Abstract
Preeclampsia (PE) is a challenge in maternal healthcare due to its complex nature, characterized by high blood pressure, protein in the urine, and damage to various organs. There is evidence linking PE to endothelial dysfunction (ED), triggered by substances released from an oxygen-deprived placenta. Previous in vitro studies have not considered the impact of in vivo elements, such as the different patterns of blood flow, and laminar (LSS) vs. oscillatory (OSS) shear stress, on the development of ED. We investigated the impact of plasma from healthy pregnant women (HP), subjects with gestational hypertension (GH), and PE patients on global gene expression of human coronary endothelial cells (HCAECs) under LSS and OSS. Our findings revealed a unique transcriptional profile of endothelial cells induced by plasma incubation in LSS. Notably, OSS resulted in similar transcriptomes irrespective of plasma treatment. Under LSS, GH plasma resulted in a proliferative profile, whereas PE plasma was linked to pro-inflammatory and antioxidant profiles compared to HP plasma. Our findings demonstrate that shear stress levels influence the endothelial cell transcriptome in response to plasma from hypertensive pregnancy patients. Both PE and GH can induce endothelial dysfunction under atheroprotective LSS, with a more significant effect observed with PE-derived plasma.
Similar content being viewed by others
Introduction
Preeclampsia (PE) is a major contributor to maternal and fetal morbidity and mortality globally1. This condition, occurring after 20 weeks of gestation, is marked by new-onset hypertension, proteinuria, and potential organ damage2,3. It poses significant risks to both mothers and fetuses, including the possibility of maternal organ damage and fetal growth restriction4. Current treatment options are mainly focused on symptom management, primarily using antihypertensive drugs, to ensure the safety of both mother and fetus and to prevent the progression to eclampsia5. In cases where medical therapy is ineffective, early delivery may be considered to safeguard the health of both mother and baby6.
The pathophysiology of PE is complex, involving factors such as placental hypoxic-ischemic injury, release of pro-inflammatory cytokines and antiangiogenic factors, and systemic endothelial dysfunction (ED)7,8. A history of PE, as well as ED, is linked to an increased risk of cardiovascular diseases (CVDs) later in life9,10,11. While the precise mechanisms connecting PE and CVDs are not fully understood, they are thought to involve long-term damage to the maternal vasculature post-gestation12,13. Hence, understanding maternal ED is crucial for advancing preventive and therapeutic strategies.
In vitro studies have been instrumental in exploring the pathophysiology of PE, particularly regarding maternal circulating factors, including extracellular vesicles and soluble factors, and their association with systemic ED14. Indeed, many studies have combined in vitro endothelial cell culture with preeclamptic women’s plasma/serum15,16,17,18,19. Traditional single-cell-type static in vitro models, however, may not completely mimic the complex interactions and environments present in the human body, including direct and indirect contact with multiple other cell types and blood mechanical forces20. Recent biotechnological advancements, such as fluidic systems and organ-on-a-chip models, offer more sophisticated methods to simulate these conditions21,22, but research in this area, particularly focusing on PE, remains limited23,24,25.
Fluidic systems, which are more accessible in terms of cost, complexity, and maintenance, have shown potential in studying endothelial cell function under different types of shear stress (SS). Laminar SS (LSS) and oscillatory SS (OSS) have notably different effects on endothelial cells, influencing factors like inflammation, oxidative stress, and nitric oxide production26,27,28,29,30. These distinctions are vital for understanding PE, as both LSS and OSS are present in the human vascular system and exert quasi-opposing effects on the endothelium, with the former displaying atheroprotective properties31,32 and the latter associated with the formation of atherosclerotic plaques28,29,30.
This study addresses a critical gap in our understanding of the pathophysiological mechanisms underlying PE by focusing on the effects of plasma from different pregnancy conditions on endothelial cells under different shear stress conditions. We compared the global transcriptomic profiles of human coronary artery endothelial cells (HCAECs) exposed to plasma from healthy pregnant women (HP), those with gestational hypertension (GH), and PE subjects, under both LSS and OSS conditions. We provide insights into the ED associated with PE and its relationship to vascular flow dynamics.
Results
The demographic and obstetric characteristics of study participants
Table 1 presents a summary of the clinical parameters for each group of pregnant women from whom plasma samples were collected for this study. It was observed that the PE group exhibited lower gestational age at the time of sample collection, and gestational age at delivery, as well as lower newborn and placental weights, compared to the other two groups. In addition, both groups GH and PE with hypertension-related conditions showed higher systolic and diastolic blood pressure levels in comparison to the HP group. Moreover, consistent with expectations, the PE group displayed elevated levels of creatinine and proteinuria, along with lower total bilirubin levels and a decreased APGAR score at 5 min post-birth, compared to the GH group and the HP group, respectively.
Impact of shear stress (SS) on endothelial cell phenotype
After 48 h, it was observed that LSS aligned endothelial cells in the flow direction across all treatment groups (Control, HP, GH, and PE), as illustrated in Supplementary Fig. 1A. Conversely, under OSS, cells across all conditions adopted a “cobblestone-like” morphology, indicative of regions in vessels prone to atherosclerosis due to turbulent flow, such as bifurcations and curves27. No significant morphological differences were detected between the plasma treatments and the control in either SS condition.
The expression levels of key SS response markers, including HIF1A, CCL2, ICAM1, VCAM1, SELE, PECAM1, CDH5, KLF4, KLF2, and NOS3, were measured in control samples without plasma. Consistent with previous reports32, samples subjected to LSS exhibited increased expression of PECAM1, CDH5, KLF4, KLF2, and NOS3, and decreased expression of HIF1A, CCL2, ICAM1, VCAM1, and SELE compared to those under OSS (Supplementary Fig. 1B).
Further analysis compared the expression of these markers between plasma treatments under both LSS and OSS conditions (Supplementary Fig. 1C). The findings indicated that plasma incubation modified the expression of these genes. Notably, under LSS, PE plasma treatment enhanced the expression of VCAM1 to levels more akin to those observed under OSS. However, the expression patterns of these genes under OSS were largely consistent across treatments.
Additionally, under OSS, plasma treatments influenced specific markers differently: CCL2 expression was elevated in the HP and PE groups but reduced in the GH group; KLF2 expression was higher in the HP group compared to both PE and GH; and NOS3 expression was lower in PE compared to HP. A few markers, such as HIF1A, PECAM1, and CDH5, showed similar expression levels between LSS and OSS conditions.
These results highlight that while different plasma treatments can modulate the expression of crucial endothelial cell markers under various SS conditions, these treatments do not induce significant morphological changes (Supplementary Fig. 1A).
Differential impact of plasma treatment on endothelial cells under laminar shear stress
We utilized Principal Component Analysis (PCA) on the transcriptome of HCAECs, which includes nearly 12,000 expressed genes, to assess the effects of plasma treatments under LSS and OSS conditions. The objective was to identify patterns that emerge from the complex, high-dimensional dataset.
In the PCA for LSS (presented in Fig. 1A), the first principal component (Dim1), accounting for 18.7% of the total variance, revealed distinct clustering based on plasma treatments, with the differences between the GH and PE groups being particularly noticeable. This indicates that plasma treatments significantly influence the endothelial cell transcriptome under LSS conditions. The second principal component (Dim2), explaining 14.8% of the variance, highlighted variability within the replicates of each group.
Transcriptome analysis for LSS. (A) Principal Component Analysis (PCA) of filtered global gene expression profile from each plasma treatment replicate in LSS; (B) of filtered global gene expression profile from each plasma treatment replicate in OSS; Venn diagram of LSS (C) and OSS (D) DEGs from each treatment: genes were considered DEGs when adjusted p-value ≤ 0.05 and |Log2foldchange|≥ 0.5. We compared both hypertensive plasma treatment with the healthy control plasma treatment and PE with the GH plasma treatment.
Under OSS conditions (Fig. 1B), the PE and GH groups showed considerable overlap in both Dim1 and Dim2, whereas the HP group demonstrated some degree of separation from these two groups across both dimensions. In this analysis, Dim1 accounted for 15.8% of the total variance, and Dim2 accounted for approximately 15.7%.
The analysis suggests that plasma treatments have a more pronounced effect on endothelial cells under LSS, with the GH and PE groups showing significant differences. In contrast, under OSS, the PE and GH groups largely overlapped, with the HP group showing some distinct differences. These findings highlight the importance of focusing on the specific effects of plasma incubation in the context of LSS due to the less distinguishable impact under OSS, which may suggest exhaustion of the buffering capacity of homeostatic control mechanisms.
PE-associated activation of endoplasmic reticulum stress, apoptosis, and inflammation
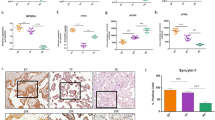
We evaluated the differential expression of genes (DEGs) under LSS and OSS conditions among PE, GH, and HP groups, revealing significant variances in gene expression profiles. In LSS a total of 20 DEGs were identified between PE and HP, 111 between GH and HP, and 113 between PE and GH, as detailed in Fig. 1C. Specifically, 5 genes were uniquely altered in PE vs. HP, 62 in GH vs. HP, and 63 in PE vs. GH comparisons. In OSS a total of 73 DEGs were identified between PE and HP, 96 between GH and HP, and 7 between PE and GH, as detailed in Fig. 1D. Specifically, 34 genes were uniquely altered in PE vs. HP, 54 in GH vs. HP, and 2 in PE vs. GH comparisons. For detailed information on these genes and their fold changes, readers are directed to the Supplementary Info.
Pathway enrichment analysis was conducted for the identified DEGs across all comparisons, utilizing a combination of Gene Ontology Biological Process, MSigDB, and BioPlanet libraries, based on the Jaccard distance methodology. The comprehensive enrichment matrix is available in Supplementary Fig. 2, with Fig. 2A providing a summary of the most notable enriched pathways unique to each condition. The analysis revealed specific pathways associated with endoplasmic reticulum stress and apoptosis (cluster k) in the PE vs. HP comparison, while the GH vs. HP comparison showed enrichment in pathways related to mitosis, proliferation (cluster n), oxidative stress (cluster l), and vascular transport (cluster u). Notably, the comparison between PE and GH identified pathways linked to endothelial dysfunction (ED) and inflammatory responses (cluster t).
Enrichment analysis utilizing combined libraries. (A) Main clusters are disposed and indicated by alphabet letters, colors indicate log10 adjusted p-value, and size indicates Odds ratio for each comparison PE vs. HP (PE), GH vs. HP (GH), and PE vs. GH. (B) Circus plot referring to cluster g pathways (on the right) and their associated genes (on the left) along their fold change for each comparison PE vs. HP (PE), GH vs. HP (GH), and PE vs. GH; (C) Circus plot referring to cluster K pathways (on the right) and their associated genes (on the left) along their fold change for each comparison PE vs. HP (PE), GH vs. HP (GH) and PE vs. GH; (D) Circus plot referring to cluster t pathways (on the right) and their associated genes (on the left) along their fold change for each comparison PE vs. HP (PE), GH vs. HP (GH) and PE vs. GH.
A circus plot was generated to visually correlate specific pathways with their related genes and fold changes, focusing on those pathways significantly impacted by PE plasma incubation. For instance, Cluster g, associated with cell cycle, migration, proliferation, and the coagulation cascade, indicative of ED, included fifteen genes (Fig. 2B). Among these, FABP4, ELOVL6, CD47, and A2M were downregulated in PE compared to HP.
The endoplasmic reticulum stress and apoptosis pathways, identified as cluster k, involved five genes, four of which (PPP1R15A, CHAC1, CEBPB, ATF4) showed increased expression in PE relative to both HP and GH (Fig. 2C).
For the inflammatory response pathways (cluster t), twelve genes were identified, including well-known markers of inflammation such as VCAM1, ICAM1, and CCL233 (Fig. 2D). These genes exhibited increased expression in PE compared to GH, except for MKI67, which was decreased. Several of these genes also showed increased expression in PE compared to HP.
The pathway enrichment analysis, leveraging combined library resources, delineated distinct pathway clusters for each comparison. This analysis underscored the unique enrichment of endoplasmic reticulum stress and apoptosis in PE vs. HP, the emphasis on mitosis, proliferation, oxidative stress, and vascular transport in GH vs. HP, and the identification of pathways related to ED and inflammatory responses in the comparison between PE and GH.
Protein interaction networks: differential effects of PE on endothelial stress and inflammation, and GH on endothelial proliferation
To gain a comprehensive understanding of the cellular processes influenced by the differential gene expression observed in PE and GH compared to HP, we conducted a protein–protein interaction network analysis. This approach allows for the elucidation of the interconnected roles of proteins within these distinct cellular contexts.
For the PE vs. HP DEGs in LSS (Fig. 3A), two clusters were identified:
Analysis of DNA packaging, gene expression, and cell division-related genes
Our analysis of the protein–protein interaction network highlighted significant expression patterns in six DEGs: RRM2, PLK1, HIST1H2AJ, HIST1H3J, HIST1H1B, and POLR1C. Among these, POLR1C was the exception, as it was not upregulated, unlike the others. Noteworthy is the presence of three histone genes (HIST1H2AJ, HIST1H3J, and HIST1H1B) in this set. These histones play a crucial role as components of nucleosomes, significantly impacting DNA accessibility and regulation34.
Cellular stress: analysis of upregulated genes across diverse functions
In the cellular stress cluster, all the genes, including SLC7A5, MTHFD2, ATF4, and CHAC1, were found to be upregulated. While these genes are primarily associated with metabolic processes, they are also involved in various critical cellular functions. For instance, SLC7A5 is linked to membrane transport, ATF4 acts as a transcription factor, and CHAC1 is involved in the regulation of apoptosis35,36.
In GH vs. HP DEGs in LSS (Fig. 3B), five clusters were identified:
Cytokine signaling and stress response: analysis of gene cluster involving direct and indirect interactions
The gene cluster identified in this context comprises 15 DEGs with direct interactions, along with two genes, INHBA and HMCN1, exerting indirect influence. Among these, four genes (LMNB1, MT1E, SERPINB2, and IL1RL1) are upregulated and primarily associated with cytokine signaling and stress response pathways37. Additionally, several downregulated genes, such as CCL2, HMOX1, and NQO1, have roles within the inflammatory network and the regulation of oxidative stress38.
Energy production: analysis of gene expression patterns in metabolic processes
Within the energy production cluster, which includes the genes CDC6, FABP3, COX7A1, SCD, FABP4, and INSR, CDC6 is the only gene that is upregulated. This gene plays a crucial role in the initiation of DNA replication39. The remaining genes, which are all downregulated, are primarily associated with fatty acid metabolism40.
Cell adhesion and signaling: examination of differential gene expression
In the cluster focusing on cell adhesion and cell signaling, almost all DEGs, including TPX2, FERMT1, ITGBL1, NEGR1, GNB5, GABBR2, and RGS4, are upregulated, with QPCTL being the notable exception. According to the Gene Cards, the majority of these genes are involved in cell signaling processes, particularly those associated with G protein signaling, as evidenced by genes like GNB5 and GABBR241,42.
Extracellular matrix regulation: analyzing gene expression in cell signaling and adhesion
This cluster comprises 14 genes that play roles in cell signaling, communication, and adhesion. Among them, eight genes are upregulated: LOX, KR, EMCN, ANTXR1, GPR116, DKK2, IL1R1, and SFRP5. Notably, some genes are markers of cell remodeling processes (LOX), angiogenesis (ANTXR1)43, and leukocyte adhesion to endothelial cells44. The remaining genes are implicated in various cell signaling pathways, such as G protein cascades (GPR116)45, and tissue development, particularly through interactions with the Wnt pathway (DKK2 and SFRP5)46.
Cell proliferation: analysis of gene expression related to cell cycle and division
In the fifth cluster of our analysis, we focused on the main axis and associated satellite genes. This cluster encompasses 33 genes, arranged in a half-moon shape starting with PRR11 at the base and culminating with CCNA2 at the top. All genes in this cluster, except for LMNB1 (which was categorized in a different cluster), are upregulated. According to the Gene Cards, these genes are predominantly involved in cell proliferation and can be grouped into several functional categories:
1. Cell cycle regulation and progression, including checkpoint control: This category includes genes such as PRR11, MYBL2, CHEK1, TYMS, HELLS, FOXM1, MCM5, DTL, MELK, SHCBP1, CDC6, TCF19, UHRF1, TK1, RRM2, and CCNA2. 2. Chromosomal integrity and separation during cell division: This group features genes like MK167, KIF23, KIF4A, NCAPG, CDC45, TOP2A, and CDC20. 3. Cell structure remodeling, including aspects of cytokinesis: Genes such as DIAPH3, ANLN, NUSAP1, RACGAP1, PRC1, DLGAP5, PLK1, WDR62, ASPM, and HURP fall into this category.
The satellite genes, positioned close to the main axis, display a varied expression profile. Three of these genes, TMOD1, ENTPD2, and ID1, are upregulated, while only one, FBXL6, is downregulated.
In PE vs. GH DEGs in LSS (Fig. 3C), two clusters were identified:
Cellular stress and inflammation: comparative gene expression analysis in PE vs. GH
The network under investigation comprises 15 genes, all of which are upregulated in PE compared to GH. This set of genes encompasses various functions, including: 1. Amino acid transport: Genes like SLC7A547 and SLC7A1148 are involved in this category, highlighting their role in nutrient transfer and metabolic processes. 2. Endoplasmic reticulum stress: CHAC147 and ATF449 are part of this group, indicating their involvement in the cellular stress response within the endoplasmic reticulum. 3. Inflammation: Several genes, including CEBPB50, VCAM1, ICAM1, CCL1, and CCL233, are associated with inflammatory processes. These genes are crucial in the regulation and mediation of inflammatory responses.
Cell cycle regulatory dynamics: analyzing gene interactions in GH and PE
This network analysis reveals a complex interaction among 13 genes, consisting of 4 upregulated and 9 downregulated genes. The network’s key aspects include 1. Downregulated genes related to mitosis and cell cycle progression, such as KIF4A, PLK1, and MKI6751. This suggests potential shifts in cell division dynamics.2. Genes like CHEK1 and RRM2, associated with DNA repair and synthesis, are indicative of potential DNA damage responses in GH plasma, a feature not as prominent in PE52. 3. Upregulated genes include CDKN2A53, which points to cell cycle arrest, and epigenetic regulators like DNMT354 and UHRF155.
In the broader context of protein–protein interaction analysis, the PE vs. HP comparison yielded two distinct clusters. The first is associated with DNA packaging, gene expression, and cell division, characterized by upregulated histones. The second cluster is linked to cellular stress. In contrast, the GH vs. HP DEGs identified five distinct clusters: cytokine signaling and stress response, energy production, cell adhesion and signaling, extracellular matrix regulation, and cell proliferation. Notably, the cell proliferation cluster highlights an upregulation of genes crucial for cell cycle regulation, chromosome integrity, and structural remodeling during cell division.
The PE vs. GH comparison further underscores a consistent pattern of cellular stress in PE, now more pronounced than in comparisons with HP and GH. This stress is coupled with the activation of pro-inflammatory genes in PE relative to GH. Additionally, there is a marked decrease in cellular proliferation indicators in PE compared to GH, highlighting the differential cellular responses in these conditions.
Phenotypic validation of pro-inflammatory, antioxidant, and proliferative responses in PE
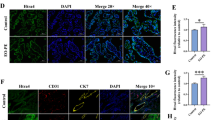
To validate the phenotypes identified in our enrichment and network analyses, HCAECs were exposed to plasma samples from each group (PE, GH, and HP) and subjected to LSS for 48 h. We used a range of specific antibodies to evaluate different cellular responses: ICAM-1 as a marker for inflammatory processes, MKI67 for cell proliferation, and GPX4 for its role in antioxidant defense and lipid peroxidation regulation, indicative of endoplasmic reticulum stress and ferroptosis. Additionally, we utilized Mitosox, a fluorogenic dye, to assess oxidative stress (refer to Fig. 4).
Experimental validation of phenotypes in HCAECs. Human coronary artery endothelial cells (HCAECs) were exposed to plasma samples from distinct groups and subjected to laminar shear stress (LSS) for 48 h to validate the identified phenotypes from enrichment analysis. Immunofluorescence staining was performed using specific antibodies to target key markers: (A) ICAM-1, for inflammatory response; (B) GPX4, for cellular stress; (C) Mitosox fluorogenic dye; and (D) MKI67, for proliferation. *p < 0.05, ** p < 0.01, *** p < 0.001, ****p < 0.0001.
The experimental outcomes provided evidence of an activated inflammatory profile in HCAECs exposed to PE plasma, with significantly elevated levels of ICAM-1 compared to HP samples (p < 0.05). However, the difference between PE and GH was not statistically significant (p = 0.07). The inflammatory profile of PE-treated cells under LSS was also demonstrated by a qPCR experiment using new pools of plasma from the different groups (Supplementary Fig. 3 and Clinical Characteristics in Supplementary Info File). Although there was only a slightly increased expression of ICAM1 and selectin E (SELE) genes in HCAEC exposed to PE plasma, these cells showed higher expression of the CCL2 and CXCL8 genes, confirming an accentuated inflammatory phenotype induced by PE treatment (Supplementary Fig. 3). In terms of oxidative stress, HCAECs exposed to PE plasma showed reduced mitochondrial reactive oxygen species (ROS) levels compared to both HP and GH (p < 0.001 and p < 0.01, respectively), alongside higher levels of the antioxidant enzyme GPX4 (p = 0.0017 and p < 0.0001, respectively, when compared to HP and GH).
Furthermore, the study revealed that plasma from GH individuals significantly increased the proliferative activity of HCAECs, with elevated levels of MKI67 observed in comparison to both PE and HP groups (both p < 0.0001).
These phenotypic results align with and reinforce our transcriptomic analysis findings. In summary, HCAECs exposed to PE plasma exhibited an activated inflammatory response, indicated by increased ICAM-1 levels relative to HP, alongside reduced mitochondrial ROS and heightened GPX4 levels, suggesting an enhanced antioxidant response. In contrast, GH plasma was associated with an increased proliferative profile in HCAECs compared to the PE and HP groups.
Discussion
The novelty of this research lies in the exploration of the role of shear stress in modulating endothelial cell responses to plasma from HP women, and patients with GH or PE. Unlike previous in vitro studies that may have overlooked the significance of flow dynamics, we provide a comparative analysis of how LSS and OSS conditions affect the transcriptome of HCAEC. The identification of distinct transcriptional profiles under LSS, especially the pro-inflammatory and antioxidant profiles induced by PE plasma, adds a new dimension to our understanding of ED in the context of pregnancy-related hypertensive disorders. These findings collectively underscore the multifaceted nature of endothelial responses in the presence of pregnancy-related hypertensive disorders and the critical role of hemodynamic forces in modulating their effects.
Each plasma sample caused distinct responses in LSS conditions, but PE and GH demonstrate high similarity when exposed to OSS suggesting a potential convergence of underlying mechanistic pathways that are predominantly caused by the OSS stimulus rather than the plasma effect. One may speculate that under OSS there may be exhaustion of the homeostatic buffering capacity. Such an observation is consistent with studies suggesting that low shear stress might diminish the endothelial cell response to various stimuli, including pharmacological treatments56.
The state of endothelial cell quiescence, characterized by non-proliferative, non-migratory, and anti-inflammatory phenotypes, is crucial for vascular health57,58. Our results indicate that both GH and PE treatments under LSS are associated with increased endothelial cell proliferation, suggesting impaired endothelial functionality59. This proliferation might be linked to increased oxidative stress, as indicated by the elevated production of ROS and changes in the expression of genes related to endothelial cell growth18. Specifically, the GH group demonstrated a decrease in the expression of antioxidant agents (such as HMOX1, NQO1, and PYCR1) and an increase in mitochondrial ROS levels compared to PE. This was accompanied by an upregulation of genes indicative of a proliferative profile, confirmed experimentally by increased Ki-67 protein levels (Fig. 4D). Interestingly, mitochondrial oxidative stress levels in GH-treated cells were similar to those in HP-treated cells. Additionally, our analysis suggested a potential energy shift toward lipid and insulin pathways in GH, aligning with previous studies on endothelial cell metabolism60,61,62.
In contrast, PE also showed increased proliferation compared to HP. This aligns with previous studies that used static conditions for incubation18,63. The PPI network analysis indicated a cluster with upregulation of histones and RRM2 and PLK1, which are genes highly expressed in cancer cells where they have a role in inducing angiogenesis and proliferation51,52. This same network also indicated downregulation of POLR1C raising questions about its impact on transcription initiation. However, in the context of proliferation/angiogenesis in PE, our results could be associated with plasma concentrations since a previous study showed that 20% plasma dilution and 10% plasma dilution caused a decreased proliferation in PE in comparison to HP, while 2% had no impact in primary HUVECs64. Our results might differ due to the presence of shear stress in our experimental setup, highlighting the significance of mechanical forces in endothelial cell biology.
Multiple studies have established that plasma from PE cases tends to exhibit a pro-inflammatory profile65. This is believed to be a result of various factors, including the release of pro-inflammatory cytokines from a hypoxic placenta into circulation and oxidative stress, both of which contribute to systemic endothelial cell dysfunction66,67,68,69,70,71,72. Notably, while these inflammatory changes in PE circulation are well-documented, our research is the first to demonstrate an inflammatory profile in HCAECs incubated with PE plasma, especially under the atheroprotective LSS condition. Key genes identified include VCAM1, ICAM1, and CCL2, which are involved in immune cell adhesion, migration, and inflammation, as well as PTGS2, associated with the production of the vasodilator prostaglandin D235. Interestingly, the inflammatory response in PE became apparent in our enrichment analysis primarily when comparing PE gene expression to that of the GH group. However, the immunofluorescence and qPCR experiments revealed that PE elicited a pro-inflammatory response, evidenced by increased ICAM1 protein expression, and CCL2 and CXCL8 gene expression compared to the other groups. The PPI network analysis for GH vs. HP also indicated immune system alterations in GH, such as the role of SERPINB2 in immune signaling, potentially linked to conditions like hypertension in preeclampsia. Additionally, IL1RL1, coding for the IL1 receptor, is crucial for inflammatory responses.
The presence of these inflammatory markers in a protective LSS environment may seem contradictory, but it aligns with clinical observations in PE and GH women. The etiology of GH and its relationship with PE remain areas of ongoing research73. Both conditions share similar risk factors and a comparable likelihood of post-pregnancy hypertension development (approximately 39% for GH and 32% for PE, compared to 1% in HP)11. Additionally, the risk of adverse outcomes like preterm birth is about three times higher in PE than in GH, which may be linked to differences in their inflammatory signatures. GH might exhibit a more effective compensatory inflammatory response compared to PE74,75.
An inflamed endothelium in PE is typically associated with reduced vasodilation, diminished nitric oxide bioavailability, and increased oxidative stress. Surprisingly, our findings indicate that PE plasma elicits an antioxidant response in endothelial cells, as evidenced in both our enrichment analysis and experimental validations. Previous work from our group has shown that PE plasma can upregulate endogenous antioxidant systems and enhance total antioxidant capacity 76,77. This response is likely an adaptive mechanism to counterbalance the elevated oxidative stress seen in PE. Increased expression of antioxidants may thus act as a protective response against the damaging effects of reactive oxygen species. This protective aspect of PE plasma is further supported by the upregulation of genes associated with endoplasmic reticulum stress. Genes such as PPP1R15A, CHAC1, CEBPB, and ATF4, which are involved in cellular stress responses and gene regulation, collectively aid the cell’s ability to adapt to stress conditions, modulate gene expression, and impact various physiological processes49. The PPI network analysis for PE vs. HP DEGs under LSS highlighted the interaction among these genes, suggesting a response to increased oxidative stress and a potential cascade of events linked to ferroptosis. Ferroptosis, a form of cell death driven by iron-dependent oxidative stress, involves a complex regulatory cascade. ATF4 indirectly regulates CHAC1 by interacting with the heat shock protein 5 family member 70, creating a complex that protects CHAC1 from degradation by oxidized lipids47. Erastin, a key player in initiating ferroptotic cell death, binds to SLC7A5, disrupting cystine uptake and leading to increased lipid peroxidation and cell death78. Additionally, ATF4 is essential for inducing MTHFD2 expression79, and its inhibition, along with reduced oxidized phospholipids, leads to increased SLC7A5 levels80. To validate these findings, we quantified the antioxidant enzyme GPX4, associated with endoplasmic reticulum stress and ferroptosis. Moreover, ATF4 is known to negatively regulate PlGF and activate a promoter in CHAC1, also upregulated in our study and implicated in the pathophysiology of pregnancy complications36. Interestingly, clinical trials using antioxidants have not shown therapeutic benefits in PE women, further emphasizing the complexity of the oxidative stress response in PE81,82.
In cluster g of our enrichment analysis, we observed the downregulation of FABP4 and ELOVL6, both of which are involved in fatty acid metabolic processes. This suggests potential alterations in lipid metabolism associated with PE. Additionally, the reduced expression of CD47 and A2M, genes implicated in blood coagulation, hints at a regulatory mechanism affecting coagulation processes in PE. The disruption of A2M-mediated negative regulation of blood coagulation, combined with the influence of CD47 on the biosynthesis and signaling of nitric oxide, highlights a potential interaction of molecular pathways that may contribute to ED83. This finding underscores the complex molecular interplay in PE, with implications for both lipid metabolism and coagulation regulation.
The results regarding the impact of PE plasma on endothelial cells are not uniform. There is evidence that HUVECs are activated by PE plasma only when co-cultured with monocytes84. Conversely, another study using PE plasma and HUVECs reported increased expression of inflammatory markers such as TNF-α, TLR-9, and ICAM-1, linking these findings to mitochondrial reactive oxygen species85. Nevertheless, it is crucial to note that these studies were conducted under static in vitro conditions. It has been observed that endothelial cells exposed to OSS in vivo and those cultured in static in vitro environments share notable similarities, potentially affecting the interpretation of their responses to various stimuli, including different types of plasma86,87. For instance, in our study, cells treated with OSS displayed only a limited number of DEGs between plasma treatments, in contrast to those under LSS. This finding suggests that the influence of OSS on endothelial cell dysfunction might overshadow the effects of plasma observed under LSS conditions. Considering the parallels between OSS and static culture conditions, this factor might also impact the results of previous studies, highlighting the importance of context in interpreting endothelial cell response to different stimuli.
It is important to acknowledge certain limitations of our study. Firstly, the sample size is relatively small, involving a limited number of patient plasma samples, which may affect the generalizability of our results. Additionally, the volume of plasma used (5%, v/v), while consistent with existing literature, might limit the robustness of the observed effects. Our reliance on two-dimensional (2D) cell culture models is another limitation, as these may not fully replicate the complexity of in vivo conditions. The study’s focus on a single cell type could also restrict the broader applicability of our findings to different types of endothelial cells. These limitations should be considered in the interpretation of our results and their application to wider clinical contexts.
In summary, the influence of shear stress on the endothelial transcriptome in the context of PE and GH is of paramount clinical importance. It sheds light on the molecular mechanisms contributing to ED, a key factor in the pathophysiology of PE, and its long-term cardiovascular implications. The findings underscore the complex interplay between mechanical forces (such as shear stress) and biochemical signals in the vascular environment during pregnancy. We uncovered distinct transcriptional profiles under LSS, especially the pro-inflammatory and antioxidant profiles induced by PE plasma, adding a new dimension to our understanding of ED in the context of pregnancy-related hypertensive disorders.
Methods
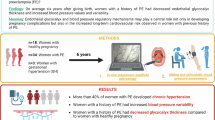
Plasma sample collection
We collected plasma from 10 HP women, 10 patients with GH, and 8 patients with PE. PE and GH patients’ diagnoses were based on the American College of Obstetricians and Gynecologists (ACOG) guidelines. Exclusion criteria consisted of twin pregnancy, chronic hypertension, hemostatic abnormalities, diabetes mellitus, history of cancer, cardiovascular disease, and autoimmune diseases. All samples were collected at the Hospital das Clínicas de Ribeirão Preto (HCFMRP-USP), and all study participants signed an informed consent. The study was approved by the Research Ethics Committee of the Faculdade de Medicina de Ribeirão Preto, São Paulo, Brazil (CAAE-37738620.0.0000.5440, October 19, 2020), following the principles of the Helsinki Declaration.
We collected 15 mL of venous blood from each patient in a standard vacutainer tube containing heparin (Becton Dickinson). We then centrifuged the tubes at room temperature at 3200 g for 10 min. Aliquots of 500 μL were stored together at − 80 °C until their use. We assembled plasma pools for each group using equal parts of each sample before experiments.
Cell culture
We used HCAECs purchased from LONZA (Lonza, USA) cultured in a specific complete medium (Lonza, EGM-2MV) in a humidified incubator (at 37 °C and 5% CO2). Cells from passage 5–7 were used for flow culture experiments.
Shear stress model
After reaching approximately 90% confluence, we resuspended the cells and seeded them in μ-Slides I Luer 0.4 (IBIDI, Germany) coated with a uniform layer of 0.1% gelatin at a density of approximately 1 × 105 cells/cm2. Then the μ-Slides were left in the incubator (37 °C with 5% CO2) for 3 h in static conditions to reach full confluence. For the time of the experiments, the cells were incubated with the pool of plasma (5%, v/v) diluted in complete medium (Lonza, EGM-2MV) and exposed to LSS of 20 dyn/cm2, and OSS at 1 Hz (± 5 dyn/cm2) for 48 h in replicates of 4 μ-Slides per group (HP, GH, and PE). We have also added a control group without any plasma. For visual inspection of cell orientation, bright-field images were acquired at 10 × magnification in a EVOS M7000 Image system (ThermoFisher Scientific, USA).
RNA isolation and Microarray gene expression profiling
At the end of the 48 h, the cells were washed with 1X PBS, and total RNA isolation was performed using the RNeasy Micro kit (Qiagen, Germany), according to the manufacturer’s protocol. Total RNA was quantified using NanoDrop (ThermoFisher Scientific, USA). To generate global gene expression (> 20,000 genes), we used the Clariom S Assay HT, human array plates (ThermoFisher Scientific, USA). The array plates were processed using the GeneTitan TM Microarray System (ThermoFisher Scientific, USA), according to the manufacturer’s protocols. Transcriptome data analysis was conducted using the R platform to analyze and visualize the profile of gene expression, differential expression analyses, and enrichment analyses of biological processes.
Microarray analysis and quality control
We used raw intensity data in CEL file format, obtained from GeneTitan™. After importing the files to the R platform, we used the ArrayQualityMetrics package to identify apparent outliers and compute measures of signal-to-noise ratio. After that, using the robust multichip average (RMA) normalization method, the probe sets with no or low expression (log2 signal intensity < 2) were removed from further analysis. We have also corrected the batch effect between samples by running BatchQC SVA. Human Clariom S assay annotation (version 8.7.0) was used for gene annotation and NA and multiple mapping probes were removed.
Differential expression analysis
We obtained differentially expressed genes (DEGs) lists using the limma package and significance was defined using the criteria of absolute |log2 fold change|≥ 0.5 and false discovery ratio (FDR) < 0.05 using Benjamini-Hochberg (BH) correction.
Enrichment analysis
We used the lists of Differentially Expressed Genes (DEGs), to conduct biological process enrichment analyses using the EnrichR package and the Gene Ontology Biological Process, MSigDB, and BioPlanet libraries. We selected the enriched terms with an adjusted p-value of less than 0.05. We then combined the three libraries to get a broad notion of the biological response associated with each treatment by grouping the terms using the k-means technique with the Jaccard distance between the sets of genes associated with the enriched terms.
Protein interactions networks
The lists of DEGs (Differentially Expressed Genes) were added to the STRING platform88, and we only used interactions obtained from databases, experiments, and co-expression analyses. The average confidence level was 0.7. Finally, the networks were exported to the Cytoscape platform89 for editing.
Immunofluorescence and image analysis
After 48 h of LSS, we incubated HCAEC with the Mitosox probe (5 µM, ThermoFisher Scientific, USA) for 30 min at 37 °C to evaluate mitochondrial superoxide generation. Then, the cells were fixed with 4% PFA for 15 min at room temperature. We followed the immunofluorescence protocol with permeabilization for 1 h with Triton (0.1% in PBS), blocking with BSA (5% in PBS) for 1 h at room temperature and incubation with the anti-ICAM1 antibody (1:200, Cell Signaling, USA, #62,133), anti-GPX4 antibody (1:100, Abcam, USA, #ab125066) and anti-Ki67 antibody (1:300, Abcam, USA, #ab16667) at 4 °C overnight. After that, we washed with PBS (2x) and followed the incubation with DAPI (1 mg/mL, ThermoFisher Scientific, USA) and with the fluorescent secondary antibodies (Alexa Fluor, ThermoFisher Scientific, USA, 1:500) for 1 h at room temperature. Finally, we performed three more washes with PBS, and the cells were kept in PBS for image acquisition in an EVOS M7000 Image system (ThermoFisher Scientific, USA). Images were obtained at ×10 magnification, with 15 images per µ-slide. After the acquisition, the raw images were analyzed using CellProfiler (Carpenter et al. 2006) software. Briefly, illumination correction was performed before image thresholding with Otsu algorithm and converting the image foreground into a single region of interest (ROI). Next, mean fluorescence intensity (MFI) of the protein of interest (i.e. ICAM1, GPX4, Mitosox) in background-corrected images was evaluated for each ROI. For Ki67 analysis, nuclei were first segmented with Otsu algorithm in DAPI images. Then nuclei with a Ki67 MFI of at least 0.1, defined as a threshold by visual inspection of several randomly selected images, were considered to be Ki67+.
Relative gene expression analysis by real-time quantitative reverse transcription (RT-qPCR)
After 48 h of LSS, we extracted total RNA from HCAEC cells using RNeasy Micro kit (Qiagen, Germany), and reverse transcribed into cDNA using SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The levels of target mRNA expression were quantified using qRT-PCR with QuantiTect SYBR Green reagent (Qiagen, Germany), and specific primers pairs (Supplementary Table 1). We performed RT-qPCR with the QuantiStudio 12 K Flex system (ThermoFisher Scientific, USA). The cyclophilin gene was used to normalize the results. The levels of gene expression were determined by 2-ΔΔCT; where ΔΔCT is the value of ΔCT subtracted from the ΔCT of the control group.
Statistical analysis
Results of fluorescence cell imaging quantification were presented as a mean ± standard error of the mean (SEM). We performed statistical analyses and graphical representations using GraphPad Prism software (GraphPad Softwares Inc.). Comparisons among 3 groups were performed using a one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test for comparison. Values of p < 0.05 were considered significant.
Data availability
All the necessary data is provided in the main text and supplementary material. Other information will be available on request to the corresponding author.
Abbreviations
- ACP5:
-
Acid phosphatase 5
- ALDH3A1:
-
Aldehyde dehydrogenase 3 family member A1
- AST:
-
Aspartate aminotransferase
- ATF4:
-
Activating transcription factor 4
- BMI:
-
Body mass index
- CCL2:
-
C–C motif chemokine ligand 2
- CDC6:
-
Cell division cycle 6
- CDH5:
-
Cadherin 5
- CEBPB:
-
CCAAT/enhancer-binding protein beta
- CHAC1:
-
ChaC glutathione-specific gamma-glutamylcyclotransferase 1
- CCL2:
-
C–C Motif chemokine ligand 2
- COX7A1:
-
Cytochrome C oxidase subunit 7A1
- CVDs:
-
Cardiovascular diseases
- DBP:
-
Diastolic blood pressure
- DEGs:
-
Differentially expressed genes
- DNA:
-
Deoxyribonucleic acid
- FABP3:
-
Fatty acid-binding protein 3
- FABP4:
-
Fatty acid-binding protein 4
- FDR:
-
False discovery ratio
- GA:
-
Gestational age
- GH:
-
Gestational hypertension
- GPX4:
-
Glutathione peroxidase 4
- HCAECs:
-
Human coronary artery endothelial cells
- HCFMRP-USP:
-
Hospital das Clínicas de Ribeirão Preto, Universidade de São Paulo
- HIF1A:
-
Hypoxia-inducible factor 1 alpha
- HMOX1:
-
Heme oxygenase 1
- HP:
-
Healthy pregnant women
- HUVECs:
-
Human umbilical vein endothelial cells
- ICAM1:
-
Intercellular adhesion molecule 1
- IL1RL1:
-
Interleukin 1 receptor-like 1
- INSR:
-
Insulin receptor
- KLF2:
-
Krüppel-like factor 2
- KLF4:
-
Krüppel-like factor 4
- LSS:
-
Laminar shear stress
- MCM5:
-
Minichromosome maintenance complex component 5
- Mitosox:
-
Fluorogenic dye for mitochondrial superoxide
- MSigDB:
-
Molecular signatures database
- MTHFD2:
-
Methylenetetrahydrofolate dehydrogenase (NADP + Dependent) 2
- MYBL2:
-
MYB proto-oncogene like 2
- N/D:
-
Not determined
- N/A:
-
Not applicable
- NOS3:
-
Nitric oxide synthase 3
- NOQ1:
-
NAD(P)H quinone dehydrogenase 1
- OSS:
-
Oscillatory shear stress
- PE:
-
Preeclampsia
- PECAM1:
-
Platelet and endothelial cell adhesion molecule 1
- PLK1:
-
Polo-like kinase 1
- PPP1R15A:
-
Protein phosphatase 1 regulatory subunit 15A
- PFA:
-
Paraformaldehyde
- PTGS2:
-
Prostaglandin-endoperoxide synthase 2
- PYCR1:
-
Pyrroline-5-carboxylate reductase 1
- RNA:
-
Ribonucleic acid
- RRM2:
-
Ribonucleotide reductase regulatory TP53 inducible subunit M2
- SBP:
-
Systolic blood pressure
- SCD:
-
Stearoyl-CoA desaturase
- SELE:
-
Selectin E
- SERPINB2:
-
Serpin family B member 2
- SFRBM:
-
Society for free radical biology and medicine
- SLC7A:
-
Solute carrier family 7 member A
- SS:
-
Shear stress
- TBST:
-
Tris-buffered saline with tween 20
- TNF-α:
-
Tumor necrosis factor alpha
- TOP2A:
-
DNA topoisomerase II alpha
- VCAM1:
-
Vascular cell adhesion molecule 1
- VEGF:
-
Vascular endothelial growth factor
- μM:
-
Micromolar (concentration unit)
References
Wang, W. et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 21, 364 (2021).
Goel, A. et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation 132, 1726–1733 (2015).
Rana, S., Lemoine, E., Granger, J. & Karumanchi, S. A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 124, 1094–1112 (2019).
Phipps, E. A., Thadhani, R., Benzing, T. & Karumanchi, S. A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 15(5), 275–289 (2019).
Brown, C. M. & Garovic, V. D. Drug treatment of hypertension in pregnancy. Drugs 74, 283–296 (2014).
Dymara-Konopka, W., Laskowska, M. & Oleszczuk, J. Preeclampsia—Current management and future approach. Curr. Pharm. Biotechnol. 19, 786–796 (2018).
LaMarca, B. Endothelial dysfunction; an important mediator in the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 64, 309 (2012).
Roberts, J. M. et al. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 161, 1200–1204 (1989).
Muijsers, H. E. C., Roeleveld, N., van der Heijden, O. W. H. & Maas, A. H. E. M. Consider preeclampsia as a first cardiovascular event. Curr. Cardiovasc. Risk Rep. 13, 1–6 (2019).
Diniz, A. L. D., Paes, M. M. B. M. & Diniz, A. D. Analyzing preeclampsia as the tip of the iceberg represented by women with long-term cardiovascular disease, atherosclerosis, and inflammation. Curr. Atheroscler. Rep. 22, 1–8 (2020).
Veerbeek, J. H. W. et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 65, 600–606 (2015).
McElwain, C. J., Tuboly, E., McCarthy, F. P. & McCarthy, C. M. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: Windows into future cardiometabolic health?. Front. Endocrinol. (Lausanne) 11, 655 (2020).
Hromadnikova, I., Kotlabova, K., Dvorakova, L. & Krofta, L. Postpartum profiling of microRNAs involved in pathogenesis of cardiovascular/cerebrovascular diseases in women exposed to pregnancy-related complications. Int. J. Cardiol. 291, 158–167 (2019).
Staff, A. C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 134–135, 1–10 (2019).
Sandrim, V. C. et al. Plasma from pre-eclamptic patients induces the expression of the anti-angiogenic miR-195-5p in endothelial cells. J. Cell. Mol. Med. 20, 1198–1200 (2016).
Caldeira-Dias, M. et al. Preeclamptic plasma stimulates the expression of miRNAs, leading to a decrease in endothelin-1 production in endothelial cells. Pregnancy Hypertens. 12, 75–81 (2018).
English, F. A. et al. Inhibition of lectin-like oxidized low-density lipoprotein-1 receptor protects against plasma-mediated vascular dysfunction associated with pre-eclampsia. Am. J. Hypertens. 26, 279–286 (2013).
Calicchio, R. et al. Preeclamptic plasma induces transcription modifications involving the AP-1 transcriptional regulator JDP2 in endothelial cells. Am. J. Pathol. 183, 1993–2006 (2013).
Sankaralingam, S., Xu, H. & Davidge, S. T. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc. Res. 85, 194–203 (2010).
Meng, X. et al. Rebuilding the vascular network: In vivo and in vitro approaches. Front. Cell Dev. Biol. 9, 639299 (2021).
Haase, K. & Kamm, R. D. Advances in on-chip vascularization. Regen. Med. 12, 285–302 (2017).
Gordon, E., Schimmel, L. & Frye, M. The importance of mechanical forces for in vitro endothelial cell biology. Front. Physiol. 11, 684 (2020).
Baker, P. N., Stranko, C. P., Davidge, S. T., Davies, P. S. & Roberts, J. M. Mechanical stress eliminates the effects of plasma from patients with preeclampsia on endothelial cells. Am. J. Obstet. Gynecol. 174, 730–736 (1996).
Rowe, J., Campbell, S. & Gallery, E. D. M. Nitric oxide production by decidual endothelial cells is not reduced in preeclampsia. Hypertens. pregnancy 22, 63–75 (2003).
Kublickiene, K. R., Lindblom, B., Krüger, K. & Nisell, H. Preeclampsia: Evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am. J. Obstet. Gynecol. 183, 160–166 (2000).
Chistiakov, D. A., Orekhov, A. N. & Bobryshev, Y. V. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. (Oxf) 219, 382–408 (2017).
Urschel, K., Tauchi, M., Achenbach, S. & Dietel, B. Investigation of wall shear stress in cardiovascular research and in clinical practice-from bench to bedside. Int. J. Mol. Sci. 22, 5635 (2021).
Jiang, Y. Z. et al. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ. Res. 115, 32–43 (2014).
Mohan, S., Mohan, N. & Sprague, E. A. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol. 273, C572 (1997).
Van Der Heiden, K., Cuhlmann, S., Luong, L. A., Zakkar, M. & Evans, P. C. Role of nuclear factor kappaB in cardiovascular health and disease. Clin. Sci. (Lond) 118, 593–605 (2010).
Hsieh, C. Y. et al. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J. Biomed. Sci. 16, 1–14 (2009).
van Thienen, J. V. et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc. Res. 72, 231–240 (2006).
Cook-Mills, J. M., Marchese, M. E. & Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 15, 1607 (2011).
Monteiro, F. L. et al. Expression and functionality of histone H2A variants in cancer. Oncotarget 5, 3428–3443 (2014).
Song, W. L. et al. Lipocalin-like prostaglandin D synthase but not hemopoietic prostaglandin D synthase deletion causes hypertension and accelerates thrombogenesis in mice. J. Pharmacol. Exp. Ther. 367, 425–432 (2018).
Mungrue, I. N., Pagnon, J., Kohannim, O., Gargalovic, P. S. & Lusis, A. J. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J. Immunol. 182, 466–476 (2009).
Boncela, J., Przygodzka, P., Wyroba, E., Papiewska-Pajak, I. & Cierniewski, C. S. Secretion of SerpinB2 from endothelial cells activated with inflammatory stimuli. Exp. Cell Res. 319, 1213–1219 (2013).
Sebastián, V. P. et al. Heme oxygenase-1 as a modulator of intestinal inflammation development and progression. Front. Immunol. 9, 394989 (2018).
Hossain, M. & Stillman, B. Opposing roles for DNA replication initiator proteins ORC1 and CDC6 in control of Cyclin E gene transcription. Elife 5, e12785 (2016).
Hasan, S. S. & Fischer, A. The endothelium: An active regulator of lipid and glucose homeostasis. Trends Cell Biol. 31, 37–49 (2021).
Sciacca, F. L. et al. Severe phenotype in a patient with homozygous 15q21.2 microdeletion involving BCL2L10, GNB5, and MYO5C genes, resembling infantile developmental disorder with cardiac arrhythmias (IDDCA). Front. Genet. 11, 399 (2020).
Zhang, H., Zhou, H., Yuan, J., Nan, Y. & Liu, J. Endothelial GABBR2 regulates post-ischemic angiogenesis by inhibiting the glycolysis pathway. Front. Cardiovasc. Med. 8, 696578 (2021).
Unamuno, X. et al. Changes in mechanical properties of adipose tissue after bariatric surgery driven by extracellular matrix remodelling and neovascularization are associated with metabolic improvements. Acta Biomater. 141, 264–279 (2022).
Zhang, G. et al. Loss of endothelial EMCN drives tumor lung metastasis through the premetastatic niche. J. Transl. Med. 20, 446 (2022).
Georgiadi, A. et al. Orphan GPR116 mediates the insulin sensitizing effects of the hepatokine FNDC4 in adipose tissue. Nat. Commun. 12, 2999 (2021).
Wang, H. et al. Concurrent hypermethylation of SFRP2 and DKK2 activates the Wnt/β-catenin pathway and is associated with poor prognosis in patients with gastric cancer. Mol. Cells 40, 45–53 (2017).
Song, X. & Long, D. Nrf2 and ferroptosis: A new research direction for neurodegenerative diseases. Front. Neurosci. 14, 484266 (2020).
Chen, Q. et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 30, 137–151 (2023).
Neill, G. & Masson, G. R. A stay of execution: ATF4 regulation and potential outcomes for the integrated stress response. Front. Mol. Neurosci. 16, 1112253 (2023).
Zhu, Y. et al. Macrophage autophagy deficiency-induced CEBPB accumulation alleviates atopic dermatitis via impairing M2 polarization. Cell Rep. 42, 113430 (2023).
Hartsink-Segers, S. A. et al. Inhibiting Polo-like kinase 1 causes growth reduction and apoptosis in pediatric acute lymphoblastic leukemia cells. Haematologica 98, 1539–1546 (2013).
Zhang, K. et al. Overexpression of RRM2 decreases thrombspondin-1 and increases VEGF production in human cancer cells in vitro and in vivo: Implication of RRM2 in angiogenesis. Mol. Cancer 8, 1–12 (2009).
Hatzistergos, K. E. et al. Tumor suppressors RB1 and CDKN2a cooperatively regulate cell-cycle progression and differentiation during cardiomyocyte development and repair. Circ. Res. 124, 1184–1197 (2019).
Chen, T., Mahdadi, S., Vidal, M. & Desbène-Finck, S. Non-nucleoside inhibitors of DNMT1 and DNMT3 for targeted cancer therapy. Pharmacol. Res. 207, 107328 (2024).
Xu, X. et al. Nuclear UHRF1 is a gate-keeper of cellular AMPK activity and function. Cell Res. 32, 54 (2021).
Salazar-Martín, A. G. et al. Single-cell RNA sequencing reveals that adaptation of human aortic endothelial cells to antiproliferative therapies is modulated by flow-induced shear stress. Arterioscler. Thromb. Vasc. Biol. 43, 2265–2281 (2023).
Amelio, G. S. et al. Endothelial dysfunction in preterm infants: The hidden legacy of uteroplacental pathologies. Front. Pediatr. 10, 1041919 (2022).
Krüger-Genge, A., Blocki, A., Franke, R. P. & Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 20, 4411 (2019).
Ricard, N., Bailly, S., Guignabert, C. & Simons, M. The quiescent endothelium: Signalling pathways regulating organ-specific endothelial normalcy. Nat. Rev. Cardiol. 18(8), 565–580 (2021).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Krützfeldt, A., Spahr, R., Mertens, S., Siegmund, B. & Piper, H. M. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell. Cardiol. 22, 1393–1404 (1990).
Mallick, R. & Duttaroy, A. K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 477(1), 15–38 (2021).
Rowe, J., Campbell, S. & Gallery, E. D. M. Plasma from preeclamptic women stimulates decidual endothelial cell growth and prostacyclin but not nitric oxide production: Close correlation of prostacyclin and thromboxane production. J. Soc. Gynecol. Investig. JSGI 8(1), 32–38 (2001).
Smarason, A. K., Sargent, I. L. & Redman, C. W. G. Endothelial cell proliferation is suppressed by plasma but not serum from women with preeclampsia. Am. J. Obstet. Gynecol. 174, 787–793 (1996).
Harmon, A. C. et al. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 130, 409–419 (2016).
Szarka, A., Rigó, J., Lázár, L., Beko, G. & Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 11, 1 (2010).
Cakmak, M. et al. Serum levels of endocan correlate with the presence and severity of pre-eclampsia. Clin. Exp. Hypertens. 38, 137–142 (2016).
Hamai, Y. et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am. J. Reprod. Immunol. 38, 89–93 (1997).
Williams, M. A. et al. Maternal second trimester serum tumor necrosis factor-alpha-soluble receptor p55 (sTNFp55) and subsequent risk of preeclampsia. Am. J. Epidemiol. 149, 323–329 (1999).
Conrad, K. P., Miles, T. M. & Benyo, D. F. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am. J. Reprod. Immunol. 40, 102–111 (1998).
Siljee, J. E. et al. Identification of interleukin-1 beta, but no other inflammatory proteins, as an early onset pre-eclampsia biomarker in first trimester serum by bead-based multiplexed immunoassays. Prenat. Diagn. 33, 1183–1188 (2013).
Jonsson, Y. et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J. Reprod. Immunol. 70, 83–91 (2006).
Ying, W., Catov, J. M. & Ouyang, P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J. Am. Heart Assoc. 7, 9382 (2018).
Shen, M. et al. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One 12, e0175914 (2017).
Amash, A., Holcberg, G., Sapir, O. & Huleihel, M. Placental secretion of interleukin-1 and interleukin-1 receptor antagonist in preeclampsia: effect of magnesium sulfate. J. Interferon Cytokine Res. 32, 432–441 (2012).
Gomes, H. F. et al. Assessment of oxidative status markers and NO bioavailability in hypertensive disorders of pregnancy. J. Hum. Hypertens. 27(6), 345–348 (2013).
Caldeira-Dias, M. et al. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 23, 205–210 (2021).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Hitzel, J. et al. Oxidized phospholipids regulate amino acid metabolism through MTHFD2 to facilitate nucleotide release in endothelial cells. Nat. Commun. 9(1), 1–18 (2018).
Roberts, J. M. et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 362, 1282–1291 (2010).
Poston, L., Briley, A., Seed, P., Kelly, F. & Shennan, A. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 367, 1145–1154 (2006).
Rogers, N. M., Sharifi-Sanjani, M., Csányi, G., Pagano, P. J. & Isenberg, J. S. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 37, 92–101 (2014).
Faas, M. M. et al. Plasma from preeclamptic women activates endothelial cells via monocyte activation in vitro. J. Reprod. Immunol. 87, 28–38 (2010).
McCarthy, C. & Kenny, L. C. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci. Rep. 6(1), 1–11 (2016).
Maurya, M. R. et al. Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proc. Natl. Acad. Sci. U. S. A. 118, e2023236118 (2021).
Qiao, C. et al. Deep transcriptomic profiling reveals the similarity between endothelial cells cultured under static and oscillatory shear stress conditions. Physiol. Genomics 48, 660–666 (2016).
Szklarczyk, D. et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
The authors express their gratitude for the technical support provided by Mariliza V. Rodrigues in conducting the microarray assays, and by Iuri C. Valadão in performing image acquisition and analysis. We also appreciate Caio M. Silva for his valuable contributions to the discussion on expression analysis strategies. We thank Profesor Jose E. Krieger for the scientific discussion during the execution of this work.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil, grant number 88887.604855/2021-00), by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil, grant number 308504/2021-6, and 408426/2021-7), and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil, grant numbers 2019/07230-8, 2021/12010-7, and 23/11925-7). VCS is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant: 308504/2021-6).
Author information
Authors and Affiliations
Contributions
Sarah Viana-Mattioli: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Miriam Helena Fonseca-Alaniz: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Iguaracy Pinheiro-de-Sousa: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. Ricardo Rosa Junior: Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. Moises Henrique Mastella: Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. Ricardo de Carvalho Cavalli: Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. Valeria Cristina Sandrim: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors have no interests to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Viana-Mattioli, S., Fonseca-Alaniz, M.H., Pinheiro-de-Sousa, I. et al. Plasma from hypertensive pregnancy patients induce endothelial dysfunction even under atheroprotective shear stress. Sci Rep 15, 4675 (2025). https://doi.org/10.1038/s41598-025-88902-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88902-8
Keywords
This article is cited by
-
Championing maternal health and reducing maternal mortality: a global multidisciplinary imperative
Scientific Reports (2025)