Abstract
Annexins (Anns) are a family of evolutionarily conserved, calcium-dependent, phospholipid-binding proteins that play critical roles in plant growth, development, and stress responses. Utilizing the pan-genome of 26 high-quality maize genomes, we identified 12 Ann genes, comprising 9 core genes (present in all 26 lines) and 3 near-core genes (present in 24–25 lines). This highlights the limitations of studying ZmAnn genes based on a single reference genome. Evaluating the Ka/Ks values of Ann genes in 26 varieties revealed that ZmAnn10 was under positive selection in certain varieties, while the remaining genes had Ka/Ks values less than 1, indicating purifying selection. Phylogenetic analysis divided ZmAnn proteins into six groups, with group VI containing only ZmAnn12. Structural variation in certain varieties altered the conserved domains, generating many atypical genes. Transcriptome analysis showed that different Ann members have distinct expression patterns in various tissues and under different abiotic and biotic stress treatments. Weighted gene co-expression network analysis of transcriptome data from various maize tissues under cold stress identified four Ann genes (ZmAnn2, ZmAnn6, ZmAnn7, ZmAnn9) involved in co-expression modules. Overall, this study utilized high-quality maize pangenomes to perform a bioinformatic analysis of ZmAnn genes, providing a foundation for further research on ZmAnn genes.
Similar content being viewed by others
Introduction
Annexin (Ann), comprising a multigene family classified as a subfamily of calcium (Ca2+)-dependent and phospholipid-binding proteins, is evolutionarily conserved and widely distributed across various taxonomic groups, encompassing prokaryotes, fungi, protists, plants, and vertebrates1. Annexins are proteins with multiple functions, featuring the distinctive annexin repeat and possessing residues associated with peroxidase, ATPase/GTPase activity, and Ca2+ channel regulatory activity. They serve to mediate Ca2+ sensing and signaling, thereby linking Ca2+ dynamics to cytoskeletal responses. This functional versatility results in numerous regulatory effects on plant growth, development, and stress response2,3. Typical mammalian membrane annexins possess a C-terminal core comprising four annexin repeats, with each repeat consisting of approximately 70 amino acids and containing a characteristic type II motif for calcium ion binding. Additionally, these annexins feature a highly variable N-terminal region, which serves as a secondary modification site that dictates interactions with other proteins. This variability in the N-terminal region is responsible for the functional diversity observed among animal annexins1,4. The diverse functions of annexins are attributed to their ability to interact with various ligands, including lipids, proteins, and ions, and to their subcellular localization in different compartments, such as the cytosol, membranes, and organelles.
Investigating the genetic mechanisms underlying crop resistance to drought and tolerance to salt, cold, heat, and other adverse conditions represents one of the most cost-effective and sustainable approaches to enhancing crop productivity and yield stability, thereby significantly contributing to the global food security agenda5,6,7. Therefore, understanding the molecular mechanisms that confer stress tolerance in crops is essential for developing new varieties that can withstand these challenges and ensure food security for a growing population. Recent investigations have demonstrated the significant role of plant annexin proteins in both plant growth and development, as well as in response to abiotic and biotic stresses. During the elongation phases of cotton fiber development, the highly expressed cotton annexin gene, GhAnn2, assumes a crucial regulatory function by modulating the flux of Ca2+ signaling in fiber development8. Oxylipin treatment upregulates Arabidopsis AtAnn7 expression, leading to callose deposition, wavy root development, and lateral root inhibition9. The expression of AtAnn5 is specifically observed in mature pollen, and silencing this gene leads to the production of abnormal pollen grains and severe male sterility10. Under long day conditions, Arabidopsis single-mutant AtAnn1 and AtAnn4 plants exhibit tolerance to drought and salt stress11. Overexpression of OsANN3 enhances sensitivity to exogenous ABA, and when plants are subjected to drought stress, may activate the ABA signaling pathway by mediating Ca²⁺ influx through interaction with phospholipids12. Overexpression of RsAnn1a in Raphanus sativus can improve plant heat tolerance13. It was demonstrated that cold activation of OST1 leads to the phosphorylation of AtAnn1, thereby enhancing its Ca2+ transport activity and further potentiating Ca2+ signaling. This unraveled a cascade linking OST1-AtAnn1 to cold-induced Ca2+ signal generation, which activates the cold response and consequently enhances freezing tolerance in Arabidopsis14. Compared to wild-type rice, CRISPR/Cas9-mediated genome editing to knock out OsAnn5 and OsAnn3 renders the plants sensitive to cold treatment, suggesting the involvement of OsAnn5 and OsAnn3 in cold stress tolerance during the seedling stage15,16. The ectopic expression of ZmAnn33/35 in Arabidopsis facilitates seedling recovery from cold stress and reinforces PM (Plasma Membrane) integrity, potentially ascribed to their roles in ROS (Reactive Oxygen Species) elimination and exocytosis17.
The recent surge in functional genomics research has revealed a high degree of genomic variation within species. This highlights the limitations of a single reference genome, which typically represents a single individual and might not capture the full range of genetic diversity18. Pan-genomics, which encompasses most of the genetic diversity of species or populations, has been proven to be a highly effective tool for studying genome evolution, species origin and domestication, and providing valuable insights for plant improvement19. Leveraging the maize pan-genome, comprising 26 high-quality genomes as publish by Hufford et al.20, this study comprehensively characterizes the maize annexin gene family. It delves into the gene structure, conserved domains, cis-regulatory elements, and phylogenetic classification of annexin genes across the pan-genome. Transcriptome data is employed to investigate gene expression patterns under diverse stress conditions. Furthermore, co-expression network analysis and GO enrichment analysis are utilized to unravel the regulatory network and biological processes involving annexin proteins under cold stress.
Results
Identification of annexin genes in the maize pan-genome
Both an HMMER search and the Blastp program were used for identification. Twelve ZmAnn genes were identified in the maize pan-genome, of which 9 were core genes, 3 were near-core genes (Table. S1). Although ZmAnn12 was not found in the B73 reference genome, a collinear segment with the gene was identified, a similar situation was observed in the M162W and NC350 genomes. The CML52 genome lacks the ZmAnn7 and ZmAnn8 gene, and the Ki11 genome lacks the ZmAnn4 gene. However, the other 24 genomes analyzed in this study contain all 12 ZmAnn genes or their corresponding collinear segments. This suggests that the absence of these genes in the CML52 and Ki11 genomes may be due to genetic variation or deletion events.
According to this analysis, the physicochemical properties of the annexin genes are shown in Table S2, The annexin genes encoded proteins ranging from 85 (B97_Ann3) to 446 aa (NC350_Ann9) in length, with isoelectric points (pIs) ranging from 5.4 (HP301_Ann10, Ki11_Ann10, Mo18W_Ann10, M126W_Ann10) to 9.6 (NC350_Ann9) and molecular weights (MWs) varying from 9.1 (B97_Ann3) to 48.6 kDa (NC350_Ann9). The instability index ranging from 30.57 (CML69_Ann5) to 84.91 (B97_Ann3) and the aliphatic index ranging from 76 (CML103_Ann9) to 104.71 (B97_Ann3). The GRAVY values ranged from − 0.534 (M126W_Ann1) to 0.232 (B97_Ann3). Except for B97_Ann3 and M126W_Ann1, all other annexin proteins are hydrophobic proteins. Furthermore, subcellular localization results revealed that the majority of the genes are located in mitochondria (58.3%), while the remaining genes are distributed in the cytoplasm (18.2%), chloroplasts (22.4%), and nucleus (0.6%) (Table S2.). Specifically, only two genes, CML247-Ann9 and M126W-Ann1, were found to be located in the nucleus. Four ZmAnns have the same localization results in 26 genomes, such as ZmAnn2, ZmAnn4, ZmAnn5, and ZmAnn12.
Phylogenetic and analysis of maize annexin genes
In order to investigate the phylogenetic relationships among maize annexin genes within maize genomes, a neighbor-joining (NJ) model tree was constructed. This tree was generated utilizing protein sequences derived from 12 distinctly identified maize annexin genes, in conjunction with 8 annexin genes sourced from Arabidopsis. The ZmAnn genes were categorized into six distinct subgroups (Fig. 1a). With the exception of group VI, each subgroup contains Ann proteins derived from Arabidopsis. Group I comprises three ZmAnn genes, while group VI consists of a single ZmAnn gene. The remaining four groups each contain two ZmAnn genes. Figure 1b illustrates the presence or absence of ZmAnn genes, across 26 maize varieties. Notably, ZmAnn7 and ZmAnn8 are absent in CML52, while ZmAnn4 is missing in Ki11. All other genes are either present or have collinear segments in all 26 genomes. This observation suggests that the Ann gene family is relatively conserved across different maize varieties, indicating a high degree of genetic stability and functional importance within the gene family.
ZmAnn is subjected to different selection pressures among maize varieties
Variations between the aligned sequences may lead to amino acid changes (nonsynonymous substitutions) or maintain the same amino acids (synonymous substitutions). Quantifying these changes provides insight into the extent of sequence alteration and the underlying evolutionary processes. The ratio of nonsynonymous substitutions per nonsynonymous site (Ka) to synonymous substitutions per synonymous site (Ks) serves as an indicator of the selective pressures acting on the protein. The Ka/Ks values of the majority of Ann genes are less than 1, suggesting that these genes have undergone purifying selection during the evolutionary process, thereby preserving their function. However, some genes in ZmAnn10 exhibited values greater than 1, indicating that they underwent positive selection in some materials, possibly contributing to adaptive evolution in certain maize varieties. (Fig. 2).
Cis-element analysis of ZmAnn
In the analysis of the 2 kb upstream sequences of the genes, the online tool PlantCARE database was utilized for cis-element prediction. This analysis identified in addition to the core cis elements, a total of 64 distinct cis-elements within the 2000 bp upstream region from the transcription start sites of the ZmAnn. These cis-elements play a significant role in various biological processes, including stress responses, hormone responses, metabolic regulation, as well as growth and development. All Ann genes contain varying numbers of light response elements, with the G-box being the most prevalent element, present in each gene. Among hormone-responsive cis-elements, the abscisic acid responsive element (ABRE) is the most abundant, with certain genes (B97-Ann2, Mo18W-Ann2, Oh43-Ann2 and Tx303-Ann2) containing as many as 15 ABRE elements (Fig. 3, Table. S3, S4). In addition, it contains various types of stress response components, such as ARE (anaerobic induction), GC-motif (anoxic specific inducibility), TC-rich repeats (defense and stress responsiveness), MBS (drought inducibility), LTR (low-temperature responsive). TCA-element (salicylic acid responsiveness). All ZmAnn9 and the majority of ZmAnn6 in the 26 genomes contain the LTR (Table. S4), suggesting that these two genes may associated with maize low-temperature stress tolerance. In the context of metabolic response processes, the O2-site, a cis-acting regulatory element involved in zein metabolism regulation, was the only element successfully identified. Notably, meristem expression elements (CAT-box) were identified in all ZmAnn8, while involved in seed-specific regulation were present in ZmAnn11, indicating potential roles in tissue-specific expression and developmental processes.
Multiple MYB binding sites (MBS, MBSI, MRE) were identified in the promoter regions of the 26 genomes (Table S3), suggesting that the Ann gene can interact with MYB transcription factors and participate in various processes, including photoresponse, drought induction, and regulation of flavonoid biosynthesis genes.
Structural variations can affect the expression pattern of ANN gene family
Abundant SVs (structural variations)were identified by aligning 26 high-quality maize genomes with reference genome (B73) in the study by Hufford et al. (2021)20. Compared with the reference genome, the main types of SVs are deletion (59), followed by insertion (14) and translocation (3), overlapped with gene regions of ZmAnn as well as upstream and downstream 2-kb regions (Fig. 4a Table. S5). SVs can impact the expression of genes by altering the composition or position of adjacent cis-regulatory sequences. We analyzed the correlation between expression values of genes containing and not containing SVs, and the results revealed a significant difference (p < 0.05) only in ZmAnn2 and ZmAnn11 (Fig. 4b). This finding suggests that the expression of ZmAnn2 and ZmAnn11 is influenced by the presence of SVs.
To investigate the impact of SVs on the gene structure within the Ann gene family, we examined the gene structures of Ann genes across 26 accessions using TBtools. The results indicate that the gene structure of most Ann is consistent with the reference genome B73. However, some Ann genes that overlap with SVs have undergone structural changes. For instance, in ZmAnn3, most genes contain 5 exons, whereas in the B97 accession, there is only one exon (Fig. 5a). A similar situation is observed in ZmAnn10, where the accessions M37W, P39, CML33, and CML103 contain two exons, with only 14 materials harboring all 10 motifs (Fig. 5b). Other genes exhibit varying degrees of structural variation, which may potentially result in functional changes.
Atypical Ann genes were widely expressed in maize
SVs are a significant factor contributing to changes in the protein spatial folding structure. To further investigate the impact of SVs on the Ann gene family, we quantified the number of typical (containing 4 annexin repeats) and atypical (containing 1–3 annexin repeats) Ann genes in the 26 genomes. In most of the materials, ZmAnn2, ZmAnn8, ZmAnn9, and ZmAnn11 were found to be typical genes, while ZmAnn1, ZmAnn6, ZmAnn7, and ZmAnn10 were identified as atypical genes (Fig. 6a). The remaining genes exhibited a coexistence of both types, a few genes with only collinear fragments cannot be counted.
The majority of the materials contain 6–8 typical genes, with only a few materials (CML103, M162W and Oh7B) having as few as 5 typical genes (Table. S6). To determine if there is a relationship between the number of Ann genes and their total expression levels among different varieties, we quantified the number and total expression of Ann genes in 26 materials (Fig. 6b). The number of Ann genes in each material is relatively consistent, ranging from 10 to 12, but the total expression levels vary significantly, with the highest RPKM at 374.14 (Ms71) and the lowest at 122.30 (Mo18W). Correlation analysis was conducted to examine the relationship between the number of typical genes, the number of atypical genes, and the total number of Ann genes and log2RPKM values. The results showed correlation coefficients (r) and significance test p-values of -0.048 and 0.818, respectively, for typical genes, 0.002 and 0.990 for atypical genes, and 0.003 and 0.985 for the total number of Ann genes (Fig.S1). These findings indicate that there is no significant correlation between these genes and their total expression levels.
Expression profiles of ZmAnn under stress conditions based on RNA-Seq data
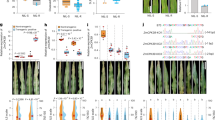
Analysis of cis-acting regulatory elements in the promoter regions of Ann genes suggests their potential involvement in various stress response pathways. To further investigate the expression patterns of Ann genes under various biotic and abiotic stress conditions, we obtained transcriptome data for biotic and non-stress treatments from public databases (Table S7). The stress conditions analyzed include Aspergillus flavus infection (Fig. 7a), aphid infestation (Fig. 7b), salt and mannitol treatments (Fig. 7c), drought treatment (Fig. 7d), cold stress (Fig. 7e) and heat stress (Fig. 7f). The expression levels of ZmAnn6 and ZmAnn8 were upregulated to different extents following Aspergillus flavus infection (Fig. 7a). Additionally, after two hours of aphid infestation, elevated expression levels were observed for ZmAnn5, ZmAnn6, ZmAnn7, and ZmAnn8 (Fig. 7b). Under salt and mannitol treatment, the expression levels of some Ann genes change in the roots and leaves. ZmAnn7 and ZmAnn10 are upregulated in the shoots under salt stress, while ZmAnn1, ZmAnn3, and ZmAnn6 are upregulated in the roots. Additionally, ZmAnn4 and ZmAnn9 are upregulated in the shoots of the knockout line R101 and the wild type under mannitol treatment (Fig. 7c). During drought treatment, the expression levels of some Ann genes are upregulated, such as ZmAnn2, ZmAnn5, ZmAnn8, and ZmAnn11 (Fig. 7d). ZmAnn4 exhibited higher expression levels during the early stages of cold treatment, specifically at 0.5 h, 1 h, and 2 h, while ZmAnn8 showed increased expression at later time points, namely 16 h and 24 h (Fig. 7e). These findings suggest that the Ann gene family plays a role in cold stress response at different time intervals. Under heat stress conditions, the expression levels of certain Ann genes in the thermosensitive maternal plants of An’nong 591 were found to be upregulated. Collectively, these results underscore the multifunctional role of the ZmAnn gene family in various stress response pathways, highlighting their importance in plant defense mechanisms.
Expression profiles of ZmAnns under diverse stresses. (a) Transcriptome analysis of 13 stages of Aspergillus flavus infection from PRJNA395908. (b) Transcriptome analysis of six stages of aphid infection from PRJNA295410. (c) Expression patterns of maize ZmCHB101 gene knockout under simulated salt and mannitol treatments from PRJNA308155. Both ZmCHB101 knockout lines and wild-type plants were subjected to salt and mannitol treatments in leaves and roots. (d) Transcriptome analysis of the drought-tolerant maize inbred line AC7643, the drought-sensitive inbred line AC7729/TZSRW, and their two recombinant inbred lines (RIL208 and RIL64) under well-watered and drought stress conditions from PRJNA506720. (e) Transcriptome analysis of maize under cold stress from PRJNA344653. (f) Transcriptome analysis of maize hybrid An’nong 591 and its parental lines under heat stress from PRJNA308155.
Co-expression network and GO enrichment analysis of ZmAnn under cold stress condition
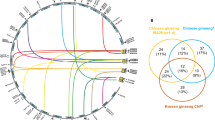
Cold reduces both the seed germination rate and seedling vigor, as exposure to low temperatures during the water absorption phase (imbibition) impairs cell membrane permeability, resulting in the loss of cellular components21. To further elucidate the role of ZmAnn in response to cold stress, we obtained expression data from Xue et al. (2021)22 on various maize tissues subjected to cold stress treatment (Table.S8) for Weighted Gene Co-Expression Network Analysis(WGCNA). This analysis revealed 19 co-expression modules, with gene counts ranging from 157 to 2810 (Fig.S2, Table.S9). Subsequently, correlation analysis was conducted on co-expressed genes utilizing diverse treatment information. Modules exhibiting higher correlation coefficients were identified in three distinct tissues: blue, brown, and turquoise (Fig.S3). The genes in these modules have similar expression patterns. A total of 4 Ann genes were found to be involved in these three co-expression modules. Specifically, ZmAnn2 and ZmAnn7 were clustered in the turquoise module, which is primarily associated with stress response processes, such as cellular protein modification, carboxylic acid metabolism, ubiquitin-like protein transferase activity, and organic acid metabolism (Fig. 8a). In addition, ZmAnn9 was also found to be clustered in the blue module, which is mainly related to various biosynthetic processes, such as amide biosynthesis, peptide biosynthesis, and organic nitrogen compound biosynthesis (Fig. 8b). ZmAnn6 is located in the brown module related to carboxylic acid metabolism, response to abiotic stimuli, and cofactor metabolism processes (Fig. 8c). Notably, ZmAnn2 and ZmAnn7 are co-expressed with three genes (Table.S10), among which LRR-RLKs (LRR-like receptor kinases) FEI1 is associated with cellulose. The loss-of-function mutant of FEI1 exhibits increased sensitivity to humidity gradients and decreased tolerance to osmotic stress23. Among the other co expressed genes, SR34A targets all alternative splicing event types, including in RNAs encoding known determinants of ABA sensitivity, to prevent ABA-responsive splicing in germinated seeds24. Expression of JMJ25 was induced significantly by darkness, suggesting that JMJ25 might be involved in stress responses25. FtsH2 may be involved in cold stress response processes by affecting the ABA-dependent signaling pathways26.
Co-expression networks based on transcriptome data under different cold stress conditions, constructed using the WGCNA method. (a) Co-expression network of ZmAnn2 and ZmAnn7 and Gene Ontology (GO) analysis of genes in the turquoise module; (b) Co-expression network of ZmAnn9 and GO analysis of genes in the blue module; (c) Co-expression network of ZmAnn6 and GO analysis of genes in the brown modul.
Validation of gene expression by RT-qPCR
To further confirm the response of ZmAnn genes to cold stress during maize seedling development, B73 seedlings were subjected to 24 h of cold stress at 4 °C, and the expression changes of six Ann genes (ZmAnn2, ZmAnn4, ZmAnn6, ZmAnn7, ZmAnn8 and ZmAnn9) were observed. The expression levels of ZmAnn2, ZmAnn4, ZmAnn6 and ZmAnn8 under cold stress were significantly higher than the control, whereas ZmAnn7 and ZmAnn9 showed no significant difference (Fig. 9). These results suggest that these four genes may play a role in the response of maize seedlings to cold stress.
Discussion
The annexin gene family in maize was first identified through the B73 reference genome (B73 RefGen_v3)27. Recently, a pan-genome consisting of 26 high-quality maize genomes has been published, which provides a more precise assembly and annotation of the B73 reference genome20. Research has demonstrated that a single reference genome is inadequate to capture the full extent of genetic diversity within a species18,28. Consequently, it is essential to identify and characterize Ann genes, based on the maize pan-genome. We utilized the maize pan-genome and identified 12 ZmAnns, including 9 core genes and 3 near-core genes. In the reference genome B73, we identified 11 Ann genes and one collinear fragment. Previous studies had identified 12 Ann genes in the B73 reference genome. Upon comparison, we found that GRMZM2G134502, which was present in the previous studies27, does not have convertible gene model IDs in subsequent versions of the B73 reference genome. This may be due to issues with early genome assembly. Regarding ZmAnn12, only collinear fragments were found in the three genomes (B73, NC350, M126W). This lack of clear annotation makes it more likely for ZmAnn12 to be overlooked when using B73 as the reference genome. Despite the variations in the number of ZmAnns, the total gene expression did not exhibit correlation. This could be attributed to the fact that certain members within the gene family have similar functions, which can compensate for each other to maintain normal physiological processes29.
SV is a common type of genetic variation, and changes in conserved domains caused by SV can potentially lead to alterations in gene structure, expression, and conserved domains. In the CSSL population derived from japonica rice NIP and indica rice 9311, a 42 kb insertion/deletion SV was identified around the qPH8.2 locus, which has an impact on plant growth30. Studies in peach trees have demonstrated that SV downstream of the OVATE gene can regulate fruit characteristics and leaf development31. This study found that SVs affects the expression of ZmAnn2 and ZmAnn11, which can be further studied. SVs can also alter the number of exons, such as ZmAnn3 and ZmAnn10, with the reference genome identifying five exons, but 1–4 exons appearing in some materials, leading to the emergence of atypical Ann genes. However, these atypical genes may also have certain functions. For example, the atypical E2F gene AtE2Ff is part of a critical regulatory network required for the differentiation of certain cell types during post-embryonic growth and development in Arabidopsis32. The atypical CC-NBS-LRR protein Pb1 in rice is known as a gene for durable resistance to rice blast, but its NBS domain lacks the P-loop essential for the function of R proteins33. Therefore, this study identified several atypical Ann genes in maize, which enhances our understanding of the functional roles of Ann in maize.
The cis-elements present in plant annexins play a crucial role in responding to environmental stress and signaling pathways during plant growth and development, and have been widely studied in various plants2,3,4. A total of 64 different cis-elements were also identified within the 2000 bp upstream region of the ZmAnn transcription start site. These cis-elements play important roles in various biological processes, including stress response, hormone response, metabolic regulation, and growth and development. All ZmAnns harbor ABRE elements, with B97-Ann2, Mo18W-Ann2, Oh43-Ann2, and Tx303-Ann2 containing up to 15 ABRE elements. Under abiotic stress conditions, ABA can trigger the activation of ABFs, which subsequently bind to ABRE elements, initiating the transcription of downstream genes to aid plants in withstanding stress34,35. AtbZIP1 is involved in the regulation of ABA signaling by binding to ABRE, thereby modulating the expression of ABA-responsive genes and contributing to the stress response in plants36. All ZmAnn6 and ZmAnn9 in 26 genomes contain LTR elements related to cold stress, indicating that these two genes may be involved in maize’s response to cold. Transcription factors (TFs) are involved in regulating various target genes responsible for plant adaptation and tolerance37. This study identified several transcription factor-related elements, including DRE, MYB, MYC, MBS, and MRE, with all ZmAnn members harboring MYB and MYC elements. DRE elements are present in all ZmAnn9; the drought-inducible MBS is found in ZmAnn8; the MBSI involved in the regulation of flavonoid biosynthesis genes is located in ZmAnn3 and ZmAnn6 in most materials. This suggests that Ann genes may bind to specific elements to participate in various life processes.
Maize is highly susceptible to cold damage, as it is a crop that thrives in high-temperature environments due to its tropical/subtropical origin. Cold stress can negatively impact seed germination, seedling development, and growth during the seedling stage, ultimately resulting in decreased grain yield38. To further elucidate the role of ZmAnns in response to cold stress, this study constructed co-expression networks using transcriptome data from different maize tissues under cold stress. Highly correlated modules were found in the shoot, root, and top-crown, specifically brown, turquoise, and blue. GO enrichment analysis found that these three modules are mainly related to stress response and secondary metabolite biosynthesis processes. A total of 4 Ann genes were found to participate in these three modules, and the interaction network of these genes was identified. The network contains multiple genes related to cold stress, such as GDSL lipases, squalene monooxygenase (SE), glycine-rich RNA-binding protein (RZ). Abiotic stress in certain plant species can induce the expression of GDSL lipases, suggesting that they play a protective role against abiotic stress39. SE1 is one of key regulators of ginsenoside biosynthesis in response to cold stress40. The transcription levels of AtRZ-1a, AtRZ-1b, and AtRZ-1c in Arabidopsis significantly increase due to cold stress treatment41. Subsequently, the expression levels of the six Ann genes under cold stress during the seedling stage were validated. The expression of ZmAnn2, ZmAnn4, ZmAnn6 and ZmAnn8 showed a significant increase. This comprehensive analysis helps to identify potential regulatory networks and key genes involved in the cold stress response.
Materials and methods
Identification of annexin genes in maize
The 26 maize genomes were obtained from a study by Hufford et al.20. The hidden Markov model (HMM) profiles of the primary annexin domain (PF00191) was retrieved from the Pfam database (http://pfam.xfam.org/). The hidden Markov model (HMM) profiles corresponding to the primary annexin domain (PF00191) were obtained from the Pfam database (http://pfam.xfam.org/). Subsequently, these profiles were employed to search against the protein sequences derived from the 26 maize genomes using HMMER v3.3.242, with a threshold of e < 1E-5. The Ann candidates were submitted to NCBI-CDD website (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)to confirm the existence of annexin domains. Utilizing the online tool ExPASy (http://web.expasy.org/protparam/), we investigated the fundamental molecular characteristics of proteins, including molecular weight (MW), theoretical isoelectric point (pI), and overall average hydrophilicity (GRAVY).
Phylogenetic analysis and presence/absence variation of ZmAnn gene family
Multiple sequence alignments were performed using the ClustalW tool to analyze the evolutionary relationships of the maize and Arabidopsis annexin genes, and the tree was constructed by the Maximum Likelihood (ML) method with MEGA X (https://www.megasoftware.net/show_eua), based on the Bayesian Information Criterion (BIC) for model selection. A total of 1000 bootstrap replications were performed to evaluate node support. The final graphics were generated using iTOL v6 (https://itol.embl.de/). The presence or absence of each Ann in the 26 accessions was visualized using a heatmap generated with the ComplexHeatmap package43.
Ka/Ks calculation
The protein and coding sequence (CDS) sequences of ZmAnn genes in 26 maize genomes were compared, and Ka/Ks values were calculated using the KaKs Calculator 2.044. The R packages ggridges and ggplot2 were used to create the Ridgeline plot of ka/ks values.
Gene structure and cis-acting elements of ZmAnns
The conserved motifs were identified using MEME V5.4.1 (https://meme-suite.org/meme/tools/meme), with the number of motifs set to 10. Genome annotation files were download from http://maize-pangenome.gramene.org to identified the gene structure. The 2-kb sequences upstream of Ann genes were analyzed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Analysis of the expression of ZmAnns that overlap with structural variants (SVs)
The SVs locations in each variety and the gene expression data from 26 accessions were obtained from Hufford et al.14. The expression data included the gene expression levels across 10 tissue types in each genome: (1) primary root at six days after planting; (2) shoot and coleoptile at six days after planting; (3) base of the 10th leaf at the Vegetative 11 (V11) growth stage; (4) middle of the 10th leaf at the V11 growth stage; (5) tip of the 10th leaf at the V11 growth stage; (6) meiotic tassel at the Vegetative 18 (V18) growth stage; (7) immature ear at the V18 growth stage; (8) anthers at the Reproductive 1 (R1) growth stage; (9) endosperm at 16 days after pollination; and (10) embryo at 16 days after pollination. For the subsequent calculations, the sum of the expression levels across all tissues in each accession was used. An in-house Perl script was employed to assess whether the ZmAnns overlapped with SVs in each variety. If a ZmAnn was found to overlap with SVs, its expression data in that variety were classified as the expression data of genes associated with SVs; otherwise, they were categorized as the expression data of genes without SVs. Pearson correlation coefficients were computed between the presence of SVs overlapped with genes and gene expression levels. ZmAnns with p < 0.05 were deemed to have significantly altered expression levels due to SVs.
Expression profile analysis of ZmAnns based on RNA-seq data
The expression profile analysis included projects from SRA database (https://www.ncbi.nlm.nih.gov/sra/?term=) such as PRJNA294848, PRJNA295410, PRJNA308155, PRJNA343268, PRJNA395908 and PRJNA506720. Raw data (raw reads) in FASTQ format were first processed with Trimmomatic v0.3945 to filter adapter sequences. These clean reads were then mapped to the reference genome sequence. Only reads with a perfect match or one mismatch were further analyzed and annotated based on the reference genome. Kallisto v0.50.146 to align reads to the B73 reference genome (https://download.maizegdb.org/Zm-B73-REFERENCE-NAM-5.0/) and count them, obtain expression data. The resulting FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values were used to quantify gene expression, which accounts for both gene length and sequencing depth. To prepare the data for downstream analysis, log2 normalization was applied to the FPKM values, adding 1 to avoid undefined values for genes with zero expression. Additionally, the data were standardized by applying z-score normalization, which centers and scales the data to account for differences in sequencing depth and expression across samples. The normalized and log2-transformed expression data were visualized using the ComplexHeatmap package in R.
Co-expression network analysis of ZmAnns
The co-expression network was constructed using a set of PRJNA705456 for expression profiling analysis, which encompassed 26 samples from various tissues, including leaves, roots, and top-crown. These samples were subjected to three distinct low-temperature treatments: 4℃ shoot + 4℃ root, 4℃ shoot + 26℃ root, 26℃ shoot + 0℃ root. The Weighted Gene Co-expression Network Analysis (WGCNA) was conducted using the TBtools software (Version 2.085)47. Subsequently, the main modules of ZmAnns were identified and subjected to a Gene Ontology (GO) enrichment analysis. The GO dataset was retrieved from the KOBAS database (http://bioinfo.org/kobas), and R packages were employed for data visualization.
Growth conditions and cold stress treatment
In this study, we used the maize inbred line B73. Forty B73 seeds were sterilized in a 1% sodium hypochlorite solution for 5 min and then placed on moist germination paper, with an additional sheet of moist paper used as a cover. The two sheets of germination paper were rolled together and positioned vertically in a sealed plastic bag, where they were incubated at 22 °C for 7 days. After germination, the seeds were transferred to two pots containing a substrate composed of peat, pine needles, and yellow clay, and cultured at 22 °C for an additional 5 days. One of the pots was then moved to 4 °C for 1 day. Leaves from three plants in each pot were collected, frozen in liquid nitrogen, and stored at -80 °C for RNA extraction. Total RNA was extracted from the leaves using the Plant RNA Extraction Kit-V1.5 (Chengdu Biofit Biotechnologies Co., Ltd., Chengdu, China). The concentration and purity of the RNA were assessed using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE).
Validation of gene expression by RT-qPCR
Actin was utilized as the reference gene to assess relative expression values, and quantitative real-time PCR (qPCR) was conducted using the CFX96 PCR System. The 10 µl reaction mixture consisted of 5 µl of 2× TB Green Premix Ex Taq II (Tli RNaseH Plus), 0.4 µl of each 10 µM primer, 1 µl of cDNA, and sterile double-distilled water to achieve the final volume. The qRT-PCR cycling conditions included an initial step at 95 °C for 3 min, followed by 39 cycles of 95 °C for 10 s and 58 °C for 30 s. After the final qRT-PCR cycle, a melt curve was generated by heating to 95 °C for 5 s and then gradually increasing the temperature from 65 °C to 95 °C at 0.5 °C increments. RT-PCR primers were designed using Primer Premier Software (version 5.0) and are provided in Table S11. Statistical analysis of the qPCR results was performed using a t-test with GraphPad Prism (v7.04), and a bar graph was generated to display the expression levels.
Data availability
The data supporting the results of this study can be obtained in the Supplementary Materials of this article and can be obtained from the corresponding author upon reasonable request.
References
Laohavisit, A., Davies, J. M. & Annexins Coding Decoding Calcium Signals Plants 111–128 (2011).
Clark, G. B., Morgan, R. O., Fernandez, M. P. & Roux, S. J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 196, 695–712 (2012).
Saad, R. B. et al. Insights into plant annexins function in abiotic and biotic stress tolerance. Plant Signal. Behav. 15, 1699264 (2020).
Mortimer, J. C. et al. Annexins: multifunctional components of growth and adaptation. J. Exp. Bot. 59, 533–544 (2008).
Shani, U. & Dudley, L. Field studies of crop response to water and salt stress. Soil Sci. Soc. Am. J. 65, 1522–1528 (2001).
Sanghera, G. S., Wani, S. H. & Hussain, W. Singh, N. Engineering cold stress tolerance in crop plants. Curr. Genom. 12, 30 (2011).
Fahad, S. et al. Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 8, 1147 (2017).
Tang, W. et al. Down-regulating annexin gene GhAnn2 inhibits cotton fiber elongation and decreases Ca2+ influx at the cell apex. Plant Mol. Biol. 85, 613–625 (2014).
Vellosillo, T. et al. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant. Cell. 19, 831–846 (2007).
Zhu, J. et al. Annexin5 plays a vital role in Arabidopsis pollen development via Ca2+-dependent membrane trafficking. PloS One. 9, e102407 (2014).
Huh, S. M. et al. Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol. 51, 1499–1514 (2010).
Li, X., Zhang, Q., Yang, X., Han, J. & Zhu, Z. OsANN3, a calcium-dependent lipid binding annexin is a positive regulator of ABA-dependent stress tolerance in rice. Plant Sci. 284, 212–220 (2019).
Shen, F. et al. Characterization of Annexin gene family and functional analysis of RsANN1a involved in heat tolerance in radish (Raphanus sativus L). Physiol. Mol. Biology Plants. 27, 2027–2041 (2021).
Liu, Q. et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 40, e104559 (2021).
Shen, C. et al. Knock out of the annexin gene OsAnn3 via CRISPR/Cas9-mediated genome editing decreased cold tolerance in rice. J. Plant. Biology. 60, 539–547 (2017).
Que, Z., Lu, Q., Li, Q. & Shen, C. The rice annexin gene OsAnn5 is involved in cold stress tolerance at the seedling stage. Plant. Direct. 7, e539 (2023).
He, F. et al. Maize annexin genes ZmANN33 and ZmANN35 encode proteins that function in cell membrane recovery during seed germination. J. Exp. Bot. 70, 1183–1195 (2019).
Bayer, P. E., Golicz, A. A., Scheben, A., Batley, J. & Edwards, D. Plant pan-genomes are the new reference. Nat. Plants. 6, 914–920 (2020).
Li, W. et al. Plant pan-genomics: recent advances, new challenges, and roads ahead. J. Genet. Genomics. 49, 833–846 (2022).
Hufford, M. B. et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 373, 655–662 (2021).
Hussain, H. A. et al. Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front. Plant Sci. 9, 393 (2018).
Xue, C. et al. Tissue-level transcriptomic responses to local and distal chilling reveal potential chilling survival mechanisms in maize. J. Exp. Bot. 72, 7610–7625 (2021).
Chang, J. et al. Defects in the cell wall and its deposition caused by loss-of-function of three RLKs alter root hydrotropism in Arabidopsis thaliana. Nat. Commun. 15, 2648 (2024).
Palusa, S. G., Ali, G. S. & Reddy, A. S. Alternative splicing of pre-mRNAs of Arabidopsis serine/arginine-rich proteins: regulation by hormones and stresses. Plant J. 49, 1091–1107 (2007).
Fan, D. et al. Histone H3K9 demethylase JMJ25 epigenetically modulates anthocyanin biosynthesis in poplar. Plant J. 96, 1121–1136 (2018).
Zhu, X. et al. Genome-wide identification and characterization of filamentation temperature-sensitive H (FtsH) genes and expression analysis in response to multiple stresses in Medicago truncatula. Mol. Biol. Rep. 50, 10097–10109 (2023).
Zhang, Z., Li, X., Han, M. & Wu, Z. Genome-wide analysis and functional identification of the annexin gene family in maize (‘Zea mays’ L). Plant. Omics 8 (2015).
Li, R. et al. Building the sequence map of the human pan-genome. Nat. Biotechnol. 28, 57–63 (2010).
Rodriguez-Leal, D. et al. Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat. Genet. 51, 786–792 (2019).
Sun, W., Sun, Q., Tian, L., Sun, Y. & Yu, S. A structure variation in qPH8. 2 detrimentally affects plant architecture and yield in rice. Plants 12, 3336 (2023).
Guan, J. et al. Genome structure variation analyses of peach reveal population dynamics and a 1.67 mb causal inversion for fruit shape. Genome Biol. 22, 1–25 (2021).
Ramirez-Parra, E., Loépez-Matas, M. A., Frundt, C. & Gutierrez, C. Role of an atypical E2F transcription factor in the control of Arabidopsis cell growth and differentiation. Plant. Cell. 16, 2350–2363 (2004).
Hayashi, N. et al. Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J. 64, 498–510 (2010).
Yamaguchi-Shinozaki, K. & Shinozaki, K. Organization of cis-acting regulatory elements in osmotic-and cold-stress-responsive promoters. Trends Plant Sci. 10, 88–94 (2005).
Xu, L. et al. Comprehensive analyses of the annexin gene family in wheat. BMC Genom. 17, 1–18 (2016).
Xiao-Li, S. et al. Arabidopsis bZIP1 transcription factor binding to ABRE cis-element regulates abscisic acid signal transduction. Acta Agron. Sinica. 37, 612–619 (2011).
Riechmann, J. L. et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290, 2105–2110 (2000).
Zhou, X., Muhammad, I., Lan, H. & Xia, C. Recent advances in the analysis of cold tolerance in maize. Front. Plant Sci. 13, 866034 (2022).
Liu, D. et al. The GDSL lipase CpGLIP1 from Chimonanthus praecox improves drought and cold tolerance in Arabidopsis and Poplar. Ind. Crops Prod. 215, 118636 (2024).
Zhang, T., Gao, Y., Han, M. & Yang, L. Changes in the physiological characteristics of Panax ginseng embryogenic calli and molecular mechanism of ginsenoside biosynthesis under cold stress. Planta 253, 1–23 (2021).
Kim, Y. O., Kim, J. S. & Kang, H. Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J. 42, 890–900 (2005).
Mistry, J., Finn, R. D., Eddy, S. R., Bateman, A. & Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 41, e121–e121 (2013).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Wang, D., Zhang, Y., Zhang, Z., Zhu, J. & Yu, J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteom. Bioinf. 8, 77–80 (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 (15), 2114–2120 (2014).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34 (5), 525–527 (2016).
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 13, 1194–1202 (2020).
Acknowledgements
We sincerely thank Professor Caiming Gou, Professor Ning Wang, and Professor Huibo Luo for their valuable suggestions during the manuscript revision process. Furthermore, we appreciate Professor Gou Caiming’s financial support for the publication of this work.
Author information
Authors and Affiliations
Contributions
Xin Liu conducted most of the analysis and wrote the manuscript; Minghu Zhang, Xin Zhao, and Mang Shen performed part of the data collection and analysis; Ruizhang Feng provided guidance on manuscript writing; Qin Wei designed the experiments and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Zhang, M., Zhao, X. et al. The evolution, variation and expression patterns of the annexin gene family in the maize pan-genome. Sci Rep 15, 5711 (2025). https://doi.org/10.1038/s41598-025-89119-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89119-5