Abstract
Cervical cancer (CC) is a significant global health issue and remains one of the leading causes of cancer-related mortality in women. Radiotherapy is a crucial treatment modality for CC; however, tumor heterogeneity and resistance to radiotherapy often result in suboptimal outcomes for some patients, including recurrence and metastasis. Periostin (POSTN), a matricellular protein within the tumor microenvironment, has been implicated in the promotion of tumor progression and treatment resistance, particularly through mechanisms such as epithelial-mesenchymal transition (EMT). Despite this, the role of POSTN in radiotherapy resistance in CC patients remains underexplored. Therefore, in this study, we investigated the prognostic significance of POSTN expression in CC patients undergoing radical radiotherapy and explored potential mechanisms underlying radiotherapy resistance. We analyzed data from 92 CC patients in The Cancer Genome Atlas (TCGA) and 153 patients from our institution, assessing POSTN expression levels through mRNA analysis and immunohistochemistry (IHC). Our findings revealed that high POSTN expression was significantly associated with advanced tumor stages, poorer radiotherapy outcomes, and worse overall survival (OS). Additionally, multivariate Cox regression analysis identified POSTN as an independent prognostic factor for CC patients undergoing radical radiotherapy. A prognostic nomogram integrating POSTN expression and clinicopathological features demonstrated superior predictive accuracy for OS. Drug sensitivity analysis suggested that high POSTN expression may be linked to resistance to multiple chemotherapeutic agents. Furthermore, weighted correlation network analysis (WGCNA) and gene set enrichment analysis (GSEA) identified EMT as a top enriched pathway in patients with high POSTN expression, suggesting it may play a critical role in radiotherapy resistance. Subsequently, in vitro experiments confirmed that POSTN knockdown significantly inhibited HeLa cell proliferation, invasion, and enhanced radiosensitivity, while promoting apoptosis. These findings indicate that high POSTN expression is a risk factor for poor prognosis in CC patients undergoing radical radiotherapy, and targeting POSTN may improve radiotherapy efficacy by reducing tumor proliferation and resistance.
Similar content being viewed by others
Introduction
Cervical cancer (CC) posed a major global health threat to women, and recent global statistics revealed that it ranked as the fourth most prevalent malignancy worldwide and the fourth leading cause of cancer-related mortality among women1. Treatment options for cervical cancer include surgical treatment, radiotherapy, and systemic therapies. Among these, radiotherapy is particularly vital, with about 80% of cervical cancer patients requiring it2. Radiotherapy for cervical cancer is categorized into adjuvant, radical, and palliative types, with radical radiotherapy also being applicable for patients at early, middle, and locally advanced stages of cervical cancer3,4. While the majority of cervical cancer patients undergoing radical radiotherapy attain favorable treatment results, factors such as tumor heterogeneity and resistance to radiotherapy mean that some patients still face uncontrolled tumor growth or recurrence and metastasis post-treatment. These challenges underscore that while radiotherapy achieves significant therapeutic outcomes for many patients, tumor heterogeneity and resistance to radiotherapy in some cases hinder its efficacy, necessitating further improvements5,6,7,8. Therefore, identifying new biomarkers for radiotherapy resistance in cervical cancer, investigating the mechanisms of this resistance, and enhancing the efficacy of radiotherapy are crucial for improving patient outcomes.
Periostin (POSTN) is a member of the extracellular matrix (ECM) protein family, functioning as a matricellular protein that interacts with cell surface receptors to facilitate intra- and inter-cellular communication9. In physiology, POSTN, as a matricellular protein, regulates various processes including embryo development, tissue repair, extracellular matrix structure, and the formation and maintenance of bones and teeth. Its role extends into pathological conditions, where recent studies have uncovered the crucial function of matricellular proteins within the tumor microenvironment10,11,12. POSTN interacts with various cell surface receptors, triggering signaling pathways that promote tumor growth. These signals involve several processes including survival of tumor cells, epithelial-mesenchymal transition (EMT), infiltration, and metastasis12,13. EMT, a frequent reversible phenotypic alteration in tumor progression, endows tumor cells with survival benefits such as stem cell traits, reduced apoptosis and aging, and increased capability for infiltration and metastasis. Post-radiotherapy, various tumor cells may undergo EMT, thereby increasing their resistance to the treatment.
In this study, we first explored the expression and prognostic significance of POSTN in CC patients undergoing radical radiotherapy. Subsequently, we developed a novel nomogram that incorporates clinicopathological features and POSTN expression levels to more precisely predict patient prognosis. We conducted weighted correlation network analysis (WGCNA), gene ontology (GO) and gene set enrichment analysis (GSEA) to further investigate potential mechanisms. Finally, we validated the oncogenic role of POSTN in cervical cancer cells through cytological experiments.
Methods
Datasets and processing
The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) database was an extensive tumor database that comprises whole-genome sequencing datasets of various tumors, along with accompanying clinical information. Our target population consisted of cervical cancer patients undergoing radical radiotherapy. From the TCGA database, we extracted all relevant follow-up information, clinical characteristics, and transcriptomic sequencing data (focusing on mRNA data for coding proteins) specifically for cervical cancer patients. Out of the 306 cervical cancer patients in the TCGA database, we initially removed those who had undergone radical surgery and those with missing survival data. Subsequently, we filtered for patients who had received radical radiotherapy, ultimately incorporating a total of 92 patients into our analysis.
Specimens
We collected data on CC patients treated at The First Affiliated Hospital of University of South China from 2013 to 2017. Out of 887 cases, 153 patients were selected who had undergone radical radiotherapy using intensity-modulated radiation therapy (IMRT). The inclusion criteria included: (1) Pathologically diagnosed at The First Affiliated Hospital of University of South China, with wax blocks still available for slicing and immunohistochemical testing; (2) No history of other malignant tumors, no distant metastasis; (3) Complete clinical pathological data (age, histological classification, staging) and 5-year follow-up records; (4) Having received radical radiotherapy. The exclusion criteria were: (1) Survival time less than 30 days; (2) Diagnosis of another malignant tumor within 5 years after treatment; (3) Failure to complete the full course of radical radiotherapy. The study was approved by the ethics committee of The First Affiliated Hospital of University of South China (2023LL041001). Given that this was a retrospective study which involved the review of patient medical records and tumor specimens, individual informed consent was not required by our ethics committee, which had approved the study as no individual patient identifiable information was utilized. All methods in this research were conducted in compliance with the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry (IHC) was carried out using standardized protocols. Sections were deparaffinized and rehydrated through sequential 10-minute xylene washes followed by 5-minute ethanol washes, followed by a 1-minute rinse in distilled water and a 1-minute PBS wash. Endogenous peroxidase was blocked by treating sections with 3% hydrogen peroxide in dark at room temperature for 25 min, followed by three 5-minute PBS washes. Non-specific binding was blocked by covering tissue sections with 3% BSA for 30 min at room temperature. The prepared primary antibody (POSTN monoclonal antibody, 66491-1-Ig, 1:200; Proteintech, China) was applied, and sections were incubated overnight at 4 °C. Post-primary antibody incubation, sections were warmed for 30 min at 37 °C and washed three times with PBS for 5 min each. The secondary antibody (HRP conjugated goat anti-mouse lgG, GB23301, 1:200; Servicebio, China) was applied and incubated for 50 min at room temperature. DAB was used for color development, and the reaction was monitored under a microscope to stop it when positive staining appeared with minimal background. Sections were rinsed with distilled water for 10 min. Nuclei were counterstained with hematoxylin for about 3 min, differentiated, and blued, followed by rinsing. Sections were dehydrated through an alcohol series, cleared in butanol and xylene, and coverslipped.
All immunohistochemical sections were diagnosed by two pathologists using a double-blind method, with five high-power fields (400x magnification) randomly selected for observation to ensure unbiased results. A consensus was reached on the results. The assessment relied on the immunohistochemical combined staining scoring method14: Staining intensity of positive cells was rated as follows: brown, 3 points; yellow, 2 points; pale yellow, 1 point; colorless, 0 points. Similarly, positive cell staining area was scored as follows: Area ≥ 75%, 4 points; area ≥ 50% and < 75%, 3 points; area ≥ 25% and < 50%, 2 points; area ≥ 0% and < 25%, 1 point. By summing the aforementioned scores, 0–3 points were classified as low POSTN expression, while 4–7 points were classified as high POSTN expression.
Construction and validation of a nomogram to predict overall survival in CC patients treated with radical radiotherapy
After analyzing median POSTN mRNA levels in the TCGA cohort, the 92 CC patients who had undergone radical radiotherapy were divided into low and high POSTN expression groups. A prognostic nomogram integrating age, histological type, stage, and POSTN expression was established to accurately predict the prognosis of CC patients from the TCGA cohort undergoing radical radiotherapy. To validate the reliability of the nomogram, the nomogram’s calibration curve was derived, and the correlation between its predicted probabilities and the actual incidence rates was examined. To further quantify the predictive accuracy, the concordance index (C-index) and area under the curve (AUC) were calculated15,16. Lastly, an external validation cohort of 153 CC patients from The First Affiliated Hospital of University of South China, all undergoing radical radiotherapy, was used to evaluate the nomogram’s predictive performance.
Drug sensitivity analysis
The NCI-60 panel was utilized to assess the relationship between POSTN expression and drug sensitivity. After excluding tumor cell lines with over 60% data loss, the analysis included 60 lines and 792 chemotherapeutic or targeted agents. Correlation analysis was conducted using the Pearson method.
WGCNA analysis and functional enrichment analysis
Weighted gene co-expression network was constructed to identify gene modules and explore correlations between the gene network and POSTN expression. The co-expression network of all genes in TCGA data set was constructed using WGCNA-R package17, and the genes with the top 5000 variogram were screened by this algorithm for further analysis. A soft threshold of 9 was set to ensure a scale-free network (when the scale-free fit index reaches 0.9, the minimal soft threshold power that satisfies the scale-free topology criterion is 9, Fig. 5a). The weighted adjacency matrix was transformed into a topological overlap matrix (TOM) to estimate network connectivity, and hierarchical clustering was used to construct the cluster tree of the TOM matrix. In this tree, different branches and colors represent various gene modules, highlighting the diversity of gene interactions. Finally, genes were classified into modules based on similar expression patterns, using weighted correlation coefficients, which facilitated the identification of functionally related gene groups. To identify the biological functions and signaling pathways associated with key WGCNA modules (notably the black module, which shows the highest correlation with POSTN), we employed the “ClusterProfiler”18 and “org.Hs.eg.db” (https://bioconductor.org/packages/org.Hs.eg.db/) R package for functional annotation. These approaches allowed for a detailed and comprehensive exploration of the functional correlations among the genes in these modules.
GSEA analysis
Gene Set Enrichment Analysis (GSEA) is a computational method used to ascertain if a defined set of genes exhibits significance, consistent differences across two groups. After analyzing median POSTN mRNA levels in the TCGA cohort, 92 CC patients treated with radical radiotherapy were classified into low and high POSTN expression groups. Subsequently, GSEA was then used to analyze the differences in Hallmark pathways between these groups18.
Cell culture
Human cervical cancer cells (HeLa) were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 medium (HyClone; Logan, UT, USA) supplemented with 10% fetal bovine serum (ExCell Bio; Shanghai, China), 100 U/ml penicillin and 100 µg/ml streptomycin (HyClone). The cells were incubated at 37 °C in a standard incubator with 5% CO2 and ambient oxygen levels.
RNA extraction and quantitative RT-PCR analysis
Total RNA was isolated using TRIzol reagent (Ambion; Austin, TX, USA) according to the manufacturer’s instructions. The concentration and purity of RNA were determined with an ultraviolet spectrophotometer (NanoDrop One, Thermo Scientific, USA). cDNA was synthesized from 1000 ng of total RNA using the PrimeScript RT reagent kit (TaKaRa; Beijing, China) for reverse transcription. Target gene expression was quantified using the SYBR Green PCR Kit (TIANGEN; Beijing, China), with GAPDH serving as the internal control. Relative mRNA expression levels were calculated using the 2 − ΔΔCt method. The qPCR primer sequences are as follows:
POSTN:
5′- CTCATAGTCGTATCAGGGGTCG − 3′ (forward).
5′- ACACAGTCGTTTTCTGTCCAC − 3′(reverse).
GAPDH:
5′- ACAACTTTGGTATCGTGGAAGG − 3′ (forward).
5′- GCCATCACGCCACAGTTTC − 3′(reverse).
POSTN knockdown
To knock down POSTN expression, two specific small interfering RNAs (siRNAs) targeting the POSTN mRNA sequence were identified and synthesized by Shanghai GenePharma Company. Transient transfection of the siRNAs was conducted using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. The sequences of the POSTN-siRNAs used are listed as follows:
POSTN-siRNA-539:
(sense: 5′- GCUUGGGACAACUUGGAUUTT − 3′; antisense: 5′- AAUCCAAGUUGUCCCAAGCTT − 3′)
POSTN-siRNA-1333:
(sense: 5′ - GCUGGCACCUGUGAAUAAUTT − 3′; antisense: 5′- AUUAUUCACAGGUGCCAGCTT − 3′)
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan) and colony formation assay were employed to assess the proliferative ability of Hela cells. In the CCK-8 assay, cells were seeded in 96-well plates (2000 cells/well), cultured for 24 h, then transfected with siRNAs. The optical density was measured at 450 nm at 24, 48, 72 h post-transfection using a microplate reader (Sunrise, Tecan; Switzerland). Each group was replicated five times. For the colony formation assay, 500 transfected cells were plated in 6-well plates and cultured for 12 days, with the medium being changed every four days. All cell colonies were fixed in methanol for one hour and stained with Giemsa for two hours at room temperature. Colonies were counted manually and images were obtained using an image scanner. Both experiments were conducted in triplicate.
Invasion assay
Matrigel matrix (Corning, USA) was thawed on ice and diluted 1:8 with FBS-free RPMI-1640 (where 1 part represents the matrix glue and 8 parts represent the FBS-free RPMI-1640 medium), then applied to cover the 8 μm pore-sized Transwell chambers, which were subsequently placed in 24-well plates. The prepared matrix gel-coated Transwell cell culture system was used for invasion assays. Cells were seeded in 6-well plates (2.5 × 105 cells/well), cultured for 24 h, then transfected with siRNAs. At 12 h post-transfection, cells were collected and reseeded in the Transwell chambers (1 × 105 cells per chamber) specifically designed for invasion assays. The upper chamber received 200 µL of FBS-free medium, and the lower chamber received 600 µL of 20% FBS medium. After 24 h, the chambers were removed; cells were fixed in methanol for 30 min and stained with Giemsa for one hour. Cells in the upper chamber were wiped off with a cotton swab. The chambers were washed with PBS and allowed to dry. For each sample, five independent fields of view were imaged under a microscope to ensure unbiased results, and photographs were taken for subsequent analysis.
Apoptosis assay
Cells transfected with siRNAs were harvested at 24 h and 48 h post-transfection. The apoptosis assay was conducted using the Annexin V-APC/PI apoptosis detection kits (KAIJI, China), as per the instructions provided. Flow cytometry (FACS) analysis was performed using BD FACS Calibur, with a minimum of 10,000 cells analyzed per sample. In the analysis, early apoptotic cells were only positive for Annexin V, late apoptotic cells were positive for both PI and Annexin V, and necrotic cells were only positive for PI. The total apoptotic cell count was calculated as the sum of early and late apoptotic cells.
Cell radiosensitivity assay
Cells were seeded in 6-well plates (2.5 × 105 cells/well) and cultured for 24 h, then transfected with siRNAs. At 12 h post-transfection, cells were collected and reseeded in 6-well plates with cell densities ranging from 500 to 6,000 cells/well and subsequently treated with varying doses of IR from 2 to 8 Gy. Following 14 days of culture, the cells were fixed in methanol and stained with Giemsa. Colonies containing more than 50 cells, visible to the naked eye, were counted. Plating efficiency (PE) was calculated as the ratio of the number of colonies formed to the number of cells seeded, multiplied by 100%. To determine the survival fraction, the number of colonies at a specific IR dose was divided by the baseline number of colonies, after correcting for plating efficiency. All data were analyzed using GraphPad Prism 8 software. Furthermore, the survival curves for the clonogenic assays were derived using the single-hit multi-target model, expressed as y = 1 - (1 - exp(-k*x))^N19.
Statistical analysis
Data analysis was performed using R language (version 4.2.0), where a significance threshold was set at p < 0.05. Simultaneously, experimental data analysis was conducted using GraphPad Prism version 9.5. For robustness, a minimum of three experimental datasets were analyzed using the t-test to assess differences, denoted as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Results
POSTN expression and clinical relevance in CC patients treated with radical radiotherapy
In order to investigate the expression of POSTN in patients receiving radical radiotherapy and its association with clinical pathological features, we selected 92 and 153 cervical cancer patients undergoing radical radiotherapy from the TCGA database and our center respectively, with specific clinical information shown in Table 1. In the TCGA database, 75.0% of patients (n = 69) were under 60 years old, while 25.0% (n = 23) were 60 years or older. Of the diagnosed patients, 89.1% had squamous cell carcinoma (n = 82) and 10.9% had adenocarcinoma (n = 10). Of the patients, 57 (62.1%) were in stages I/II, and 32 (34.5%) were in stages III/IV. Radiotherapy outcomes were CR in 39.1% of cases (n = 36) and NCR in 22.8% (n = 21). Regarding survival status, 63.0% of patients were alive (n = 58), while 37.0% were deceased (n = 34). At our center, 69.3% of patients were under 60 years old (n = 106), while 30.7% were 60 years or older (n = 47). Of the patients, 94.8% had squamous cell carcinoma (n = 145) and 5.2% had adenocarcinoma (n = 8). Of the patients, 97 (63.4%) were in stages I/II, and 56 (36.6%) were in stages III/IV. Radiotherapy outcomes were CR in 58.8% of cases (n = 90) and NCR in 20.3% (n = 31). Survival statuses were 69.9% alive (n = 107) and 30.1% deceased (n = 46).
We then analyzed POSTN mRNA expression in 92 cervical cancer patients from the TCGA database who were undergoing radical radiotherapy. The Wilcoxon test results showed that POSTN mRNA levels were significantly associated with patients’ stages, radiotherapy outcomes, and survival status (Fig. 1a–e). Specifically, patients with more advanced stages (P = 0.0058), less effective radiotherapy responses (P = 0.034), and poorer survival outcomes (P = 0.001) exhibited higher POSTN expression levels. Subsequently, using immunohistochemistry, we assessed POSTN protein expression in 153 cervical cancer patients undergoing radical radiotherapy at our center, it was observed that POSTN protein predominantly localized to the interstitial cells (see Fig. 1f–i). Furthermore, Chi-square test results indicated that POSTN protein expression levels significantly correlated with patients’ age, stages, radiotherapy outcomes, and survival status (refer to Fig. 1j–n), Patients who were older (P = 0.009), had more advanced stages (P = 0.001), less effective radiotherapy responses (P = 0.028), and poorer survival status (P < 0.001) showed higher levels of POSTN expression.
POSTN expression and clinical relevance in CC patients treated with radical radiotherapy. Clinical significance of POSTN expression with age (a), histological type (b), stage (c), radiotherapy responses (d) and vital status (e) from the TCGA database. Low POSTN expression in CC tissues (f: magnification 200×, j: magnification 400×). High POSTN expression in CC tissues (h: magnification 200×, i: magnification 400×). Clinical significance of POSTN expression with age (j), histological type (k), stage (l), radiotherapy responses (m) and vital status (n) from our center. TCGA, the cancer genome atlas database; CC, cervical cancer.
High levels of POSTN expression is associated with poor prognosis in CC patients treated with radical radiotherapy
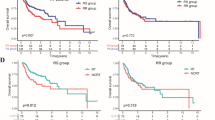
To determine if POSTN expression levels associated with the prognosis of cervical cancer patients treated with radical radiotherapy, we conducted Kaplan-Meier survival analysis, univariate COX regression, and multivariate COX regression using data from both the TCGA database and our center. The Kaplan-Meier survival analysis showed that in both the TCGA database (Fig. 2a) and our center (Fig. 2b), POSTN expression levels were significantly associated with prognosis in cervical cancer patients undergoing radical radiotherapy, with patients exhibiting high POSTN expression having shorter overall survival. Subsequently, univariate COX regression analysis revealed that clinical staging (TCGA cohort: HR = 2.965, 95% CI = 1.495–5.880, P = 0.002; Our cohort: HR = 2.594, 95% CI = 1.451–4.636, P = 0.001) and POSTN expression levels (TCGA cohort: HR = 3.307, 95% CI = 1.573–6.952, P = 0.002; Our cohort: HR = 4.767, 95% CI = 2.663–8.535, P < 0.001) were prognostic factors affecting patients undergoing radical radiotherapy for cervical cancer (Fig. 2c, d). Furthermore, multivariate COX regression analysis showed that clinical stage (TCGA cohort: HR = 2.300, 95% CI = 1.134–4.664, P = 0.021; Our cohort: HR = 1.850, 95% CI = 1.009–3.390, P = 0.047) and POSTN expression levels (TCGA cohort: HR = 2.721, 95% CI = 1.263–5.880, P = 0.011; Our cohort: HR = 4.005, 95% CI = 2.182–7.348, P < 0.001) were the independent prognostic factors for patients undergoing radical radiotherapy for cervical cancer (Fig. 2e, f). These findings underscore that high POSTN expression was associated with a poorer prognosis in cervical cancer patients undergoing radical radiotherapy.
High POSTN expression is associated with poor prognosis in CC patients treated with radical radiotherapy. Kaplan-Meier survival analysis (a,b), univariate COX regression analysis (c,d), and multivariate COX regression analysis (e,f) of POSTN expression in CC patients treated with radical radiotherapy from the TCGA database and our center. CC, cervical cancer; TCGA, the cancer genome atlas database.
Nomogram construction and validation
To improve prognosis prediction for cervical cancer patients undergoing radical radiotherapy, we developed a nomogram model based on POSTN expression levels and clinicopathological characteristics within the TCGA cohort, as illustrated in Fig. 3a. Calibration curves for predicting 1-, 3-, and 5-year overall survival (OS) indicated that the nomogram performed ideally, as demonstrated in Fig. 3b–d. Moreover, the C-index of the nomogram was significantly higher than that of any single index, including stage, POSTN expression levels, age, and histological type, as detailed in Fig. 3e. Kaplan-Meier survival analysis demonstrated that high-risk patients exhibited significantly poorer overall survival, as depicted in Fig. 3f. Additionally, the nomogram’s ROC AUC for predicting 1-, 3-, and 5-year survival was superior to those derived from POSTN expression and stage alone, as confirmed in Fig. 4g–i.
Nomogram construction and validation. The nomogram model (a) based on POSTN expression levels and clinicopathological characteristics were constructed to predict OS of CC patients underwent radical radiotherapy. Calibration curves of the nomogram for predicting 1- (b), 3- (c), and 5-year (d) OS. The C-index of the nomogram and other clinicopathological characteristics (e). Kaplan-Meier survival analysis (f, g), 1-year (h, i), 3-year (j, k), and 5-year (l, m) ROC analysis for nomogram from the TCGA database and our center. OS, overall survival; CC, cervical cancer; C-index, concordance index; ROC, receiver operating characteristic; TCGA, the cancer genome atlas database.
The overexpression of POSTN was negatively correlated with drug sensitivity. The correlation between POSTN expression and drug sensitivity, including Debio 0932 (a), Lapatinib (b), Onalespib (c), Bromopyruvic acid (d), Allopurinol (e), Hypothemycin (f), the product of Debio 0932 (g), Gefitinib (h), and Parthenolide (i).
To further validate our nomogram’s accuracy, we employed Kaplan-Meier survival analysis and ROC analysis to re-assess its predictive performance using an external dataset from our center. Kaplan-Meier survival analysis revealed that, within our center’s dataset, high-risk patients had significantly poorer overall survival compared to low-risk patients (Fig. 3j). Subsequently, the ROC AUC values for the nomogram’s 1-, 3-, and 5-year survival predictions were 0.733, 0.778, and 0.734, respectively, as presented in Fig. 3k, m. These results confirm that our nomogram model demonstrates satisfactory accuracy and reliability.
The relationship between POSTN and drug sensitivity in CC patients treated with radical radiotherapy
To elucidate why cervical cancer patients with high POSTN expression had a poorer prognosis, we assessed the correlation between POSTN expression and drug sensitivity using the spearman method. Our analysis revealed a positive correlation between POSTN expression and sensitivity to several drugs, including Debio 0932, Lapatinib, Onalespib, Bromopyruvic acid, Allopurinol, Hypothemycin, the product of Debio 0932, Gefitinib, and Parthenolide (Fig. 4a–i). This suggests that high POSTN expression might lead to enhanced drug resistance in cervical cancer patients undergoing radical radiotherapy, thereby worsening prognosis. Furthermore, this also implies that concurrent use of these drugs during radical radiotherapy for cervical cancer could potentially enhance therapeutic outcomes.
WGCNA analysis, functional enrichment analysis and GSEA analysis
Using the TCGA dataset, we constructed a WGCNA network to investigate the regulatory network associated with POSTN in cervical cancer patients undergoing radical radiotherapy. We set the soft threshold β to 9 (Fig. 5a), and identified 12 gene modules via the TOM matrix (Fig. 5b), which were named as follows: black (966 genes), magenta (343), brown (1272), yellow (1251), purple (239), pink (540), turquoise (2003), green (1242), red (1232), blue (1781), green yellow (222), and grey (6088) (Fig. 5c). Further analysis revealed that the black module showed the highest correlation with POSTN expression (correlation coefficient = 0.49, p = 9e-07) (Fig. 5c, d). GO enrichment analysis results indicated that genes in this module were primarily enriched in extracellular matrix organization (BP), collagen-containing extracellular matrix (CC), and extracellular matrix structural constituent (MF) (Fig. 5e). Additionally, Hallmark pathway enrichment analysis demonstrated that these genes were predominantly involved in EMT, myogenesis, UV response, angiogenesis, KRAS signaling, and other pathways (Fig. 5f).
WGCNA and functional enrichment analysis. Scale-free fit index analysis (a) of each soft threshold for CC patients treated with radical radiotherapy. Modules for mRNA co-expression networks (b). Heatmap depicting the traits relationship between low- and high-POSTN expression groups (c). Black module gene scatter plots (d). GO (e) and hallmark pathway (f) enrichment analysis of black module genes. CC, cervical cancer; GO, gene ontology.
We conducted GSEA analysis to elucidate POSTN’s biological functions in cervical cancer patients treated with radical radiotherapy. Our Hallmark pathway enrichment analysis revealed several POSTN-associated signaling pathways in the TCGA database, including interferon response, angiogenesis, epithelial mesenchymal transition, E2F targets, DNA repair, TGF-β signaling, and KRAS signaling, among others (Fig. 6a). In the high-POSTN expression group, pathways such as epithelial mesenchymal transition (Fig. 6b), TGF-β signaling (Fig. 6c), angiogenesis (Fig. 6d), KRAS signaling up (Fig. 6e), coagulation (Fig. 6f), TNF-α signaling via NF-κB (Fig. 6g), and hypoxia (Fig. 6h) were significantly activated. Conversely, pathways such as interferon alpha response, interferon gamma response, E2F targets, KRAS signaling down, DNA repair, and the P53 pathway were significantly suppressed in the high-POSTN expression group (Fig. 6i).
GSEA analysis. GSEA analysis of the hallmark pathway (a). EMT (b), TGF-β signaling (c), angiogenesis (d), KRAS signaling up (e), coagulation (f), TNF-α signaling via NF-κB (g), and hypoxia (h) were significantly activated in the high-POSTN expression group. The suppressed pathways in the high-POSTN expression group (i). GSEA, gene set enrichment analysis; EMT, epithelial-mesenchymal transition.
Within the WGCNA and GSEA hallmark pathway analysis results, the epithelial mesenchymal transition showed the most significant enrichment. Consequently, we performed a correlation analysis between POSTN and genes associated with the EMT pathway, with the genes ranked in the top 16 for correlation displayed in Fig. 7a–l. Many previous studies have shown that EMT is critically involved in the resistance to cancer treatments, including radiotherapy resistance. Based on the integrated results, we hypothesize that the poor prognosis in cervical cancer patients with high POSTN expression undergoing radical radiotherapy may be attributed to the activation of the EMT pathway.
In vitro assays
As indicated by the hallmark pathway enrichment results (Figs. 5f and 6a), the pro-carcinogenic pathways such as EMT, TGF signaling, angiogenesis, and KRAS signaling are significantly activated in patients with high POSTN expression. Building on these findings, we further confirmed through cytological experiments the regulatory effects of POSTN on cell proliferation, invasion, cell cycle, apoptosis, and radiosensitivity. POSTN-siRNAs were transfected into Hela cells, successfully knocking down POSTN expression (Fig. 8a). Subsequently, CCK-8 analysis demonstrated that POSTN knockdown significantly inhibited Hela cell proliferation (Fig. 8b). The results of clone formation assay revealed that POSTN knockdown could decreased the clone formation capability in Hela cells (Fig. 8c). The invasion ability of Hela cells was suppressed after knocking down the expression of POSTN (Fig. 8d). Apoptosis levels were significantly higher in si-POSTN groups compared to the si-NC group (Fig. 8e), suggesting that POSTN may play an important role in CRC cell survival. Subsequently, after transfecting Hela cells with POSTN-siRNAs, we assessed their clonogenic ability at radiation doses of 0, 2, 4, 6, and 8 Gy. Parameters of a multitarget/single-hit model were then calculated based on these clonogenic survival assays. The resulting data, as shown in Fig. 8f, indicate that POSTN knockdown increased the radiosensitivity of Hela cells relative to controls, thereby confirming its role in cervical cancer cell radiosensitivity.
Discussion
Radiation therapy is a key treatment option for cervical cancer, with approximately 80% of cervical cancer patients requiring radiotherapy2. While most patients treated with curative radiotherapy have favorable outcomes, the heterogeneity of tumors and resistance to radiotherapy mean that some patients still face uncontrolled tumor growth or recurrence and metastasis post-treatment. This challenge highlights the importance of understanding the factors that contribute to radiotherapy resistance5. Increasing research indicates that the tumor microenvironment is a crucial factor affecting patient prognosis20,21. The extracellular matrix, an important component of the tumor microenvironment, is composed of proteins, polysaccharides, and other molecules, providing structural support to the tumor. Besides providing structural support, the extracellular matrix also plays a role in tumor cell proliferation, migration, invasion, and treatment resistance by modulating cellular behaviors. POSTN, a member of the extracellular matrix ECM protein family, facilitates both intracellular and intercellular communication by interacting with cell surface receptors10,11,12,22,23. Recent studies have highlighted POSTN’s crucial role in the tumor microenvironment, where it triggers oncogenic signaling pathways by interacting with various cell surface receptors13,24. These signals facilitate processes such as tumor cell survival, EMT, infiltration, and metastasis. Despite its importance, the literature currently lacks reports on POSTN’s role in radiotherapy resistance, highlighting a significant knowledge gap.
In this study, we initially explored the expression status of POSTN in cervical cancer patients receiving curative radiotherapy. Utilizing data from the TCGA database, we discovered that high expression of POSTN mRNA was significantly associated with advanced staging, poorer radiotherapy outcomes, and worse survival status in these patients. Next, using immunohistochemistry, we assessed the POSTN expression in 153 cervical cancer patients receiving curative radiotherapy at our institution. It was observed that the POSTN protein is mainly expressed in the interstitial spaces. Similarly, high expression of POSTN protein was significantly associated with older age, advanced staging, poorer radiotherapy outcomes, and worse survival status in patients. These results indicate that high expression of POSTN is associated with adverse clinicopathological features in patients with cervical cancer undergoing curative radiotherapy, echoing findings from previous research such as the study by Wei et al.25, which showed that high expression of POSTN in cervical cancer is significantly related to later stages, vascular invasion, and lymph node metastasis.
Recent studies indicate that high expression of POSTN is often significantly associated with poor prognosis. Jaakko S Knuutila et al. found that patients with high POSTN expression in cutaneous squamous cell carcinoma exhibit worse prognoses26. Research by Md Roman Mogalet et al. in pancreatic cancer shows that high POSTN expression is associated with significantly shorter overall and disease-free survival times compared to patients with lower POSTN expression27. Similarly, Song’s research highlights POSTN as an adverse prognostic factor in breast cancer, with those exhibiting high POSTN expression facing significantly worse outcomes28. To date, there has been no reported data on the prognostic significance of POSTN in cervical cancer patients treated with radical radiotherapy; thus, we further assessed the prognostic value of POSTN in such patients using Kaplan-Meier survival analysis, univariate, and multivariate Cox analyses. Our survival analysis results indicate that patients with high POSTN expression have significantly shorter overall survival times than those with low POSTN expression. Moreover, multivariate results reveal that the stage and the level of POSTN expression are independent prognostic factors affecting overall survival, with patients at advanced stages and those with high POSTN expression having a worse prognosis. Subsequently, we created a novel nomogram using the TCGA dataset, integrating clinical pathological characteristics and POSTN expression levels. This nomogram accurately predicts the 1-year, 3-year, and 5-year survival rates of patients with cervical cancer undergoing radical radiotherapy, and its C-index is higher than that of single clinical pathological features or POSTN expression alone. Furthermore, its predictive accuracy has also been validated in a larger external validation cohort.
In cervical cancer patients undergoing curative radiotherapy, those with high POSTN expression have poorer radiotherapy efficacy and prognosis. Consequently, investigating potential therapeutic drugs for use during concurrent radiotherapy could potentially mitigate this adverse outcome. Through drug sensitivity correlation analysis, we found that POSTN expression levels are significantly negatively correlated with drugs such as Debio 0932, Lapatinib, Onalespib, Bromopyruvic acid, Allopurinol, Hypothemycin, Gefitinib, and Parthenolide. Based on these findings, we speculate that these drugs could potentially be effective in treating patients with high POSTN expression in cervical cancer undergoing curative radiotherapy. To move forward, clearly, further basic experiments are needed to validate our hypothesis.
To further explore the underlying mechanisms behind the significant differences in radiotherapy efficacy and prognosis between high and low POSTN expression groups in patients undergoing curative radiotherapy for cervical cancer, we assessed the differences in biological processes between these groups using WGCNA and GSEA analysis. The results indicated that EMT was the top-ranked pathway in both analyses, with EMT significantly activated in patients with high POSTN expression. This activation suggests a critical role of EMT in modulating radiotherapy responses. EMT is a reversible phenotype change that frequently occurs during tumor progression, providing cancer cells with a survival advantage29,30,31,32. When subjected to radiotherapy, many cancer cell types can undergo EMT to enhance their resistance. For example, breast cancer cells treated with ionizing radiation exhibit increased expression of CD146, which encourages EMT, DNA damage repair, and radioresistance33. Yang et al.‘s study demonstrated that DLL4 inhibited the progression and increased the radiosensitivity in cervical cancer cells by reversing EMT34. Similarly, Che et al. found that TRIP4 can promote EMT in cervical cancer by activating PI3K/AKT and MAPK/ERK signaling, ultimately leading to the proliferation of cervical cancer cells and resistance to radiotherapy35. Therefore, we hypothesize that the potential biological mechanism behind the poorer radiotherapy efficacy and prognosis in cervical cancer patients with high POSTN expression undergoing curative radiotherapy may be due to the activation of the EMT pathway.
Finally, we further verified POSTN’s function through cytological experiments. We observed that knockdown of POSTN expression significantly decreased HeLa cell proliferation and invasion capabilities. Additionally, apoptosis and clonogenic survival assays showed that downregulation of POSTN expression inhibited cell survival and radioresistance. Consequently, POSTN holds potential as a molecular target for cervical cancer treatment.
This study presents some limitations. Firstly, being retrospective in nature, it inherently carries a certain level of bias. Moreover, this study only confirmed the effects of POSTN on the proliferation, apoptosis, and radiosensitivity of cervical cancer cell lines at the cytological phenotype level and did not extend to a deeper investigation into the underlying mechanisms. Therefore, more comprehensive experiments are needed in the future to elucidate the specific biological mechanisms of POSTN.
Conclusions
In conclusion, our study identifies high POSTN expression as an independent risk factor for overall survival (OS) in cervical cancer patients undergoing radical radiotherapy. To enhance clinical utility, we developed nomograms that demonstrated superior prognostic discrimination and predictive accuracy for OS in these patients. These nomograms are designed to predict individualized survival rates, guiding tailored treatment strategies for these patients. Additionally, we discovered that reducing POSTN expression inhibits proliferation, survival, and radiation resistance in cervical cancer cells.
Data availability
The datasets analyzed in the current study are available from The Cancer Genome Atlas (TCGA) repository (https://portal.gdc.cancer.gov/).
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Moreno-Acosta, P. et al. Biomarkers of resistance to radiation therapy: A prospective study in cervical carcinoma. Radiat. Oncol. 12, 120 (2017).
Chargari, C. et al. Radiotherapy of cervical cancer. Cancer Radiother. 26, 298–308 (2022).
Polgár, C., Major, T. & Varga, S. [Radiotherapy and radio-chemotherapy of cervical cancer]. Magy Onkol. 66, 307–314 (2022).
Mayadev, J. S. et al. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer. 32, 436–445 (2022).
Zhang, H. et al. Review of possible mechanisms of radiotherapy resistance in cervical cancer. Front. Oncol. 13, 1164985 (2023).
Zhou, J. et al. Recent progress of the tumor microenvironmental metabolism in cervical cancer radioresistance. Front. Oncol. 12, 999643 (2022).
Sun, Q., Wang, L., Zhang, C., Hong, Z. & Han, Z. Cervical cancer heterogeneity: A constant battle against viruses and drugs. Biomark. Res. 10, 85 (2022).
Du, J. & Li, M. Functions of Periostin in dental tissues and its role in periodontal tissue regeneration. Adv. Exp. Med. Biol. 1132, 63–72 (2019).
Xia, Y., Chen, L., Lu, J., Ma, J. & Zhang, Y. The comprehensive study on the role of POSTN in fetal congenital heart disease and clinical applications. J. Transl Med. 21, 901 (2023).
Ackerman, J. E. et al. Identification of Periostin as a critical niche for myofibroblast dynamics and fibrosis during tendon healing. Matrix Biol. 125, 59–72 (2024).
Chen, C. et al. Single-cell and spatial transcriptomics reveal POSTN(+) cancer-associated fibroblasts correlated with immune suppression and tumour progression in non-small cell lung cancer. Clin. Transl Med. 13, e1515 (2023).
Wasik, A., Ratajczak-Wielgomas, K., Badzinski, A., Dziegiel, P. & Podhorska-Okolow, M. The role of Periostin in angiogenesis and lymphangiogenesis in tumors. Cancers (Basel) 14 (2022).
Zhu, T. et al. ALDH1B1 predicts poor survival for locally advanced nasopharyngeal carcinoma patients. Transl Cancer Res. 11, 382–391 (2022).
Robin, X. et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 12, 77 (2011).
Blanche, P., Dartigues, J. F. & Jacqmin-Gadda, H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat. Med. 32, 5381–5397 (2013).
Langfelder, P. & Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Spring, E. & Holmberg, P. Evaluation of experimental irradiation fractionation with the single-hit, multi-target model. Acta Radiol. Ther. Phys. Biol. 7, 297–306 (1968).
Trujillo-Cirilo, L., Weiss-Steider, B., Vargas-Angeles, C. A., Corona-Ortega, M. T. & Rangel-Corona, R. Immune microenvironment of cervical cancer and the role of IL-2 in tumor promotion. Cytokine 170, 156334 (2023).
Li, Y. et al. Tumor microenvironment promotes lymphatic metastasis of cervical cancer: its mechanisms and clinical implications. Front. Oncol. 13, 1114042 (2023).
Lee, D. et al. Endothelial periostin regulates vascular remodeling by promoting endothelial dysfunction in pulmonary arterial hypertension. Anim. Cells Syst. (Seoul). 28, 1–14 (2024).
You, T. et al. POSTN secretion by extracellular matrix cancer-associated fibroblasts (eCAFs) correlates with poor ICB response via macrophage chemotaxis activation of akt signaling pathway in gastric cancer. Aging Dis. 14, 2177–2192 (2023).
Dorafshan, S. et al. Periostin: Biology and function in cancer. Cancer Cell. Int. 22, 315 (2022).
Wei, W. F. et al. Periostin(+) cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol. Oncol. 15, 210–227 (2021).
Knuutila, J. S. et al. Cancer-associated fibroblast activation predicts progression, metastasis, and prognosis of cutaneous squamous cell carcinoma. Int. J. Cancer. 155, 1112–1127 (2024).
Mogal, M. R. et al. Integrated bioinformatics analysis reveals upregulated extracellular matrix hub genes in pancreatic cancer: Implications for diagnosis, prognosis, immune infiltration, and therapeutic strategies. Cancer Rep. (Hoboken) 7, e2059 (2024).
Song, J. et al. Identification of a novel cancer-associated fibroblasts gene signature based on bioinformatics analysis to predict prognosis and therapeutic responses in breast cancer. Heliyon 10, e29216 (2024).
Pastushenko, I. & Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell. Biol. 29, 212–226 (2019).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell. Biol. 20, 69–84 (2019).
Liu, Q. et al. Nuclear isoform of RAPH1 interacts with FOXQ1 to promote aggressiveness and radioresistance in breast cancer. Cell. Death Dis. 14, 803 (2023).
Youssef, K. K. & Nieto, M. A. Epithelial-mesenchymal transition in tissue repair and degeneration. Nat. Rev. Mol. Cell. Biol. 25, 720–739 (2024).
Liang, Y. et al. CD146 interaction with integrin β1 activates LATS1-YAP signaling and induces radiation-resistance in breast cancer cells. Cancer Lett. 546, 215856 (2022).
Yang, S. S. et al. Inhibition of Delta-like Ligand 4 enhances the radiosensitivity and inhibits migration in cervical cancer via the reversion of epithelial-mesenchymal transition. Cancer Cell. Int. 20, 344 (2020).
Che, Y. et al. TRIP4 promotes tumor growth and metastasis and regulates radiosensitivity of cervical cancer by activating MAPK, PI3K/AKT, and hTERT signaling. Cancer Lett. 452, 1–13 (2019).
Acknowledgements
We acknowledge the TCGA database for providing the platform and its contributors for uploading meaningful datasets.
Funding
This work was supported by National Natural Science Foundation of China (82304083 to Jun-yan He), Natural Science Foundation of Hunan Province (2023JJ40584 to Jun-yan He) and Natural Science Foundation of Hunan Province (2022JJ40394 to Cui-qin Huang).
Author information
Authors and Affiliations
Contributions
J.H. and Z.L. designed the research. C.H. collected and analysed the data. C.H. and Z.L. drafted the manuscript. W.X. and X.Y. prepared the figures. J.H. revised the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Cq., Xiao, Wt., Yao, Xr. et al. Elevated POSTN expression predicts poor prognosis and is associated with radioresistance in cervical cancer patients treated with radical radiotherapy. Sci Rep 15, 4174 (2025). https://doi.org/10.1038/s41598-025-88908-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88908-2