Abstract
Nocturia is an increasingly prevalent chronic disease that affects the quality of an individual’s life. The prevalence of nocturia exceeds 50% among older adults in the U.S. Inflammation and oxidative stress are considered potential factors affecting nocturia, according to prior research. Flavonoids have attracted considerable interest due to their anti-inflammatory, antioxidant, and anti-aging properties. The association between flavonoid consumption and nocturia has been rarely investigated. For this cross-sectional study, we selected 5926 middle-aged and older adults over 40 from the 2007–2010 National Health and Nutrition Examination Survey (NHANES). Daily dietary flavonoid consumption was assessed through two 24-hour dietary recall interviews and the principal outcome was assessed based on a standardized questionnaire administered by trained interviewers. Weighted multivariate logistic regression and stratified analysis were employed to evaluate the impact of dietary total flavonoid consumption on nocturia. Restricted cubic spline (RCS) was utilized to explore whether there is a non-linear association between total flavonoid intake and nocturia. In addition, we performed weighted logistic regression and weighted quantile sum (WQS) regression by six flavonoid species (isoflavones, anthocyanins, flavan-3-ols, flavanones, flavonoids and flavonols) to estimate a combined mixture sum effect as well as the contribution of each mixture component. Subgroup analysis help us identify heterogeneity in these relationships and enhances the applicability of the research findings across different groups. After fully adjusting for confounders, weighted logistic regression models indicated that dietary flavonoid intake was significantly associated with a decreased prevalence of nocturia (OR: 0.93; 95% CI: 0.88-1.00, P-value = 0.036). This trend remained significant in the analysis stratified by quartile level of flavonoid intake. RCS analysis demonstrated an inverse linear correlation between total flavonoid consumption and nocturia. Analysis conducted through quartile stratification of six flavonoid species and WQS regressions showed that anthocyanins, flavan-3-ols, and flavonols were crucial elements. Within these subclasses, Peonidin in anthocyanins, Catechin and Theaflavin-3,3’-digallate in flavan-3-ols, and Isorhamnetin, Kaempferol, and Quercetin in flavonols were also investigated and may have contributed significantly. Our study revealed an inverse association between dietary flavonoid intake and nocturia in middle-aged and older adults, and anthocyanins, flavan-3-ols and flavonols were protectively associated with nocturia.
Similar content being viewed by others
Introduction
Nocturia is defined as urination that occurs during the main sleep period by the International Continence Society (ICS)1,2. Two or more episodes of voiding per night are considered to be correlated with adverse clinical endpoints1,3. The prevalence of nocturia among the United States population is 30.5% in men and 34.9% in women4, and it has been reported to be over 50% among older adults5. A study has shown a general increasing trend in the prevalence of nocturia in the middle-aged and older adults over the last decade6. However, patients and physicians often tend to underestimate its serious negative influence on the life quality of the individuals, health care systems and socio-economic burdens7,8. Nocturnal urination directly interrupts the sleeping process, causing fatigue, lack of concentration and low mood during the daytime among disturbed individuals9. Patients with nocturia have a substantially increased risk of cardiovascular disease, with the mortality rising by 23%10,11. A variety of factors cause nocturia: increased nocturnal urine production, decreased bladder storage capacity (including cystitis and nocturnal detrusor overactivity), and impaired circadian rhythms associated with sleep disorders1,9. The current main medications for nocturia are desmopressin and antidiuretic hormones. However, these therapeutic strategies have some side effects such as hyponatremia, and some patients develop resistance or adverse effects due to long-term continuation of the drug usage12,13. Therefore, it is imperative to seek preventive and therapeutic strategies for nocturia from the perspective of daily dietary supplementation.
Flavonoids are a category of natural polyphenols that have received extensive attention for their beneficial effects such as anti-inflammatory, antioxidant and anti-aging properties14. These properties make flavonoids a promising candidate for investigating their potential role in managing various diseases15,16. Inflammatory mediators can cause damage to the urothelial cells and irritation of the bladder mucosa, leading to nocturia17. Studies on dietary management of nocturia have indicated that certain dietary compounds, including flavonoids, alkaloids and glycosides, were linked to the pathogenesis of nocturia, but sorely lacking specific evidence18,19. A cross-sectional study of 785 Japanese elderly individuals (mean age: 61.7 years) found that increased intakes of fruits and vegetables were inversely associated with nocturia, possibly due to the potential role of phytochemicals with antioxidant activity20. However, there was no definitive evidence to attribute this benefit to flavonoids. To date, studies on the association between dietary flavonoid consumption and nocturia have not yet been carried out, and the number of animal experiments and clinical studies in this field is quite limited.
Therefore, we performed a cross-sectional analysis to evaluate the connection between dietary flavonoid consumption and nocturia based on complete data from the 2007–2010 National Health and Nutrition Examination Survey (NHANES). The results may provide novel insights into daily dietary management and prevention strategies for middle-aged and older adults with nocturia.
Materials and methods
The participants in the study
The NHANES, conducted across the country, employs a multistage, intricately stratified sampling design at random and is devoted to gathering data regarding the health risk factors and nutritional status of populations. Each subject has already signed a written informed consent prior to being added to the database. Information and data are publicly accessible for this survey. The Research Ethics Review Committee examined and ratified all NHANES protocols in strict adherence to the tenets of the Declaration of Helsinki.
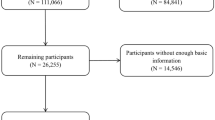
Our study utilized available data from NHANES 2007–2010 based on the completeness of the data required for the study, with a total of 20,686 participants recruited. Nocturia is a common clinical condition, with its prevalence increasing with age and primarily affecting middle-aged and older adults; therefore, our study selected adults aged 40 years and above as the main research subjects. Participants who were ≤ 40 years of age (N = 12,752), were pregnant (N = 2), had prostate cancer (N = 198), and had comorbidities associated with neurogenic bladder (e.g., stroke) (N = 437) were excluded. Subsequently, participants with missing information on nocturia (N = 941), missing data on dietary flavonoid intake (N = 115), and missing data on covariates (N = 303) were also excluded. After the above screening, 5938 participants were finally included (Fig. 1).
Dietary flavonoid intake assessment
The dietary intake information for NHANES was obtained via two 24-hour dietary recollection interviews. The first interview of dietary recollection was performed at the Mobile Examination Center (MEC), followed by a subsequent telephone interview three to ten days later. All dietary interviewers were required to complete an intensive one-week training program and participate in supervised practice interviews before conducting fieldwork independently. This rigorous training and retraining process ensured the accuracy and consistency of the dietary data collected. The Food and Nutrition Database for Dietary Studies (FNDDS) carried out by the United States Department of Agriculture (USDA) is the platform for coding and calculating dietary nutrient intake for NHANES21. Flavonoid counts of all foods and beverages are provided in FNDDS versions 4.1 and 5.0. The database comprises data on 29 flavonoids of the 6 main flavonoid subclasses in line with the NHANES Dietary Data, including isoflavones, anthocyanidins, flavan-3-ols, flavanones, flavones, and flavonols. Isoflavones consist of daidzein, genistein, and glycitein. Anthocyanidins include cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin. Flavan-3-ols contain epicatechin, epicatechin 3-gallate, epigallocatechin, epigallocatechin 3-gallate, catechin, gallocatechin, theaflavin, theaflavin-3,3’-digallate, theaflavin-3’-gallate, theaflavin-3-gallate, and tthearubigins. Flavanones include eriodictyol, hesperetin, and naringenin. Flavones include apigenin and luteolin. Flavonols comprise isorhamnetin, kaempferol, myricetin, and quercetin. The total flavonoid intake was obtained by summing up the six flavonoid subclasses. The daily flavonoid consumption was calculated by averaging the dietary recollection data from two days (in milligrams per 100 g of food or beverage per day). This process is standardized to mitigate confusion arising from varying numbers of dietary recalls.
Measurement and definition of nocturia
Nocturia, serving as the principal outcome, was assessed using a standardized questionnaire administered by trained interviewers via the Computer-Assisted Personal Interviewing (CAPI) system within the Mobile Examination Center (MEC). This was determined by asking, “How many times per night did you most typically get up to urinate from bedtime to rising in the morning during the past 30 days?” The available response choices included the following: “zero nocturnal voids,” “one nocturnal void,” “two nocturnal voids,” “three nocturnal voids,” “four nocturnal voids,” and “five or more nocturnal voids.” As defined by the International Continence Society in 2018, participants who experienced two or more voids per night were considered nocturia1,3.
Covariates
Based on previous studies, we identified several potential confounders associated with dietary flavonoids and nocturia including sex, age, race/ethnicity, body mass index (BMI), cigarette use, alcohol consumption, diabetes mellitus, hypertension, cardiovascular disease (CVD), stress urinary incontinence, urge urinary incontinence, urinary albumin creatinine ratio (UACR), daily energy intake, daily carbohydrate intake, daily moisture intake and daily sodium intake. Continuous variables have been categorized. The study stratified the smoking status into three separate categories: (1) Never smoker, characterized by who has inhaled less than one hundred cigarettes in a lifetime; (2) Former smoker, characterized by who has consumed over 100 cigarettes in a lifetime but having not smoked any cigarettes until now; (3) Current smoker, characterized by who has consumed over 100 cigarettes in a lifetime and now smoking occasionally or on a daily basis. The definition of drinking status is as follows: (1)non-drinkers: alcohol consumption < 12 times per year; (2) drinkers: alcohol consumption ≥ 12 times per year. BMI was divided into three groups: (1) ≤ 24.9 kg/m2; (2) 25.0–29.9 kg/m2;(3) > 30 kg/m2. The diagnosis of hypertension is established when the individual meets any of the following criteria: (1) Being told to have hypertension by doctors previously; (2) Using the antihypertensive medication; (3) Having high blood pressure satisfying the diagnostic criteria (systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg)22. Our research employed the following diagnostic criteria for diabetes mellitus: (1) Being diagnosed with diabetes by a physician; (2) The glycated hemoglobin>6.5%; (3) Fasting glucose levels exceeding 7 mmol/L; (4) Random plasma glucose levels equal to or more than 11.1 mmol/L; (5) Two hours glucose levels in oral glucose tolerance test equal to or greater than 11.1 mmol/L23. Participants were diagnosed with diabetes if they met any of the criteria above. Cardiovascular disease (CVD) was defined as a diagnosis of at least one disease, including congestive heart failure, coronary artery disease, angina pectoris or heart attack by a physician. Stress urinary incontinence was considered any episode of urinary leakage or loss of control while coughing, weightlifting, or exercising within the previous twelve months. Urge urinary incontinence was considered involuntary urine leakage or loss of control due to the urge or pressure to urinate before reaching a lavatory in time.
Statistical analysis
The extraction, integration and analysis of data from NHANES were all performed with R software (Version 4.3.1). The sampling design incorporated stratification, clustering, and varying selection probabilities. “SDMVSTRA” facilitated stratification by grouping the sample based on geographic and demographic factors, thereby addressing population heterogeneity. “SDMVPSU” was used to address clustering by representing the primary sampling unit (PSU), capturing the correlation among observations within each PSU. Unequal selection probabilities were addressed through the use of “WTINT2YR”, the interview weight, which ensured that the sample accurately represents the US population. Initially, demographic characteristics were compared between nocturia and non-nocturia. For the baseline analysis, the Wilcoxon rank-sum test was utilized as the primary method for analyzing continuous variables, while the chi-square test was applied for the assessment of categorical variables. Three weighted logistic regression models were then applied to evaluate the link between dietary flavonoid intake and nocturia. Total flavonoid intake was introduced into models as a continuous variable after logarithmic transformation. Subsequently, intakes of total flavonoids and flavonoid subclasses were categorized by quartiles based on their absolute values, with the lowest quartile being the reference group. Model 1 adjusted for no variables. Model 2 adjusted for statistically significant sociodemographic characteristics including age, race, and BMI. Model 3 adjusted for all significant variables in the baseline analysis. Restricted cubic spline (RCS) was utilized to explore whether there is a non-linear association between total flavonoid intake and nocturia. Weighted quantile sum (WQS) regression models by six flavonoid species (isoflavones, anthocyanins, flavan-3-ols, flavanones, flavonoids and flavonols) were performed to estimate a combined mixture sum effect as well as each mixture component’s contributions. The process of WQS regression modeling comprised two stages: (1) Conducting significance tests to determine the relationship between the WQS index and outcome regression coefficient and (2) Calculating the appropriate weights of critical components in bootstrap samples. The data were separated into a 60% validation set and a 40% training set. One hundred bootstrap samples were generated from the training set in order to estimate the weights of flavonoid subclasses, and the effects values were tested in the validation set. Details of the calculation of WQS were presented in the literature24. Subgroup analysis helps us identify heterogeneity in these relationships and enhances the applicability of the research findings across different groups. Finally, another weighted multivariate logistic regression with full adjustment were performed to investigate the influential individual flavonoids within the primary contributing subgroups. All statistical analysis were considered to be significant with a threshold of P-value < 0.05 (two-tailed).
Results
Demographic features of the study group
The demographic characteristics between nocturia and non-nocturia are presented in Table 1. A total of 5,938 participants over 40 years of age were eventually included, comprising 2231 with nocturia (30%) and 3707 without nocturia (70%). Differences in sex, age, race, body mass index (BMI), smoking status, drinking status, hypertension, diabetes mellitus, cardiovascular disease (CVD), stress urinary incontinence, urge urinary incontinence, urinary albumin creatinine ratio (UACR), daily energy intake, daily carbohydrate intake, daily moisture intake and daily sodium intake between the two groups were statistically significant (P-value<0.05). The characteristics of dietary flavonoid consumption are illustrated in Table 2, and our study revealed individuals with nocturia were more inclined to consume fewer total flavonoids (P-value = 0.002), Flavan-3-ols (P-value < 0.001), Anthocyanidins (P-value < 0.001), Flavones (P-value = 0.008) and Flavonols (P-value < 0.001).
Association between total flavonoid consumption and nocturia
In all unadjusted and adjusted models, there was a significant correlation between the overall consumption of flavonoids (mg/day) and the decreased prevalence of nocturia when examined as a continuous variable (Table 3), and this association was still significant after fully adjusting for confounding factors in Model 3 (OR: 0.93; 95% CI: 0.88-1.00, P-value = 0.036). We categorized total flavonoid consumption by quartiles for a more detailed examination and found a salient reduction regarding the prevalence of nocturia in the 2nd, 3rd, and 4th quartiles in comparison with the minimum intake group. In Model 1, individuals involved in the 2nd quartile (OR: 0.72; 95% CI: 0.59–0.88, P-value = 0.002), the 3rd quartile (OR: 0.76; 95% CI 0.64–0.89, P-value = 0.001) and the 4th quartile (OR: 0.72; 95% CI: 0.59–0.89, P-value = 0.003) exhibited a lower likelihood of nocturia than those in the reference quartiles. In Model 2, after adjusting for sociodemographic characteristics, significant negative associations were also perceived in the 2nd quartile (OR: 0.73; 95% CI: 0.58–0.93, P-value = 0.012), the 3rd quartile(OR: 0.73;95% CI: 0.61–0.88, P-value = 0.002) and the 4th quartile(OR: 0.77; 95% CI: 0.62–0.95, P-value = 0.018). Even after fully adjusting for all confounders in Model 3, the 2nd (OR: 0.73; 95% CI: 0.56–0.97, P-value = 0.032), the 3rd (OR: 0.77;95% CI: 0.62–0.95, P-value = 0.020) and the 4th (OR: 0.77; 95% CI: 0.59-1.00, P-value = 0.049) quartile of total flavonoid consumption were considerably associated with the diminished occurrence of nocturia. We implemented a weighted RCS analysis to further discuss the non-linear relationship between the overall consumption of flavonoids (mg/day) and nocturia (Fig. 2). After adjusting for confounders, no statistically significant non-linear relationship was discovered (p for non-linear = 0.1428).
Associations between six flavonoid subgroup consumption and nocturia
Analysis conducted through quartile stratification further assessed the influence of six specific flavonoid subgroups consumption (mg/day) on nocturia (Table 4). In Model 1, a prominent negative correlation was captured between the highest quartile of Isoflavones, Anthocyanidins, and Flavones with nocturia, as well as Flavan-3-ols and Flavonols in all quartiles. In Model 2, the negative association was verified in all quartiles of Flavan-3-ols, the 2nd and 3rd quartiles of Flavonols, and the 4th quartile of Anthocyanidins. In Model 3 (Table 4; Fig. 3), this inverse association was mainly established in the following three flavonoid subclasses: the intakes of Flavan-3-ols in the 2nd (OR: 0.76, 95% CI: 0.62–0.93, P-value = 0.012), the 3rd (OR: 0.71, 95% CI: 0.57–0.89, P-value = 0.008) and the 4th (OR: 0.77, 95% CI: 0.60–0.99, P-value = 0.040) quartile; Anthocyanidins in the 4th (OR: 0.70, 95% CI: 0.56–0.89, P-value = 0.007) quartile; Flavonols in the 2nd (OR: 0.77, 95% CI: 0.60-1.00, P-value = 0.049), the 3rd (OR: 0.74, 95% CI: 0.59–0.92, P-value = 0.013) and the 4th (OR: 0.74, 95% CI: 0.56–0.99, P-value = 0.042) quartile. The WQS model (Fig. 4) demonstrated their mixed effect was related to a decreased prevalence of nocturia (OR: 0.79, 95% CI: 0.72–0.87, p<0.01). In terms of their individual effect estimates, Anthocyanidins (39.60%), Flavan-3-ols (21.40%) and Flavonols (14.80%) were the top 3 components, followed by Flavones (9.33%), Isoflavones (7.56%) and Flavanones (7.40%).
Subgroups analysis
To gain a deeper understanding of the relationship between the consumption of total flavonoids, Flavan-3-ols, Anthocyanidins, Flavonols, and nocturia, we conducted subgroups and interaction analyses (Supplementary Figure S1–S4) across various subgroups defined by age, race, BMI, cigarette use, drinking status, hyperlipidemia, diabetes and cardiovascular disease. The subgroup analysis of total flavonoid intake revealed no significant heterogeneity across the different subgroups (Supplementary Figure S1). This finding suggested that the potential confounders such as age, may successfully be controlled in our study, and that the exposure factor under investigation may have a similar effect across diverse subgroups.Significant interactions were identified between Flavan-3-ols and nocturia within the race subgroup (P for interaction = 0.035) (Supplementary Figure S2). The interaction effect between Anthocyanidins and nocturia was found to be significant in the smoking status subgroup (P for interaction = 0.033) (Supplementary Figure S3). A significant interaction effect between Flavonols and nocturia was observed within both the Age subgroup (P for interaction = 0.043) and the Hypertension subgroup (P for interaction = 0.022) (Supplementary Figure S4). As shown in the Supplementary Figure S4, the 60–79 age group exhibited a noticeable deviation compared with the other two age groups. This deviation could potentially be attributed to several factors, such as the sample size or the specific characteristics of the participants within this age group. Subgroup analysis helps identify heterogeneity in these relationships and enhances the applicability of the research findings across different groups.
Individual flavonoids in anthocyanins, flavan-3-ols, and flavonols
Since anthocyanins, flavan-3-ols, and flavonols significantly contributed to the overall effect of flavonoids, we further investigated the influential individual flavonoids within the primary contributing subgroups via weighted multivariate logistic regression with full adjustment (Supplementary Table S1). Among anthocyanins, significant differences were observed in the 4th quartile of Peonidin (OR: 0.75, 95% CI: 0.57-1.00, p = 0.048) and Petunidin (OR: 0.73, 95% CI: 0.54–0.97, p = 0.034). In flavan-3-ols, the 4th quartile of Catechin (OR: 0.74, 95% CI: 0.56–0.98, p = 0.037) and the 3rd quartile of Theaflavin-3,3’-digallate (OR: 0.83, 95% CI: 0.71–0.96, p = 0.018) exhibited significant differences. In flavonols, the 4th quartile of Isorhamnetin (OR: 0.78, 95% CI: 0.65–0.94, p = 0.016) and Kaempferol (OR: 0.76, 95% CI: 0.58-1.00, p = 0.049), as well as the 3rd quartile of Quercetin (OR: 0.76, 95% CI: 0.61–0.95, p = 0.023) were also found to be related to a decreased prevalence of nocturia.
Discussion
To the best of our understanding, this study represents the inaugural endeavor to explore the correlation between dietary flavonoids intake and nocturia. First, the logistic regression model demonstrated that the dietary consumption of total flavonoid was strongly correlated with a reduced prevalence of nocturia among 5926 participants. This tendency remained significant in the analysis stratified by quartile levels of flavonoid intake. Analysis conducted through quartile stratification and WQS regressions revealed that anthocyanins, flavan-3-ols, and flavonols played major roles in the six subclasses. The exact processes underlying the association between dietary flavonoid consumption and a lower prevalence of nocturia remain unclear, and we have collated the existing evidence and proposed several possibilities.
Ameliorating bladder detrusor overactivity at night may serve as a potential therapeutic avenue to reduce nocturia25,26 and this possibility is supported by several studies. A prospective clinical study found that after supplemental treatment with flavonoids, postmenopausal women with overactive bladder disease had a lower mean number of voiding episodes in 24 h compared to a placebo group27. Flavonoid galangin has been reported to inhibit porcine detrusor contractions by blocking intracellular Ca2+ release, as demonstrated in an ex vivo study using porcine bladder strips at various concentrations28. As shown in another preclinical studies using rat bladder strips, flavonol glycosides have been suggested to play a role in suppressing rat bladder smooth muscle contractions29. According to the findings above, the inhibitory effect of flavonoids on the detrusor muscle is well-established and might be a viable therapeutic modality to facilitate nocturia caused by detrusor overactivity.
Circadian rhythm disruption regulated by clock genes may contribute to nocturia30. An animal experiment observed that Clock gene mutants had impaired urine storage function and urine production rhythms of the bladders due to loss of circadian transcriptional regulation, causing nocturia in mice31. Polymethoxy flavonoids differentially affect the amplitude, period, and phase of circadian rhythms in PER2::LUC knock-in mice, which may have beneficial effects on circadian disorders32. Cyclocarya paliurus flavonoid has been proven to be beneficial for intestinal microbiota to regenerate the intestinal microenvironment and regulate the release of advantageous metabolites, thereby treating circadian rhythm disruption33. Another study also indicated that proanthocyanidins have the ability to regulate peripheral molecular clocks34. These indicate that intake of flavonoids, as therapeutically active dietary supplements, could improve nocturia due to their potential benefits for circadian rhythm disruption.
Flavonoids have been extensively studied for their anti-inflammatory, antioxidant damage and free radical scavenging properties. Inflammatory mediators can cause damage to the urothelial cell and irritation of the bladder mucosa, leading to urinary urgency, frequency and nocturia17. Anthocyanins exhibit potent anti-inflammatory effects because they suppress the release of inflammatory cytokines including TNF-α and NO35. Flavan-3-ols inhibit inflammatory responses by down-regulating the transcription of genes associated with the PI3K-Akt and the TNF pathway, as well as suppressing the secretion of IL-1β, IL-6 and TNF-α36. Moreover, oxidative stress activates cell survival signals and causes ultrastructural damage to bladder smooth muscle cells, leading to frequent urination, increased urine output and nocturia37. Anthocyanins isolated from black rice can protect rats against cytotoxicity induced by H2O2 for inhibiting oxidative stress38. Another study found that anthocyanins in blueberries may prevent the development of overactive bladder syndrome by inhibiting bladder remodeling and performing as antioxidants39. Flavonols have been proven to protect against oxidative damage induced by tert-butyl hydroperoxide and other cytotoxic compounds, as well as scavenge ROS/RNS formed in organisms and exert antioxidant modulating activity40. These shreds of evidence imply that flavonoid compounds with anti-inflammatory and antioxidant properties have the potential to treat and prevent diseases related to bladder storage dysfunction.
This study has several strengths. First of all, this is the first investigation to reveal the relationship between dietary flavonoid consumption and nocturia in middle-aged and older adults and provide evidence for the beneficial association based on the large population. Secondly, we consider appropriate sampling weights in our analysis, which can be representative of the characteristics of the entire U.S. population, to mitigate oversampling bias and bolster the validity of our conclusions. Additionally, we constructed three different models adjusted for several confounding variables for our analysis and performed stratified and subgroup analysis, confirming the robustness of our results.
Nevertheless, our study is subject to several limitations. Firstly, we employed a validated questionnaire to assess nocturia but did not require participants to complete any symptom score scale or bladder diary as nocturia diagnostic tools. Secondly, the estimation of daily dietary flavonoid intake was derived from a two 24-hour dietary recalls regarding dietary consumption, which could potentially compromise the accuracy of our findings. Furthermore, we ensured the effective exclusion of relevant confounders, but there were potential confounders that we could not completely eliminate. Finally, as a cross-sectional investigation, we still need additional clinical trials and prospective longitudinal cohort studies to validate the health benefits of flavonoids.
Conclusion
Our study revealed an inverse association between dietary flavonoid intake and nocturia in middle-aged and older adults, and anthocyanins, flavan-3-ols and flavonols was protectively associated with nocturia. Further clinical and experimental studies are required to explore the precise mechanisms, and to validate its efficacy in human metabolism underlying the intrinsic connection between dietary flavonoid intake and nocturia.
Data availability
This study used data from a free and open public database, which can be found here: www.cdc.gov/nchs/nhanes/.
References
Hashim, H. et al. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol. Urodyn. 38, 499–508. https://doi.org/10.1002/nau.23917 (2019).
Everaert, K. et al. International Continence Society consensus on the diagnosis and treatment of nocturia. Neurourol. Urodyn. 38, 478–498. https://doi.org/10.1002/nau.23939 (2019).
Chen, J. et al. Relationship between nocturia and hypertension: findings from the NHANES 2005–2016. Front. Cardiovasc. Med. 10 https://doi.org/10.3389/fcvm.2023.1165092 (2023).
Moon, S. et al. The Association between Obesity and the Nocturia in the U.S. Population. Int. Neurourol. J. 23, 169–176. https://doi.org/10.5213/inj.1938062.031 (2019).
Bosch, J. L. H. R. & Weiss, J. P. The prevalence and causes of Nocturia. J. Urol. 189. https://doi.org/10.1016/j.juro.2012.11.033 (2013).
Soysal, P. et al. Trends and prevalence of nocturia among US adults, 2005–2016. Int. Urol. Nephrol. 52, 805–813. https://doi.org/10.1007/s11255-019-02361-5 (2019).
Dmochowski, R. et al. Economic burden of illness in adult patients with Nocturia. J. Managed Care Specialty Pharm. 25, 593–604. https://doi.org/10.18553/jmcp.2019.18067 (2019).
Jhaveri, J., Gauthier-Loiselle, M., Gagnon-Sanschagrin, P. & Wu, E. Q. The Economic Burden of Nocturia on the U.S. Health Care System and Society: a National Health and Nutrition Examination Survey Analysis. J. Managed Care Specialty Pharm. 25, 1398–1408. https://doi.org/10.18553/jmcp.2019.19191 (2019).
Dani, H., Esdaille, A. & Weiss, J. P. Nocturia: aetiology and treatment in adults. Nat. Reviews Urol. 13, 573–583. https://doi.org/10.1038/nrurol.2016.134 (2016).
Moon, S. et al. Association of nocturia and cardiovascular disease: data from the National Health and Nutrition Examination Survey. Neurourol. Urodyn. 40, 1569–1575. https://doi.org/10.1002/nau.24711 (2021).
Moon, S. et al. The relationship between Nocturia and Mortality: Data from the National Health and Nutrition Examination Survey. Int. Neurourol. J. 26, 144–152. https://doi.org/10.5213/inj.2142370.185 (2022).
Weiss, J. P. & Everaert, K. Management of Nocturia and Nocturnal Polyuria. Urology 133, 24–33. https://doi.org/10.1016/j.urology.2019.09.022 (2019).
Dossche, L., Walle, J. V. & Van Herzeele, C. The pathophysiology of monosymptomatic nocturnal enuresis with special emphasis on the circadian rhythm of renal physiology. Eur. J. Pediatrics. 175, 747–754. https://doi.org/10.1007/s00431-016-2729-3 (2016).
Kopustinskiene, D. M., Jakstas, V., Savickas, A. & Bernatoniene, J. Flavonoids as Anticancer agents. Nutrients 12 https://doi.org/10.3390/nu12020457 (2020).
Li, M. et al. Evidence of Flavonoids on Disease Prevention. Antioxidants 12. https://doi.org/10.3390/antiox12020527 (2023).
Shen, N. et al. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 383. https://doi.org/10.1016/j.foodchem.2022.132531 (2022).
Grover, S., Srivastava, A., Lee, R., Tewari, A. K. & Te, A. E. Role of inflammation in bladder function and interstitial cystitis. Ther. Adv. Urol. 3, 19–33. https://doi.org/10.1177/1756287211398255 (2011).
Alwis, U. S. et al. Dietary considerations in the evaluation and management of nocturia. F1000Research 9. https://doi.org/10.12688/f1000research.21466.1 (2020).
Matsuo, T., Miyata, Y. & Sakai, H. Effect of salt intake reduction on nocturia in patients with excessive salt intake. Neurourol. Urodyn. 38, 927–933. https://doi.org/10.1002/nau.23929 (2019).
Furukawa, S. et al. Dietary intake habits and the prevalence of nocturia in Japanese patients with type 2 diabetes mellitus. J. Diabetes Invest. 9, 279–285. https://doi.org/10.1111/jdi.12709 (2017).
Sebastian, R. S. et al. A New Database facilitates characterization of flavonoid intake, sources, and positive associations with Diet Quality among US adults. J. Nutr. 145, 1239–1248. https://doi.org/10.3945/jn.115.213025 (2015).
Williams, B. et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Classification and Diagnosis of Diabetes:Standards of Medical Care in Diabetes—2021. Diabetes Care 44, S15–S33. https://doi.org/10.2337/dc21-S002 (2021).
Wei, M. et al. Associations of multiple metals with bone mineral density: a population-based study in US adults. Chemosphere 282 https://doi.org/10.1016/j.chemosphere.2021.131150 (2021).
Gordon, D. J., Emeruwa, C. J. & Weiss, J. P. Management strategies for Nocturia. Curr. Urol. Rep. 20 https://doi.org/10.1007/s11934-019-0940-2 (2019).
Al-Zahrani, A. A. & Gajewski, J. Urodynamic findings in women with refractory overactive bladder symptoms. Int. J. Urol. 23, 75–79. https://doi.org/10.1111/iju.12954 (2015).
Betschart, C. et al. Randomized, double-blind placebo-controlled trial with Bryophyllum pinnatum versus placebo for the treatment of overactive bladder in postmenopausal women. Phytomedicine 20, 351–358. https://doi.org/10.1016/j.phymed.2012.10.007 (2013).
Fürer, K. et al. Inhibition of porcine detrusor contractility by the flavonoid fraction of Bryophyllum pinnatum – a potential phytotherapeutic drug for the treatment of the overactive bladder syndrome. Phytomedicine 22, 158–164. https://doi.org/10.1016/j.phymed.2014.11.009 (2015).
Shimoda, H., Takeda, S., Shimizu, N., Hirano, M. & Hitoe, S. Suppressive effect of triterpenoids and a flavonol glycoside in seaberry extract on carbacol-induced contraction of bladder smooth muscle and TGF-β-induced contraction of collagen gel containing bladder smooth muscle cells. J. Funct. Foods. 31, 152–159. https://doi.org/10.1016/j.jff.2017.01.043 (2017).
Ihara, T. et al. G protein-coupled receptor 55 activated by palmitoylethanolamide is associated with the development of nocturia associated with circadian rhythm disorders. Life Sci. 332 https://doi.org/10.1016/j.lfs.2023.122072 (2023).
Ihara, T. et al. Different effects of GsMTx4 on nocturia associated with the circadian clock and Piezo1 expression in mice. Life Sci. 278 https://doi.org/10.1016/j.lfs.2021.119555 (2021).
Oster, H. et al. Potent effects of Flavonoid Nobiletin on Amplitude, Period, and phase of the circadian clock rhythm in PER2::LUCIFERASE Mouse embryonic fibroblasts. Plos One. 12 https://doi.org/10.1371/journal.pone.0170904 (2017).
Sun, Y. et al. The Modulatory Effect of Cyclocarya paliurus flavonoids on intestinal microbiota and hypothalamus clock genes in a circadian rhythm disorder mouse model. Nutrients 14 https://doi.org/10.3390/nu14112308 (2022).
Ribas-Latre, A. et al. Chronic consumption of dietary proanthocyanidins modulates peripheral clocks in healthy and obese rats. J. Nutr. Biochem. 26, 112–119. https://doi.org/10.1016/j.jnutbio.2014.09.006 (2015).
Gao, Q. et al. Anti-glycation and anti-inflammatory activities of anthocyanins from purple vegetables. Food Funct. 14, 2034–2044. https://doi.org/10.1039/d2fo03645b (2023).
Yang, Q. et al. Asymmetric synthesis of flavanols via Cu-catalyzed kinetic resolution of chromenes and their anti-inflammatory activity. Sci. Adv. 8 https://doi.org/10.1126/sciadv.abm9603 (2022).
Witthaus, M. W. et al. Bladder oxidative stress in Sleep Apnea contributes to Detrusor instability and Nocturia. J. Urol. 193, 1692–1699. https://doi.org/10.1016/j.juro.2014.11.055 (2015).
Palungwachira, P. et al. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa L. in Primary Dermal Fibroblasts. Oxidative Medicine and Cellular Longevity 1–18. (2019). https://doi.org/10.1155/2019/2089817 (2019).
Miyazaki, N., Katsura, R., Hamada, K. & Suzutani, T. Blueberry prevents the bladder dysfunction in bladder outlet obstruction rats by attenuating oxidative stress and suppressing bladder remodeling. Nutrients 12 https://doi.org/10.3390/nu12051285 (2020).
Barreca, D. et al. Food flavonols: nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 117, 194–204. https://doi.org/10.1016/j.tifs.2021.03.030 (2021).
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (grant number: 82274419), Guangdong Basic and Applied Basic Research Foundation (No: 2021A1515220177) and the Natural Science Foundation of Guangdong Province (grant number: 2020A1515010775).
Author information
Authors and Affiliations
Contributions
Y.C. and Y-C.L. designed this study, analyzed, wrote, and revised the manuscript, X-Y.H., and L.L. assisted in editing the manuscript and analyzing data, H-L.L., D-L.L. and S-F.C. supervised and reviewed the manuscript. All authors reviewed and approved the final manuscript, which had not been previously published. The work was not under consideration for publication elsewhere.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Each participant provided a written informed agreement before inclusion in the NHANES database, which was examined and allowed by the National Center for Health Statistics Ethics Review Board. Anonymously processing the data makes it available to the public. The researchers then can transform the data into a form suitable for analysis following privacy-preserving. Based on the study’s data usage guidelines, all data will be analyzed statistically, and all studies will comply with all relevant laws and standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, Y., Liang, YC., Hu, XY. et al. Inverse association between dietary flavonoid intake and nocturia in middle-aged and older adults from NHANES 2007–2010. Sci Rep 15, 4423 (2025). https://doi.org/10.1038/s41598-025-88681-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88681-2