conven_onal transseptal puncture and transseptal needle puncture with radiofrequency energy. J. Interv. Card Electrophysiol. 31(3), 237–42 (2011).
Park, Y. J. et al. Effec_veness and safety of single transseptal abla_on for atrial fibrilla_on in real-word prac_ce. Clin. Cardiol. 44(4), 547–554 (2021).
Acknowledgements
The authors have nothing to declare regarding grants, patents or external funding in regard to the above study. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Contributions
RK wrote the manuscript, collected data and performed statistical analysis. All other authors contributed equally in supervision and review of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jauvert, G., Kerley, R.N., Fitzpatrick, N. et al. The safety of direct transeptal puncture using a radiofrequency guidewire combined with a 17Fr pulsed field ablation sheath. Sci Rep 15, 8500 (2025). https://doi.org/10.1038/s41598-025-88447-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88447-w