Abstract
Whether surgical treatment of tubal pregnancy affects the outcomes of concomitant live normally sited (eutopic) pregnancies is unknown. The purpose of this study was to investigate the outcomes of live eutopic singleton pregnancies following surgical treatment for concomitant fallopian pregnancies. A total of 446 patients who conceived tubal heterotopic pregnancies (HPs) via in vitro fertilization–embryo transfer and underwent surgical treatment to remove ectopic pregnancies (EPs) were included. For each study patient, one matched patient who conceived a live eutopic singleton pregnancy was selected randomly as a control. The rates of early miscarriage, late miscarriage, live birth, preterm birth, low birth weight and perinatal mortality were not significantly different between the study and control groups. The birth weight were also similar between these two groups. However, the caesarean section rate was significantly higher in the study group than that in the control group(P < 0.001). Additionally, laparotomy and laparoscopy for tubal HPs yielded similar pregnancy outcomes. Compared with controls, patients who conceived tubal pregnancies and concomitant live eutopic singleton pregnancies had similar pregnancy outcomes after proper surgical treatment of EPs. These findings can be used for counselling women who have conceived tubal HPs regarding the risks associated with surgical treatment.
Similar content being viewed by others
Introduction
Heterotopic pregnancy (HP) is defined as the simultaneous occurrence of a normal site (eutopic) pregnancy and an ectopic pregnancy (EP)1. HPs have been reported to occur in 0.003% (1:30,000) of natural pregnancies and in 1% of assisted reproduction technology (ART) pregnancies2,3,4. Women who receive ART have an increased prevalence of tubal pathology, which increases their risk of EP5,6. The prevalence of HP in ART pregnancies has declined because the practice of transferring multiple embryos is no longer as prevalent as it once was, probably reflecting the move towards single embryo transfer7,8.
Concomitant EP can occur in any location outside the uterine cavity, with the most common site being the fallopian tube9,10. Women usually have no obvious symptoms9. Clinicians often view the reassuring sign of a normal site pregnancy and might therefore ignore the possibility of an EP, resulting in potentially delayed or missed diagnoses with an increased risk of ruptured EP. Ruptured tubal pregnancies can lead to haemorrhage, increase the risk of miscarriage of the concomitant live eutopic pregnancy, and even threaten the pregnant woman’s life. Therefore, early diagnosis and proper management of EP are priorities for women. Concomitant tubal pregnancy can be treated surgically or expectantly or by ultrasound-guided aspiration, with the aim of preserving the concomitant live eutopic pregnancy11,12.
The tubal pregnancies in HPs are at risk of rupture, and most HPs must be surgically removed. Surgery to treat tubal pregnancy is generally safe for pregnant women, but it is not known whether surgical treatment affects the outcomes of concomitant live normally site pregnancies13,14.
Reported data regarding pregnancy outcomes after surgical treatment of tubal HPs are limited. The vast majority of available studies are small retrospective studies that lack case controls for potential confounding factors14,15, which makes it difficult to determine whether surgical treatment increases the risk of miscarriage in concomitant live eutopic pregnancy. Moreover, previous studies of HP have reported widely varying rates of miscarriage and live birth rates of concomitant eutopic pregnancies16,17. Thus, the aim of this study was to investigate pregnancy outcomes following surgical treatment of tubal HPs after in vitro fertilization–embryo transfer (IVF-ET). To our knowledge, this study is the largest series to date to describe tubal HPs after IVF-ET at a single reproductive centre .
Materials and methods
This was a retrospective, single-centre matched cohort study conducted at the Reproductive and Genetic Hospital of CITIC-Xiangya, Changsha City, China. The study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (reference number: LL-SC-2023-016). Owing to the retrospective nature of the study, the requirement for informed consent was also waived by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya. This research has been performed in accordance with the Declaration of Helsinki.
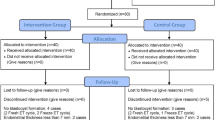
All patients who conceived a tubal HP via IVF-ET from January 2005 to December 2021 were recruited (for a flow diagram, see Fig. 1). The following inclusion criteria were used: patients had indications for IVF and underwent IVF/intracytoplasmic sperm injection (ICSI), tubal HPs were diagnosed by ultrasound at our hospital, and each of the HPs included a live normal site pregnancy at the time of diagnosis. The exclusion criteria were as follows: use of preimplantation genetic testing (PGT), other types of HP, tubal pregnancies concomitant with multiple eutopic pregnancies, tubal pregnancies that ruptured preoperatively, miscarriage of eutopic pregnancies or eutopic pregnancies without cardiac activity before EP surgery, missed cases, termination of pregnancy due to congenital foetal structural or chromosomal abnormalities or maternal complications, incomplete pregnancy and birth information, and loss to follow-up.
For each patient in the study group, one control patient who conceived a normal site pregnancy during the same period was selected randomly. The selected controls all had initial scans showing a live eutopic singleton pregnancy. Patients in the two groups were matched for maternal age (MA; ±2 year), gestational age (GA) at diagnosis (± 3 days), number of embryos transferred, type of embryo transferred, grade of embryo transferred and type of frozen embryo transfer cycle.
The serum beta-human chorionic gonadotropin (β-hCG) level was routinely checked at 14 or 12 days after the transfer of day 3 embryos or day 5 blastocysts, respectively. If the serum β-hCG level was above 200 mIU/ml, a transvaginal sonography (TVS) scan (GE Voluson E10/E8/730; GE Healthcare, Zipf, Austria) equipped with a 5 to 9 MHz endocavitary transducer was scheduled for 2 weeks later to determine the presence of a gestational sac (GS, indicating clinical pregnancy) with or without a foetal pole and embryonic/foetal cardiac activity. If a low serum β-hCG level (< 200 mIU/ml) was measured, an earlier TVS scan was performed on days 21–22 after ET to exclude EP. A tubal HP was diagnosed via ultrasound scanning if a normally sited GS was observed and if the patient met all of the following diagnostic criteria18: (1) a heterogeneous adnexal mass or ‘blob sign’ adjacent to the ovary; (2) an empty extrauterine GS with a hyperechoic ring; or (3) a yolk sac (YS) and/or foetal pole with or without cardiac activity in an extrauterine GS. According to the European Society of Human Reproduction and Embryology (ESHRE) definition, interstitial pregnancy (IP) is classified as a tubal pregnancy1.
The EPs were further divided into five categories according to their morphological characteristics: GS with an embryo and cardiac activity (type I), GS with an embryo and no cardiac activity (type II), GS with a YS and no embryo (type III), empty GS (type IV) and solid inhomogeneous swelling (type V)19.
A live pregnancy was defined by the presence of an embryo/foetus with visible cardiac activity on an ultrasound scan. Early embryonic demise was diagnosed if any of the following criteria were met: embryo length of ≥ 7 mm with no cardiac activity, mean GS diameter ≥ 25 mm without an embryo, absence of an embryo with cardiac activity ≥ 2 weeks after a GS without a YS was shown by a scan, or absence of an embryo with cardiac activity ≥ 11 days after a GS with only a YS was shown by a scan20.
The treatment approach was selected by the same postpregnancy management specialist team of the gynaecological minimally invasive surgery centre at our hospital. Management strategies were formulated depending on the clinical manifestations, EP location, EP morphological features, size of the EP mass, viability of the EP and patients’ preference. The benefits and risks of each treatment option were explained to women, and patients’ preferences were fully considered.
Surgical treatment was recommended for women with EP showing a YS, with/without a foetal pole and/or cardiac activity on an ultrasound scan or evidence of rupture of the EP mass. The indications for expectant treatment were as follows: haemodynamically stable; asymptomatic or mild abdominal pain and/or vaginal bleeding; a nonviable EP mass (type IV and V) < 3 cm; a strong desire for nonsurgical management; and a willingness to undergo potentially prolonged follow-up. Patients receiving expectant treatment were advised to be hospitalized or stay near the hospital to facilitate close ultrasound examination to monitor changes in the EP. If a nonviable EP mass that developed further than 3–4 cm or presented with a YS, with/without a foetal pole, as observed in serial follow-up examinations, abdominal pain and/or vaginal bleeding increased, surgical treatment was performed immediately.
Surgical treatment included salpingectomy and salpingostomy. Salpingectomy was recommended for women with a well-developed EP and noninterstitial tubal pregnancy. The indications for salpingostomy were as follows: heterotopic interstitial pregnancy; tubal EP combined with EP dysplasia; and a strong desire for a reserved fallopian tube.
The pregnancy and perinatal outcomes were defined as follows: miscarriage was defined as spontaneous loss of a eutopic pregnancy by 22 weeks of gestation; preterm delivery referred to birth at ≥ 22 weeks but < 37 weeks of gestation; term birth was defined as delivery ≥ 37 weeks of gestation but < 42 weeks of gestation; and a live birth was defined as an infant born alive with heartbeat or breathing signs after completing at least 22 weeks of gestation. Perinatal mortality was defined as foetal or neonatal death occurring during late pregnancy (≥ 22 completed weeks of gestation), during childbirth or up to 7 completed days after birth. Low birth weight was defined as a birth weight < 2500 g21.
All patients were followed until the end of pregnancy by a dedicated team at our centre. Information on GA at delivery, delivery mode, neonatal birth weight and foetal survival was obtained from the patients via telephone or fax. The maternal demographic characteristics, details of the IVF/ICSI procedure, and ultrasound findings were obtained from the hospital’s electronic medical records. The GA was calculated from the date of ET plus 17 or 19 days for day 3 embryo or day 5 blastocyst transfers, respectively22.
Statistical analysis
Statistical analyses were conducted using SPSS version 24.0 software (SPSS, Inc., Chicago, IL, USA). Student’s t test was used for comparisons of continuous variables, and the chi-square test or Fisher’s exact test was used for comparisons of categorical variables between the HP and control groups. The results are presented as the means ± SDs or n (%). Associations between discrete factors and outcomes were assessed by odds ratios (ORs) and their 95% confidence intervals (CIs). A P value < 0.05 was considered statistically significant.
Results
During the study period, the incidence of tubal HPs was 0.52% in our centre23. And 686 tubal HPs were confirmed by surgery and histological results; among them, 630 cases were diagnosed by TVS at our hospital, and 56 cases were missed. The diagnostic accuracy of TVS was 91.8%. Among the patients diagnosed in our hospital, 602 had concomitant eutopic singleton pregnancies, and 28 had concomitant eutopic multiple pregnancies. Among the eutopic singleton pregnancies accompanied by tubal pregnancies, excuding 2 cases diagnosed in the second trimester, 145 cases of miscarriage of the eutopic pregnancies or normally sited GS without cardiac activity before EP surgery, 6 cases ruptured before surgery, and 3 pregnancies were terminated due to congenital foetal structural or chromosomal abnormalities or maternal complications. Ultimately, a total of 446 patients with live single eutopic and concomitant tubal pregnancies were enrolled. We also identified 446 matched control cases.
At the time of the initial ultrasound diagnosis of tubal HPs, according to the morphological characteristics, 169 (37.9%) of the tubal pregnancies were Type I, 4 (0.9%) were Type II, 87 (19.5%) were Type III, 134 (30.0%) were Type IV, and 52 (11.7%) were Type V.
Among the women with tubal HPs, 401 underwent laparoscopic treatment (including 274 (68.3%) for salpingectomy and 127 (31.7%) for salpingostomy), and 45 were treated with laparotomy (including 22 (48.9%) for salpingectomy and 23 (51.1%) for salpingostomy).
The maternal demographic characteristics are shown in Table 1. The tubal HP group and the control group were matched for MA (median, range) (30 (22–47) vs. 30 (21–45) years, P = 0.492), number of embryos transferred (2.08 (rang 2–3) vs. 2.08 (rang2-3), P = 0.803), type of transfer cycle (P = 0.985), embryo grade ( P = 0.555) and median gestational age at diagnosis (6+ 3 (5+ 6-11+ 3) vs. 6+ 3 (5+ 2-11+ 1) weeks, P = 0.520). Two embryos were transferred in 410 patients (91.9%), and three embryos were transferred in 36 patients (8.1%) in the study group. A total of 81.4% (363/446) of the tubal HPs were first diagnosed before 7 gestational weeks, and the average time of the first diagnosis was 6+ 3 (range 5+ 6-11+ 3) gestational weeks. Half of the women treated surgically were asymptomatic at the time of the first diagnosis (n = 231, 51.8%), and mild vaginal bleeding and/or slight abdominal pain were the most common presenting symptoms (n = 215, 48.2%). Four-fifths of women with tubal HPs underwent surgery before 7 gestational weeks (n = 358, 80.3%). The average time of surgical treatment was 6+ 4 (range 6–11+ 5) gestational weeks.
The rates of early miscarriage (< 12 weeks; 10.1% vs. 9.9%; odds ratio (OR) (95% confidence interval (CI)) 0.975 (0.629–1.511), P = 0.911), late miscarriage (12–22 weeks, 0.7% vs. 0.9%; OR (95% CI) 1.336 (0.297–6.006), P = 1.000), live birth (88.3% vs. 88.8%; OR (95% CI) 1.045 (0.692-1.579), P = 0.833), preterm birth (6.1% vs. 5.8%; OR (95% CI) 0.961 (0.551–1.674), P = 0.887), low birth weight (4.3% vs. 4.0%; OR (95% CI) 0.939 (0.467–1.885), P = 0.859) and perinatal mortality (1.0% vs. 0.5%; OR (95% CI) 0.497 (0.091–2.732), P = 0.682) were not significantly different between the study and control groups. Birth weight (3281.8 ± 436.4 vs. 3277.1 ± 465.4 g, P = 0.929) was similar between these two groups. But the caesarean section (CS) rate (75.6% vs. 58.0%; OR (95% CI) 0.446 (0.329–0.604), P < 0.001) in study group was significantly higher than that in the control group and the GA at delivery (38.6 ± 1.9 vs. 38.9 ± 1.8 weeks, P < 0.001) was significantly different between the study and control groups. (Table 2). In the tubal HP group, 45 women experienced early miscarriages due to embryo growth cessation; among the 3 women who experienced late miscarriage, induced labour was performed in 2 patients due to premature rupture of the membrane, 1 patient had vaginal fluid at 21+5 weeks of pregnancy and experienced induced labour due to stillbirth without amniotic fluid after half a month.
Compared with laparotomy for tubal HPs, laparoscopic treatment was associated with similar rates of preterm birth (6.2% vs. 4.4%; OR (95% CI) 0.700 (0.160–3.056), P = 0.882) and perinatal mortality (1.1% vs. 0%; OR (95% CI) 0.891 (0.861–0.922), P = 1.000). The rates of early miscarriage (10.7% vs. 4.4%; OR (95% CI) 0.387 (0.091–1.655), P = 0.287) and live birth (87.5% vs. 95.6%; OR (95% CI) 3.063 (0.720–13.036), P = 0.112) were not significantly different between the laparoscopic groupand the laparotomy groups (Table 3).
Discussion
In the present study, we analysed the pregnancy outcomes following surgical treatment of tubal HPs in the first trimester after IVF-ET and found that four-fifths of the tubal HPs underwent surgical treatment before 7 gestational weeks, and surgical treatment of the tubal pregnancy was not associated with an increased risk of miscarriage of the concomitant live normally sited pregnancy compared with the control group, with a similar live birth rate. Compared with laparotomy for tubal HPs, laparoscopy was associated with similar pregnancy outcomes.
An early and accurate diagnosis of HP is critical because a delayed diagnosis or missed HP may lead to EP rupture, hypovolemic shock or even a risk to eutopic pregnancy and maternal life13. However, HP is often difficult to diagnose early because most women with EP are asymptomatic or symptomatic nonspecifically9,23. In this study, most women with tubal HPs also had no obvious symptoms or only slight abdominal pain and/or vaginal bleeding. Previous reports have shown that 76-92.4% of HPs can be correctly diagnosed by early TVS9,24. Although the present data showed that the diagnostic accuracy of tubal HP was 91.8%, only 77.6% (346/446) of women were suspected to have tubal HPs at the time of the initial TVS scan in our centre. In addition, enlarged ovaries and pelvic fluid can also result in atypical ultrasound presentations of HP after ART and increase the difficulty of exploring EP9. A total of 72.6% (324/446) of women with tubal HPs underwent a fresh embryo transfer cycle, and 68.2% (304/446) of women had pelvic fluid. Therefore, the accessory areas should be scanned carefully, and routine follow-up TVS scans are necessary to minimize the probability of a missed diagnosis of EP in women who have undergone ART, especially those who have undergone ovulation induction and multiple embryos ETs.
The challenge for clinicians was not only to diagnose HPs but also to formulate the most appropriate treatment strategy for each woman. Because women who have received ART have a strong desire to preserve concomitant live normally sited pregnancies, the influences of treatment methods should be evaluated to determine the balance between maternal risk and preservation of live eutopic pregnancies when determining treatment methods. Surgical treatment is a first-line treatment option for women with tubal HPs; it can be the primary treatment or a rescue treatment complementary to the failure of other treatment methods11.
Some previous studies on HPs reported a spontaneous miscarriage rate of concomitant normally sited pregnancies between 20% and 33%25,26, which is much higher than the miscarriage rate of 10.1% in the study group in our investigation. We speculate that this might be because women with tubal HPs who had a miscarried eutopic pregnancy before surgery were not included in our analysis. JinBo Li et al. analysed the treatment and pregnancy outcomes of tubal HPs with a live eutopic pregnancy, and the results revealed that the miscarriage rate in the surgical treatment group was 15.4% (8/52)11, which is much higher than the rate of 10.1% that we observed. The differences in the miscarriage rate is potentially due to an inadequate number of women with tubal HPs received surgical treatment in the JinBo Li et al. study. A recent study demonstrated that, in women with HPs, minimally invasive surgery to treat the EPs did not increase the risk of miscarriage of the concomitant live normally sited pregnancies and had a similar live birth rate compared with women in the control group, which was in agreement with our study19. These results indicate that surgical treatment of tubal HP with a live normally sited pregnancy is safe and efficient.
In women who received surgical treatment for tubal EP, there were an increased risk of CS, which was possibly due to consideration of the safety of pregnant women and the fetus in third trimester pregnancy after EP surgery, as well as the women’s anxiety about the intrauterine fetus, the clinical indication for CS were loosened for tubal HPs women with surgical treatment. Therefore, the GS at delivery in these women was also relatively low.
Laparoscopy or laparotomy may be used for the treatment of HP, both of which have been reported previously17,27. Compared with laparotomic surgery, laparoscopic treatment has the advantages of minimal invasiveness, a reduced risk of postoperative adverse events and a shortened hospital stay. However, general anaesthesia, CO2 aeration, and relatively difficult manoeuvring during surgery may impair concomitant normally sited pregnancies27. A recent report revealed that the postoperative perinatal and neonatal outcomes of women with HP were comparable between laparoscopy and laparotomy28. S A Solangon et al. also reported that the pregnancy outcomes of laparoscopy and laparotomy recipients were similar19, which is consistent with our data.
In the present study, two or three embryos were transferred to each patient. Previous studies have indicated that the transfer of a single frozen blastocyst could reduce the risk of EP29. We speculate that the transfer of multiple embryos may also lead to an increased risk of tubal HP and that transferring a single embryo selectively may help to decrease the occurrence of tubal HP.
To our knowledge, this is the largest study to examine pregnancy outcomes following surgical treatment of tubal HPs after IVF-ET, and the main strength of this study is the application of statistical methods to control for potential confounding factors, including carefully matched controls. However, there are limitations as well; for example, this was a retrospective study the study period was relatively long (2005–2021). During this time, much progress has occurred in ART, such as changes in stimulation protocols and techniques used to cryopreserve embryos; these changes may have affected reproductive outcomes. In addition, our institution is a reproductive centre at which most patients merely undergo TVS, returning to other centres for treatment. Thus, details on the women’s surgical treatment, complications and postoperative care were obtained via telephone calls and were thus limited.
Conclusions
In conclusion, the majority of tubal HPs were diagnosed via detailed TVS during early follow-up after IVF-ET in the present study. Compared with women who conceived live eutopic singleton pregnancies with similar characteristics, those who underwent surgical treatment for tubal EP did not have an increased risk of miscarrying concomitant live normally sited singleton pregnancies with similar live rates. Laparotomy and laparoscopy for tubal HPs yielded similar pregnancy outcomes. Our data can assist physicians in counselling women with tubal HPs. We recognize that the most effective measure to prevent unnecessary HPs is to restrict the number of embryos transferred in women undergoing ART and to encourage selective single embryo transfer.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EP:
-
Ectopic pregnancy
- HP:
-
Heterotopic pregnancy
- MA:
-
Maternal age
- GA:
-
Gestational age
- ART:
-
Assisted reproduction technology
- IVF-ET:
-
In vitro fertilization–embryo transfer
- ICSI:
-
Intracytoplasmic sperm injection
- PGT:
-
Preimplantation genetic testing
- β-hCG:
-
Beta-human chorionic gonadotropin
- TVS:
-
Transvaginal sonography
- GS:
-
Gestational sac
- YS:
-
Yolk sac
- ESHRE:
-
European Society of Human Reproduction and Embryology
- IP:
-
Interstitial pregnancy
- OR:
-
Odds ratio
- CI:
-
Confidence intervals
References
ESHRE working group on Ectopic Pregnancy, et al. Terminology for describing normally sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice. Human reproduction open, 2020(4), hoaa055. https://doi.org/10.1093/hropen/hoaa055 (2020).
Habana, A., Dokras, A., Giraldo, J. L. & Jones, E. E. Cornual heterotopic pregnancy: contemporary management options. American journal of obstetrics and gynecology, 182(5), 1264–1270. https://doi.org/10.1067/mob.2000.103620 (2000).
Marcus, S. F., Macnamee, M. & Brinsden, P. The prediction of ectopic pregnancy after in-vitro fertilization and embryo transfer. Human reproduction, 10(8), 2165–2168. https://doi.org/10.1093/oxfordjournals.humrep.a136254 (1995).
Svare, J. et al. Heterotopic pregnancies after in-vitro fertilization and embryo transfer—a Danish survey. Human reproduction (Oxford, England), 8(1), 116–118. https://doi.org/10.1093/oxfordjournals.humrep.a137858 (1993).
Weiss, A. et al. Ectopic pregnancy risk factors for ART patients undergoing the GnRH antagonist protocol: a retrospective study. Reprod Biol Endocrinol., 14: 12. https://doi.org/10.1186/s12958-016-0146-0 (2016).
Karadağ, C. & Çalışkan, E. Ectopic pregnancy risk with assisted reproductive technology. Current Obstetrics and Gynecology Reports, 9, 153–157 (2020).
Dooley, W. M. et al. Predictive value of presence of amniotic sac without visible embryonic heartbeat in diagnosis of early embryonic demise. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 57(1), 149–154. https://doi.org/10.1002/uog.23533 (2021).
Santos-Ribeiro, S., Tournaye, H. & Polyzos, N. P. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Human reproduction , 31(2), 393–402. https://doi.org/10.1093/humrep/dev315 (2016).
Li, X. H., Ouyang, Y. & Lu, G. X. Value of transvaginal sonography in diagnosing heterotopic pregnancy after in-vitro fertilization with embryo transfer. Ultrasound Obstet. Gynecol., 41(5), 563–569. https://doi.org/10.1002/uog.12341 (2013).
Barnhart K. T. Clinical practice. Ectopic pregnancy. The New England journal of medicine, 361(4), 379–387. https://doi.org/10.1056/NEJMcp0810384 (2009).
Li, J., Luo, X., Yang, J. & Chen, S. Treatment of tubal heterotopic pregnancy with viable intrauterine pregnancy: Analysis of 81 cases from one tertiary care center. Eur. J. Obstet. Gynecol. Reprod. Biol., 252, 56–61. https://doi.org/10.1016/j.ejogrb.2020.06.005 (2020).
Mohr-Sasson, A., Tamir, M., Mugilevsky, D., Meyer, R. & Mashiach, R. Should expectant management of heterotopic pregnancy be considered?. Arch. Gynecol. Obstet., 306(4), 1127–1133. https://doi.org/10.1007/s00404-022-06628-8 (2022).
Maleki, A., Khalid, N., Rajesh Patel, C. & El-Mahdi, E. The rising incidence of heterotopic pregnancy: Current perspectives and associations with in-vitro fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol., 266, 138–144. https://doi.org/10.1016/j.ejogrb.2021.09.031 (2021).
Goldstein, J. S., Ratts, V. S., Philpott, T. & Dahan, M. H. Risk of surgery after use of potassium chloride for treatment of tubal heterotopic pregnancy. Obstet. Gynecol., 107(2 Pt 2), 506–508. https://doi.org/10.1097/01.AOG.0000175145.23512.5e (2006).
Balafoutas, D., Diessner, J., Kiesel, M., Woeckel, A. & Joukhadar, R. Laparoscopic Management of a Heterotopic Pregnancy in the Tubal Stump. Journal of minimally invasive gynecology, 28(4), 752–753. https://doi.org/10.1016/j.jmig.2020.07.008 (2021).
Zhu, S., Fan, Y., Lan, L., Deng, T. & Zhang, Q. Heterotopic Pregnancy Secondary to in vitro Fertilization-Embryo Transfer: Risk Factors and Pregnancy Outcomes. Frontiers in medicine, 9, 864560. https://doi.org/10.3389/fmed.2022.864560 (2022).
Lv, S., et al. Management strategies of heterotopic pregnancy following in vitro fertilization-embryo transfer. Taiwanese journal of obstetrics & gynecology, 59(1), 67–72. https://doi.org/10.1016/j.tjog.2019.11.010 (2020).
Condous, G., et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet. Gynecol., 26(7), 770–775. https://doi.org/10.1002/uog.2636 (2005).
Solangon, S. A. et al. The risk of miscarriage following surgical treatment of heterotopic extrauterine pregnancies. Human reproduction open, 2022(1), hoab046. https://doi.org/10.1093/hropen/hoab046 (2022).
Doubilet, P. M. et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. The New England journal of medicine, 369(15), 1443–1451. https://doi.org/10.1056/NEJMra1302417 (2013).
Zegers-Hochschild, F. et al. The International Glossary on Infertility and Fertility Care, 2017. Human reproduction, 32(9), 1786–1801. https://doi.org/10.1093/humrep/dex234 (2017).
Li, X. et al. Pregnancy outcomes of women with a congenital unicornuate uterus after IVF-embryo transfer. Reproductive biomedicine online, 35(5), 583–591. https://doi.org/10.1016/j.rbmo.2017.07.015 (2017).
Zheng, M. et al. Surgical treatment of fallopian tubal pregnancy and interstitial pregnancy has no differential effect on intrauterine pregnancy after in vitro fertilization-embryo transfer. BMC pregnancy and childbirth, 24(1), 762. https://doi.org/10.1186/s12884-024-06943-9 (2024).
Lyu, J. et al. Diagnosis and management of heterotopic pregnancy following embryo transfer: clinical analysis of 55 cases from a single institution. Arch. Gynecol. Obstet., 296(1), 85–92. https://doi.org/10.1007/s00404-017-4384-y (2017).
Talbot, K., Simpson, R., Price, N. & Jackson, S. R. Heterotopic pregnancy. J. Obstet. Gynaecol., 31(1), 7–12. https://doi.org/10.3109/01443615.2010.522749 (2011).
Jeon, J. H. et al. The Risk Factors and Pregnancy Outcomes of 48 Cases of Heterotopic Pregnancy from a Single Center. J. Korean Med. Sci., 31(7), 1094–1099. https://doi.org/10.3346/jkms.2016.31.7.1094 (2016).
Cohen, A. et al. Laparoscopy versus laparotomy in the management of ectopic pregnancy with massive hemoperitoneum. Int. J. Gynaecol. Obstet., 123(2), 139–141. https://doi.org/10.1016/j.ijgo.2013.05.014 (2013).
Chen, X., et al. Treatment effects of laparoscopy versus laparotomy on heterotopic pregnancy after in vitro fertilization and embryo transfer. Int. J. Gynaecol. Obstet., 163(2), 689–696. https://doi.org/10.1002/ijgo.14919 (2023).
Wei, D. et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet., 393(10178), 1310–1318. https://doi.org/10.1016/S0140-6736(18)32843-5 (2019).
Acknowledgements
The authors thank the staff of the Reproductive Center and the Imaging Department for their contribution to the smooth conduct of this study.
Funding
This work was funded by the Research Grant of CITIC-Xiangya (YNXM-202310), the Hunan Provincial Grant for Innovative Province Construction (2019SK4012) and the National Natural Science Foundation of China (82302234).
Author information
Authors and Affiliations
Contributions
P.C. and M.Z. collected, analysed, interpreted the data and drafted the article. Y.P. and Y.M. collected data and performed statistical analyses. F.G., H.C. and L.G organized, supervised data collection and revised the article. X.L and Y.O. were responsible for the conception and design of the study, contributed to analysis and interpretation of data and revised the article critically for intellectual content. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the local ethics committee (LL-SC-2023-016). Owing to the retrospective nature of the study, the requirement for informed consent was waived.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, P., Zheng, M., Peng, Y. et al. The effect of surgical treatment of tubal pregnancy on concurrent intrauterine pregnancy after in vitro fertilization. Sci Rep 15, 4328 (2025). https://doi.org/10.1038/s41598-025-88519-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88519-x