Abstract
The Salt Lake Mahai in Qaidam Basin, western China contains large and thick lithium-rich clay sediments that exhibit great economic potential for lithium exploration. This study analyzed the occurrence of lithium and related dissolution mechanisms in these clay through mineral identification, chemical analyses, and monitoring of brine composition evolution. Our results show that lithium-rich clay mainly occurred as interbeds between salt layers and fillings between salt crystals. The dominant clay mineral is illite, followed by chlorite, kaolinite, and an illite–smectite mixed layer. The leached lithium content in brine was less than 10% of the total lithium content in the clay samples. Lithium commonly occurred as structurally incorporated or adsorbed pattern within the clay minerals, particularly illite, leading to a relatively slow dissolution rate in brine during leaching. Consequently, optimizing the solvent injection points based on the distribution of silt-bearing and clay-bearing halite, particularly in the eastern and northwestern sections of Salt Lake Mahai where leached lithium concentrations are higher (45 ~ 70 mg/L), and extending the contact time between solvent and ore layers could further enhance lithium recovery.
Similar content being viewed by others
Introduction
Lithium is a strategic metal element due to its high melting and boiling points, low density and hardness1,2,3,4. The rapid growth of the new energy industry and widespread use of lithium batteries notably increased the global demand for lithium resources5,6,7,8,9,10,11. China’s demand for lithium resources is predicted to exceed one million tons in the next decade12,13. As such, lithium has become a crucial energy storage metal used in various applications14,15,16,17,18.
Economic lithium mainly includes liquid lithium from salt lake brine and solid lithium from pegmatites. The lithium products extracted from salt lake brine account for 75% of the world’s lithium production19, due to the low energy consumption and extraction cost compared with those of solid lithium ore. The exploration of deep underground brine lithium in oil-gas basins has received attentions for the continuous expansion of the demand for lithium resources20,21,22,23,24.
Lithium-rich clastic sediments have recently been a focus as a new resource for their wide distribution and large amounts. These deposits mainly include: (1) Lithium-rich clays come from bauxite sediments as found in the Daoshitou Formation in Yunnan, China, with lithium mainly hosted in montmorillonite and illite25,26,27,28. (2) Lacustrine lithium-rich clay sediments mainly consist three subtypes: (i) the Jadar type, found in the Miocene lacustrine formations in the Jadar Basin, Serbia, containing lithium in zeolitic borosilicate minerals29,30; (ii) volcanic sedimentary sediments that associated with rock-water reaction of volcanic ash as seen in McDermitt and Clayton Valley, USA, and Sonora, Mexico31,32; and (iii) clay sediments in salt lake, where lithium occurs mainly in illite as seen in the Salt Lake Balun Mahai in Qadaim Basin, China3,33.
The solid potash sediments in the shallow part of Salt Lake Mahai in Qaidam Basin have a maximum thickness of 18.52 m. They are characterized by a relatively low KCl content of 2% and thus extracted by solution mining. The lithium content in leached brine can generally reach approximately 20 mg/L. It increased up to 80 –90 mg/L during evaporation and concentration in salt pond, thus showing a great economic potential for lithium extraction. In this contribution, we focused the lithium-rich sediments in Salt Lake Maihai and investigated their distribution, occurrence and related dissolution cheminism using mineralogy, geochemistry and hydrological data. Our findings will provide a basis for the better use of the associated lithium resources in potash ores.
Geological setting
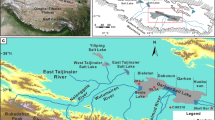
Salt Lake Mahai is situated in the northeastern Qaidam Basin (Fig. 1). The formation of this lake is associated with tectonic movements since the Late Pliocene that finally isolated the Mahai Basin from surrounding areas during the late Middle Pleistocene34,35. In the late Pleistocene, strike-slip and compressional movements of the Altyn Tagh fault system caused continuous uplift in the central and northern Qaidam Basin, transitioning the sub-basins from semi- to fully enclosed units36. In the mid-to-late Holocene, the arid and cold climate led to the rapid shrinkage and concentration of Salt Lake Mahai, resulting in a present-day playa environment37.
Geographical location of the Qaidam Basin and study area. (a) Satellite imagery shows the area of the Qaidam Basin (from Google Earth, https://earth.google.com); (b) Distribution of salt lakes in the Qaidam Basin and location of the study area. (modified by Zhao, 2024)38. These maps were created using CorelDRAW software version 2020, https://www.coreldraw.com.
The sediments in the JIV ore layer of Salt Lake Mahai are characterized by salt-bearing series based on 96 borehole core data39. Their thickness and distribution are seen in Table 1 and in Fig. 2, respectively. Halite is thickest in the northeastern part of the lake. Silt-bearing halite, which is more continuous near the gas pipeline, provides favorable conditions for solution mining due to its higher permeability. In contrast, clay-bearing halite is less continuous and often forms isolated zones, impacting the efficiency of solvent penetration.
Isopleth maps showing thickness variations of lithologic strata in the JIV ore layer within the Salt Lake Mahai solution mining area. These maps were created using Surfer software version 15, https://www.goldensoftware.com/products/surfer.
Samples and methods
Samples
To accurately assess lithium content and solubility in shallow solid ores and their intercalations, we collected 86 core samples of mud, clay, silt, and salt from 13 boreholes at depths ranging from 0.25 to 30.13 m (Fig. 3a). Brine samples were collected from hydrological observation wells within the solution mining area (Fig. 3b) and from each borehole between April and August 2023. A depth sampler was used for three rounds of sampling in June, July, and August, targeting both shallow (approximately 2.5 m) and deep (approximately 6.5 m) depths.
Distribution maps of geological boreholes (a) and hydrological observation wells (b) in the Salt Lake Mahai. From Google Earth, https://earth.google.com.
Methods
Before element contents of solid samples measured preparations including oven drying, crushing, milling, and sieving through a 200-mesh screen have been conducted. Two methods were employed to analysed the elemental composition of the samples. Water leaching was used to measure the mobilizable elements in halite and other easily soluble minerals, as well as the ions adsorbed onto clay mineral surfaces. This method provides insights into the soluble fractions of elements such as potassium and magnesium, which are readily extracted under solution mining conditions. Total acid digestion involved the complete dissolution of the rock matrix using a combination of acids (HCl, HNO3, HF, and HClO4), allowing for the quantification of total elemental content. This approach captures both the soluble and structurally bound elements of studied samples.
Most analysis were conducted at MNR Key Laboratory of Metallogeny and Mineral Assessment, Institute of Mineral Resources, Chinese Academy of Geological Sciences. Inductively coupled plasma optical emission spectrometry (ICP-OES) was used for element composition measurement employing a German SPECTRO ARCOS SOP instrument. Relative standard deviation (RSD) of ICP-OES quantification was less than 2%. X-ray diffraction (XRD) analysis used a Bruker D8 Discovery. The X-ray source was a copper target with a power of 40 KV and 40 mA, and the detector used was a LYNXEYE_XE_T (1D mode). The relative abundance of crystalline phases of studied sample was quantitatively analysed using the Reference Intensity Ratio method that based on upon scaling all diffraction data to the diffraction of standard reference materials. The abundance data was calculated using software of Jade 6.5 version and PDF-4 + 2023 Database. Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS) was conducted at the Institute of Geological Sciences of the Chinese Academy of Geological Sciences. Before testing, the freshness and flatness of the analysed sections were ensured. Gold powder was sprayed on the sections to increase the conductivity of the sample. An OXFORD X-Max (50 mm2) energy spectrometer was employed using an FEI NOVA NanoSEM 450 scanning electron microscope operating at 20 kV.
Results
Element contents of 86 core samples in the Mahai mining area are summarized in Tables 2 and 3. The lithium contents by water leaching of clay samples were 3.3 ~ 6.5 µg/g, showing relative low content. The lithium contents using total acid digestion method were 50 ~ 120 µg/g, with an average value of 85 µg/g. The average potassium and magnesium content in clay-bearing silt by total acid digestion is 4,479.65 µg/g and 22,572.25 µg/g, respectively. However, the potassium and magnesium by water leaching is 1,536.08 µg/g and 12,327.5 µg/g, respectively.
In total, 17 minerals were identified by XRD analysis (Fig. 4). Mineral of halite, quartz, calcite, gypsum, albite, illite, chlorite, and kaolinite are more common and aragonite, dolomite, anhydrite, bassanite, polyhalite, syngenite, glauberite, epsomite, and bischofite were only found in few samples.
SEM-EDS results show that clay minerals are common in the solid samples and dominated by illite, with minor illite–smectite mixed layers (I/S) and chlorite. Illite is characterised by scale-like (Fig. 5a) and plate-like (Fig. 5b) structures, with an incomplete crystal form, and frequently coexists with other clay minerals in a hybrid form. Some particles are embedded within halite crystals (Fig. 5c). In addition, illite coexists with minerals such as dolomite, quartz, albite, carnallite, and polyhalite (Fig. 5d). Chlorite exhibits a plate-like structure (Fig. 5e) and a thin-layered structure (Fig. 5f) with irregular shapes. I/S is a composite mineral formed by the alternating arrangement of illite and smectite in specific proportions, primarily displaying a xenomorphic-granular structure.
Scanning electron microscopy images of mineral structures. (a) Scale-like illite; (b) Plate-like illite; (c) Iillite particles present in the halite crystals; (d) Illite coexist with carnallite and polyhalite; (e) Plate-like chlorite and xenomorphic-granular I/S; (f) Thin-layered chlorite. C: chlorite; Car: carnallite; Dol: dolomite; Ha: halite; I/S: illite–smectite mixed layer; It: illite.
The lithium contents in the brine samples collected from the hydrologic observation wells in the solution mining area from April to August 2023 are listed in Table 4.
Discussion
Lithium occurs in various forms in nature: (1) Isomorphous substitution, where lithium’s small ionic radius (68 pm) allows it to replace elements like magnesium (66 pm), iron (74 pm), and aluminium (51 pm). This substitution is particularly common with magnesium due to their similar crystal chemistry, enabling extensive heterovalent isomorphous replacement26,40. (2) Independent minerals, where lithium is found in distinct mineral forms such as spodumene and petalite41. (3) Ion adsorption, where lithium exists as adsorbed ions in clay minerals such as montmorillonite, illite, and chlorite41,42,43. (4) Free ions, which occur in salt lake brines typically found in relatively young (mainly Quaternary), closed, and tectonically active basin44. Clay minerals are the primary lithium carriers in sedimentary lithium deposits due to their unique adsorption properties. The form of lithium present influences the mining method, extraction efficiency, and economic value of the resource.
Comparison of lithium content using two dissolution methods
As shown in Fig. 6, the sodium and sulfate levels obtained from total acid digestion were generally consistent with those from water leaching, with minor discrepancies possibly due to testing errors. This similarity indicates that sodium and sulfate are primarily sourced from readily soluble salts. In contrast, larger discrepancies for lithium, potassium, and magnesium were observed in clay and mud due to their low soluble salt content. For silt, which contains more soluble salts than clay or mud, the differences were smaller, while salt samples showed minimal variation between methods. The presence of lithium, potassium, and magnesium in clay and silt associated with halite warrants attention. Solution mining should investigate the potential and conditions for extracting these valuable elements from halite-bound interbedded and interstitial clastic minerals.
The differences in the lithium content between the total acid digestion and water leaching tests for the solid samples of different lithologies were compared (Fig. 7). The analysis indicated a notable difference between the two tests for clay and mud, whereas the difference was relatively small for salt and silt. The water leaching tests extracted less than 10% of the actual lithium content, attributable to the high adsorption capacity of mud and clay and the low concentration of soluble salts. When considering the long-term perspective of the solution mining process, lithium has a higher sustained conversion capacity than potassium-magnesium. Although the potassium-magnesium content rapidly decreases, the lithium content in dissolved brine may remain more stable if the proportion of lithium derived from interbedded and interstitial clay is relatively large.
Occurrence of lithium states
To increase the accuracy of identifying the occurrence of lithium states in solid ores, we statistically analysed and compared the semiquantitative XRD results for mineral components with total acid digestion data (Figs. 8 and 9). Among the 86 samples, illite had the highest maximum content at 50%, with an average of 19%; the highest kaolinite content was 14%, with an average of 5%; and the chlorite content was as high as to 6%, with an average of 2%. In solid samples, lithium, potassium, and magnesium contents positively correlated with chlorite and kaolinite, although their influence was minimal. The contents of these three elements were more closely related to the illite content.
Illite, a mica-like clay mineral with a layered 2:1 dioctahedral structure, has the ideal chemical formula of K < 1(Al, R2+)2[(Si, Al)Si3O10][OH]2·nH2O, where K < 1 indicates that the potassium site is not fully occupied and has an occupancy of less than 1, meaning some positions can be vacant or substituted by other cations. Here, R2+ represents divalent metal cations such as magnesium and iron. Chlorite, with the general formula Yx[(Si, Al)4O10](OH)8 (x = 5–6), and Y mainly includes cations such as magnesium, iron, and aluminium, with smaller amounts of lithium and manganese. Chlorite has a 2:1 layered silicate structure.
The radius of lithium is similar to those of magnesium, aluminium, and iron, allowing for isomorphous substitution, which increases the lithium content in clay minerals. This substitution can lead to the formation of independent lithium minerals, such as lithium illite and cookeite45. In addition, lithium is easily adsorbed between clay minerals layers. During the acid digestion process, the layered structures of illite and chlorite are disrupted, releasing lithium, potassium, and magnesium. However, owing to the relatively low chlorite content in the samples, the contribution to the contents of these element is limited. Kaolinite, with a 1:1 dioctahedral layer structure and the molecular formula Al4[Si4O10](OH)8·(H2O)n, has minimal substitution of aluminum and silicon, and alkali and alkaline earth metals are primarily incorporated through ion adsorption46. Unlike illite, kaolinite has a lower lithium adsorption capacity, and releases fewer cations during acid digestion due to its simpler structure and reduced cation exchange capacity47.We further examined the statistical relationship between lithium, potassium, and magnesium contents and halite, gypsum, and albite. No significant correlation was observed between these elements and gypsum, while halite and albite contents were inversely correlated with lithium, potassium, and magnesium contents.
The low lithium content detected via water leaching method may be due to lithium’s association with clay minerals in an insoluble form, such as via ion adsorption or ion exchange, impeding its transfer from solid to liquid phase. The results of the comprehensive analysis indicated that the total lithium, potassium, and magnesium contents in the solid samples are primarily controlled by clay mineral content, particularly illite.
Relationship between solid ore distribution and brine composition
To explore the relationship between solid ore distribution and brine composition, results48 of our prior laboratory solution mining experiment conducted in the Mahai area were used. This experiment used simulated extraction solvents with primary chemical compositions resembling those in the Mahai mining area: Baume 10, Baume 20, and deionized water (Table 5). Solid samples were divided into three groups: untreated sample, sieved halite, and sieved “clay” (with minor halite content). Each sample was placed in a 500 mL beaker, partially filled, and then topped with solvents to 400 mL. Beakers were sealed and kept in a controlled environment (15 °C, 20% relative humidity). Sample compositions were analyzed at multiple intervals up to 726 h.
The dissolution effects are shown in Fig. 10. The “clay” sample yielded the highest potassium and lithium leaching in all solvents, though with slightly slower dissolution rates, and lithium required more time to reach saturation. Most lithium-rich clay sediments do not form independent lithium minerals during their formation; instead, lithium is mainly present within clay minerals through ion adsorption or structural incorporation via isomorphic substitution30. Thus, lithium requires extended time to transition from solid to liquid phase.

(modified from48).
Temporal variation of lithium and potassium contents in a laboratory solution mining experiment at 15 ℃.
Reservoir brine storage characteristics were statistically analyzed, focusing on porosity (pore volume as a percentage of rock volume) and specific yield (brine volume extractable by gravity) from a verification report41. Among lithologies, salt has the highest storage capacity due to extensive, connected pore spaces. Finer-grained silt and clay have lower storage capacities because of smaller, less connected pores (Fig. 11). Salt porosity and permeability also vary with mineral composition: silt-bearing halite exhibits variable characteristics, while clay-bearing and mud-bearing halite generally show lower porosity and permeability, particularly the latter (Fig. 12).
Lithium extraction efficiency from different lithologic strata indicates that clastic-bearing halite is most effective for lithium release under current solution mining techniques. Monitoring data from observation wells in 2023 show consistently high lithium levels in the eastern and northwestern mining areas, correlating with the distribution of silt-bearing and clay-bearing halite (Fig. 13). However, no clear association was observed between lithium-rich brine areas and the clay layers in the northern region.
A comparison of brine samples collected on April 29 and May 25 reveals that after approximately one month of solvent leaching, lithium contents in the brine slightly decreased, yet the spatial extent of high-lithium zones expanded. From June to August, these high-lithium areas gradually increased in both shallow and deep brine layers, suggesting a slow release of lithium from solid to liquid phase due to prolonged rock-water interactions, which incrementally raised lithium contents.
Optimizing lithium extraction may involve adjusting or adding solvent injection channels based on the distribution of silt-bearing and clay-bearing halite. Given lithium’s slower dissolution rate compared to potassium, extending the contact time between the solvent and ore layers may further enhance lithium levels in the brine.
Isopleth map of lithium contents in hydrologic observation wells in 2023 within the Salt Lake Mahai solution mining area. These maps were created using Surfer software version 15, https://www.goldensoftware.com/products/surfer.
Conclusion
A comparison of the results of total acid digestion and water leaching tests indicated that the lithium content using water leaching was less than 10% of the actual content in the clay and mud samples. The clay minerals in the study area are mainly illite, followed by chlorite, kaolinite, and an illite–smectite mixed layer. Lithium mainly occurs in the form of structural or adsorbed lithium in clay minerals, particularly illite.
Hydrological monitoring from April to August 2023 showed that high lithium concentrations (45 – 70 mg/L) were sustained in the silt-bearing and clay-bearing halite zones, particularly in the eastern and northwestern areas of Salt Lake Mahai. Although lithium levels in brine initially declined after early-stage solvent extraction of low-grade potash, the high-lithium zones gradually expanded, leading to stabilized lithium contents. This suggests that, in prolonged solution mining, lithium-rich clay interbedded with salt layers and filling salt crystal spaces provides a more sustainable and stable lithium source.
Optimizing the solvent injection strategy based on the lithological characteristics of the ore layers could further enhance the recovery efficiency of lithium in solution mining operations.
Data availability
The data are available from the corresponding author upon reasonable request.
References
Bibienne, T., Magnan, J., Rupp, A. & Laroche, N. From mine to mind and mobiles: Society’s increasing dependence on lithium. Elements 16, 265–270 (2020).
Bowell, R. J., Lagos, L., Hoyos, C. D. & Declercq, J. Classification and characteristics of natural lithium resources. Elements 16, 259–264 (2020).
Fan, E. et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 120, 7020–7063 (2020).
Khakmardan, S. et al. Comparative life cycle assessment of lithium mining, extraction, and refining technologies: A global perspective. Procedia CIRP. 116, 606–611 (2023).
Gruber, P. W. et al. Global lithium availability. J. Ind. Ecol. 15, 760–775 (2011).
Heredia, F., Martinez, A. L. & Urtubey, V. S. The importance of lithium for achieving a low-carbon future: Overview of the lithium extraction in the ‘lithium triangle’. J. Energy Nat. Resour. L. 38, 213–236 (2020).
Pan, T. et al. Characterization and resource potential of Li in the clay minerals of Mahai Salt Lake in the Qaidam Basin, China. Sustainability 15, 14067 (2023).
Shen, P. et al. Lithium deposits in the central Asian metallogenic domain: Metallogenic regularity and model. Acta Petrol. Sin. 39, 3185–3209. https://doi.org/10.18654/1000-0569/2023.11.01 (2023). (in Chinese with English abstract).
Su, T., Guo, M., Liu, Z. & Li, Q. Comprehensive review of global lithium resources. J. Salt Lake Res. 27, 104–111. https://doi.org/10.12119/j.yhyj.201903015 (2019). (in Chinese with English abstract).
Tabelin, C. B. et al. Towards a low-carbon society: a review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Min. Eng. 163, 106743 (2021).
Xin, X. et al. Extraction of lithium with functionalized lithium ion-sieves. Prog Mater. Sci. 84, 276–313 (2016).
Dai, H. Z. et al. New progress in lithium prospecting abroad (2019–2021) and its significance to China’s strategic mining resources exploration. Acta Geo Sin. 97, 583–595. https://doi.org/10.19762/j.cnki.dizhixuebao.2023012 (2023). (in Chinese with English abstract).
Han, C. M. et al. Source-sink process of brine-type lithium deposits in the salt lakes in the Qaidam Basin and its spatial mineralization association with hydrogeomorphic evolution of Nalinggele River. J. Salt Lake Res. 31, 9–18. https://doi.org/10.12119/j.yhyj.202302003 (2023). (in Chinese with English abstract).
Han, J. H. et al. Analysis of existing circumstance of supply and demand on China′s lithium resources. Inorg. Chem. Ind. 53, 61–66. https://doi.org/10.19964/j.issn.1006-4990.2021-0002 (2021). (in Chinese with English abstract).
Xue, Y. Y., Liu, H. Y. & Sun, W. D. The geochemical properties and enrichment mechanism of lithium. Geotecton et Metallog. 45, 1202–1215. https://doi.org/10.16539/j.ddgzyckx.2021.06.006 (2021). (in Chinese with English abstract).
Yan, K. et al. Origin and evolution of deep lithium-rich brines in the southwest Jianghan Basin, central China: Evidence from hydrochemistry and stable isotopes. J. Hydrol. 626, 130163 (2023).
Zhang, Z. N., Zhang, Z. Z., Wu, Q., Pan, Z. S. & Xu, H. Y. Chinese lithium mineral resource demand forecast. China Min. Mag. 29, 9–15. https://doi.org/10.12075/j.issn.1004-4051.2020.07.006 (2020). (in Chinese with English abstract).
Zhao, H. N., Ling, K. Y., Qin, S. Q., Lei, M. R. & Wen, H. J. Modes of occurrence of lithium in black shale in the Nandan area, Guangxi, SW China: Implications for clay-type resources. Ore Geol. Rev. 157, 105409 (2023).
Chen, L. et al. Investigation and analysis of lithium-containing gas field water resource in southwest oil and gas field. Chem. Eng. Oil Gas. 52, 41–47. https://doi.org/10.3969/j.issn.1007-3426.2023.02.007 (2023a). (in Chinese with English abstract).
Chen, Y., Xu, F., Cheng, H. F., Chen, X. Z. & Wen, H. J. Lithium isotope geochemistry-a review. Earth Sci. Front. 30, 469–490. https://doi.org/10.13745/j.esf.sf.2023.2.51 (2023b). (in Chinese with English abstract).
Li, H. P., Zheng, M. P., Hou, X. H. & Yan, L. J. Control factors and water chemical characteristics of potassium-rich deep brine in Nanyishan structure of western Qaidam Basin. Acta. Geo .Sin.. 36, 41–50. https://doi.org/10.3975/cagsb.2015.01.05 (2015). (in Chinese with English abstract).
Liu, C. L. et al. A tentative discussion on regional metallogenic background and mineralization mechanism of subterranean brines rich in potassium and lithium in South China Block. Min. Depos. 35, 1119–1143. https://doi.org/10.16111/j.0258-7106.2016.06.001 (2016a). (in Chinese with English abstract).
Liu, C. L. et al. Characteristics, distribution regularity and formation model of brine type Li deposits in salt lakes in the world. Acta Geo Sin. 95, 2009–2029. https://doi.org/10.19762/j.cnki.dizhixuebao.2021230 (2021). (in Chinese with English abstract).
Yu, X. C. et al. Genesis of lithium brine deposits in the Jianghan Basin and progressin resource exploration: A review. Earth Sci. Front. 29, 107–123. https://doi.org/10.13745/j.esf.sf.2021.8.11 (2022). (in Chinese with English abstract).
Liu, L. J. et al. The main types, distribution features and present situation of exploration and development for domestic and foreign lithium mine. Geol. China. 44, 263–278. https://doi.org/10.12029/gc20170204 (2017). (in Chinese with English abstract).
Fan, H. P., Ye, L. & Huang, Z. L. The associated lithium resource in bauxite (bauxite-bearing rock). Acta Min. Sin. 41, 382–390. https://doi.org/10.16461/j.cnki.1000-4734.2021.41.090 (2021). (in Chinese with English abstract).
Wang, H. et al. Types, distribution, development and utilization of lithium mineral resources in China: Review and perspective. Geotecton et Metallog. 46, 848–866. https://doi.org/10.16539/j.ddgzyckx.2022.05.002 (2022). (in Chinese with English abstract).
Wen, H. J. et al. Carbonate-hosted clay-type lithium deposit and its prospecting significance (in Chinese). Chin. Sci. Bull. 65, 53–59. https://doi.org/10.1360/TB-2019-0179 (2020). (in Chinese with English abstract).
Zhao, Y. Y., Fu, J. J. & Li, Y. Super large lithium and boron deposit in Jadar basin, Serbia. Geol. Rev. 61, 34–44. https://doi.org/10.16509/j.georeview.2015.01.001 (2015). (in Chinese with English abstract).
Wang, H. et al. The bottleneck problem and some thoughts on the process of exploration and development of clay-type lithium deposit. Geol. Rev. 69, 1298–1312. https://doi.org/10.16165/j.ggeorevie.2023.01.023 (2023). (in Chinese with English abstract).
Castor, S. B. & Henry, C. D. Lithium-rich claystone in the McDermitt Caldera, Nevada, USA: Geologic, mineralogical, and geochemical characteristics and possible origin. Minerals 10, 68 (2020).
Xu, L. et al. Lithium extraction from clay-type ore in China: Status and prospects. Multipur Util. Min. Resour. 44, 12–18. https://doi.org/10.3969/j.issn.1000-6532.2023.04.002 (2023). (in Chinese with English abstract).
Wang, W. et al. Geological characteristics and genetic mechanism of the lacustrine sedimentary clay type lithium deposit. Bull. Mineral. Petrol. Geochem. 43, 64–78. https://doi.org/10.3724/j.issn.1007-2802.20240001 (2024). (in Chinese with English abstract).
Wei, X. J., Jiang, J. X. & Wang, M. L. Sedimentary characteristics of quaternary and evolution of saline lake of Mahai Potash deposit. Qinghai Geol. 1, 40–52 (1992). (in Chinese with English abstract).
Wang, Z. Q., Hu, X. M., Wu, Y. & Wang, C. Y. Sedimentology and evolution of sedimentary facies for the cenozoic in the Mahai Region on the Northern Margin of the Qaidam Basin. Acta Geol. Sichuan. 30, 129–131 (2010). (in Chinese with English abstract).
Ma, J. Y., Hu, S. Z. & Tian, X. D. Sedimentary enviroment and exploitation of Mahai Potash deposits in Qaidam basin. J. Salt Lake Res. 18, 9–17 (2010). (in Chinese with English abstract).
Yao, F. J. et al. Research of remote sensing recognition of concealed brine-controlling structures in dry salt lake area: A case study of Mahai salt lake. Acta Geol. Sin. 95, 2225–2237 (2021).
Zhao, Y. J. et al. Contribution of deep material sources to shallow potash formation of the quaternary Mahai salt lake in the Qaidam Basin: Evidence from isotopes and trace elements. Ore Geol. Rev. 171, 106166 (2024).
Yang, H. et al. Verification Report of Resource Reserves of Mahai Potash Mining Area, Leng Lake Town, Mangya City, Qinghai Province. Report, Qinghai Zhonghang Resources Co., Ltd., 27–105. (2020).
Liu, P., Liao, Y. C. & Zhang, Y. J. Sedimentary characteristics of sedimentary bauxite and ore-bearing rock series in corroded depression: A case study of the Houcao mining area in Zunyi. Geol. China. 43, 546–563. https://doi.org/10.12029/gc20160215 (2016b). (in Chinese with English abstract).
Gu, H. N. et al. Leaching efficiency of sulfuric acid on selective lithium leachability from bauxitic claystone. Min. Eng. 145, 106076. https://doi.org/10.1016/j.mineng.2019.106076 (2020).
Liu, X. F., Wang, Q. F., Feng, Y. W., Li, Z. M. & Cai, S. H. Genesis of the Guangou karstic bauxite deposit in western Henan, China. Ore Geol. Rev. 55, 162–175 (2013).
Wanner, C., Bucher, K., Strandmann, P. P., Waber, H. N. & Pettke, T. On the use of Li isotopes as a proxy for water-rock interaction in fractured crystalline rocks: A case study from the Gotthard rail base tunnel. Geochim. Cosmochim. Acta. 198, 396–418e (2019).
Gourcerol, B., Gloaguen, E., Melleton, J., Tuduri, J. & Galiegue, X. Re-assessing the European lithium resource potential-A review of hard-rock resources and metallogeny. Ore Geol. Rev. 109, 494–519 (2019).
Liao, J. L., Wei, M. D. & Liang, X. D. Analysis on late Permian Heshan formation coal accumulation basin lithium resource features in Guangxi. Coal Geol. China. 32, 122–127. https://doi.org/10.3969/j.issn.1100-1986.2020.01.011 (2020). (in Chinese with English abstract).
Zhong, H. R. et al. Bauxite (aluminum)-type lithium resources and analysis of its development and utilization potential. Min. Depos. 38, 898–916. https://doi.org/10.16111/j.0258-7106.2019.04.014 (2019). (in Chinese with English abstract).
Zhao, Y. J. et al. Experimental study on the adsorption of Li+ by clay minerals-implications for the mineralization of clay-type lithium deposit. Acta Mineral. Sin. 42, 141–153. https://doi.org/10.16461/j.cnki.1000-4734.2021.41.118 (2022). (in Chinese with English abstract).
Cui, Z. H. et al. Experimental study of lithium extraction in the solid-liquid conversion of low-grade solid potash ore. Minerals 14, 116 (2024).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFC2906502), and the Qinghai Zhonghang Resources Co, Ltd. Commissioned Science and Technology Research Project (zhzyb20210126-01).
Author information
Authors and Affiliations
Contributions
Y.Z.: Conceptualization, Formal Analysis, Methodology, Investigation, Resources, Writing - Original Draft; P.L.: Conceptualization, Data Curation, Investigation, Writing - Review & Editing; H.Z.: Investigation, Writing - Review & Editing; Y.H.: Data Curation, Formal Analysis; Z.C.: Investigation, Formal Analysis; Y.Z.: Investigation, Formal Analysis; M.Z.: Investigation, Resources; Q.W.: Investigation; S.H.: Investigation, Resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Y., Long, P., Zhang, H. et al. The occurrence characteristic and dissolution mechanism of lithium-rich sediments in Salt Lake Mahai of Qaidam Basin, NW China. Sci Rep 15, 4291 (2025). https://doi.org/10.1038/s41598-025-88674-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88674-1