Abstract
IgA nephropathy (IgAN) imposes a substantial burden of illness and death. However, a systematic evaluation of the impact of the COVID-19 pandemic on the incidence and pathology of IgAN has yet to be performed. In this study, we analyzed kidney biopsy results from two medical institutions, covering the timeframe from January 2016 to May 2023. We conducted statistical analyses on various glomerular diseases according to their corresponding pathological diagnoses. Our objective was to compare the incidence of different glomerular diseases before and during the COVID-19 pandemic. The study focused on variations in the incidence of IgAN, and collected clinical and pathological data to assess pathological changes using the Oxford Classification (MEST-C). The findings revealed a significant increase in the incidence of IgAN during the COVID-19 pandemic, from 39.9% prior to the pandemic to 46.3% during it, representing a net increase of 6.4% (P < 0.001). Although clinical manifestations and laboratory indicators of disease activity in IgAN patients remained consistent over both periods, observable changes occurred in pathological features. Specifically, the proportions of M1 and E1 lesions according to the Oxford classification significantly increased during the pandemic, with odds ratios of 11.764 (95% CI 5.583–24.789, P < 0.001) and 1.718 (95% CI 1.114–2.649, P = 0.014), respectively. Our results indicate that the incidence of IgAN has risen during the COVID-19 pandemic, along with exacerbated renal damage and elevated proportions of M1 and E1 in the Oxford classification.
Similar content being viewed by others
Introduction
Since the 2019 coronavirus disease (COVID-19) pandemic, it is increasingly recognized that the virus may have broader effects on human health beyond the respiratory system. One of the affected organs is the kidney, with reports suggesting a possible association between COVID-19 and kidney damage, including IgA nephropathy (IgAN)1,2,3,4. IgAN is the most common glomerulonephritis globally5, the typical clinical presentation involves gross or microscopic hematuria in children or young adults resulting from upper respiratory tract infections6, up to 20–40% of IgAN patients develop renal failure within 10 to 20 years after diagnosis7, which is related to significant morbidity and mortality8. However, the role of COVID-19 in the onset and progression of IgAN remains unclear. Some studies have predicted that IgAN might become a severe complication of COVID-19 and COVID-19 mRNA vaccines, potentially leading to an increased incidence in the future9. However, there is insufficient evidence to support this hypothesis. Further investigation into changes in IgAN incidence during the COVID-19 pandemic could provide valuable insights into the relationship between viral infection and this glomerular disease.
The clinical diagnosis of IgAN involves histopathological examination of kidney biopsy tissue, revealing IgA and related immune complex deposition in the glomerular mesangium. The primary pathological process involves mucosal immune dysfunction10. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a mucosal-targeting virus, can trigger a strong mucosal immune response, leading to excessive IgA secretion11. Consequently, COVID-19 infection and/or vaccination could be potential triggers for IgAN. In addition, currently proposed mechanisms of renal injury include effects on the renin-angiotensin-aldosterone system, hemodynamic instability, coagulation dysfunction, and cytokine storm12,13. However, these all require associations with pathological changes to inform the mechanism hypothesis, and to date there is limited information available on the pathological characteristics of IgAN patients during the COVID-19 pandemic.
In this study, we aim to investigate epidemiological trends in IgAN incidence during the COVID-19 pandemic by analyzing a large patient cohort, and collect clinical and pathological data to assess pathological changes using the Oxford Classification (MEST-C)14.
Methods
Study design and population
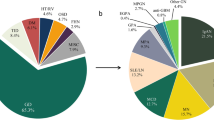
A retrospective study was conducted using renal biopsy data from two hospitals, Shenzhen Second People’s Hospital and Shenzhen People’s Hospital. This retrospective study was carried out using the opt-out method for the case series of our hospitals. The Ethics Committee of Shenzhen Second People’s Hospital approved the study, conducted in accordance with the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards. The Institutional Review Board, also known as the Ethics Committee of Shenzhen Second People’s Hospital, waived the requirement for informed consent because the study was retrospective. Patients who underwent renal biopsy at the aforementioned hospitals between 1 January 2016 and 5 May 2023 were included, with the following inclusion criteria: (1) first-time kidney puncture patients; (2) patients with a clear pathological diagnosis and complete pathological data. Exclusion criteria were as follows: (1) patients lacking a renal pathological diagnosis; (2) patients with incomplete pathological data; (3) patients who underwent repeated renal biopsies. The World Health Organization (WHO) declared COVID-19 a global pandemic on March 11, 2020, and announced on May 5, 2023 that COVID-19 no longer constituted a Public Health Emergency of International Concern. To this end, we conducted a statistical analysis of pathological diagnoses for various glomerular diseases, comparing their incidence before (Pre-COVID-19, January 1, 2016 to March 10, 2020) and during the COVID-19 pandemic (COVID-19, March 11, 2020 to May 5, 2023). These diseases included minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), IgA nephropathy (IgAN), lupus nephritis (LN), and diabetic nephropathy (DN). The study focuses on examining changes in the incidence and pathological features of IgAN.
Regional COVID-19 overview
The population of Shenzhen during the study period was approximately 17.6 million people. The first confirmed COVID-19 case in Shenzhen was reported on January 19, 2020. Between January 19 and December 31, 2020, a total of 670 confirmed COVID-19 cases, including asymptomatic cases, were reported. The number of cases rose to 757 in 2021 and surged to 9,758 cases between January 1 and December 16, 2022. Daily updates on the epidemic were discontinued after December 18 (Data source: Shenzhen Municipal Health Commission, https://www.sz.gov.cn/szzt2010/yqfk2020/qktb/). The COVID-19 pandemic can be divided into two phases: Pre-vaccination and Post-vaccination, based on the implementation of the vaccination program. Mass COVID-19 vaccinations in Shenzhen began on March 27, 2021. By the end of 2022, the full vaccination rate reached 108.08% of the target population, and 93.65% of the population had received booster shots. A vaccination rate exceeding 100% indicates the inclusion of non-permanent residents in the vaccination campaign.
Pathological analysis and categorization
Renal biopsy specimens were examined using light microscopy with hematoxylin-eosin, periodic acid-Schiff, periodic acid-silver methenamine, Masson staining, and direct immunofluorescence staining (for IgA, IgG, IgM, Fib, C1q, and C3), as well as electron microscopy. For patients with suspected damage from specific components, additional special stains, such as Congo red staining, were performed. The pathological diagnosis followed the WHO histological classification of glomerular diseases, revised in 1982 and 199514,14. If a patient has two or more types of kidney disease, each type is classified separately.
Pathological grading of IgAN
IgAN is graded according to the Oxford classification, which evaluates each specimen based on five key pathological features, collectively referred to as the MEST-C score14. Mesangial hypercellularity was classified as M0 if more than 50% of the glomeruli had fewer than three cells per mesangial area, or as M1 if more than 50% of glomeruli had more than three cells per mesangial area. Endocapillary hypercellularity was categorized as absent (E0) or present (E1). Segmental glomerulosclerosis was categorized as absent (S0) or present (S1). The tubular atrophy/interstitial fibrosis score was determined by the ratio of tubular atrophy to interstitial fibrosis within the total interstitium and classified as T0 (0–25%), T1 (26–50%), or T2 (> 50%). The crescent score was determined by the ratio of glomeruli with cellular or fibrocellular crescents to the total number of glomeruli and classified as C0 (0%), C1 (> 0% and < 25%), or C2 (≥ 25%). Due to the low number of patients with T2 and C2, these categories were combined with T1 and C1, respectively. All histologic findings were derived from pathology reports of renal biopsies, as evaluated by renal pathologists.
Clinical data collection of IgAN patients
Data collection includes inpatient data at the time of renal biopsy. Patient demographic information is collected, including age, sex, and body mass index (BMI). Clinical manifestations are recorded, including hypertension, edema, recurrent macroscopic hematuria, microscopic hematuria, nephrotic syndrome, acute renal failure, and chronic renal failure. Laboratory parameters are collected, including hemoglobin (Hb), serum albumin (Alb), serum creatinine (Scr), estimated glomerular filtration rate (eGFR), 24-hour urine total protein (24-h UTP), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum immunoglobulin G (IgG), serum immunoglobulin M (IgM), serum immunoglobulin A (IgA), complement C3, and complement C4. Renal pathological features are documented, primarily including Oxford classification and Lee’s pathological grade, as detailed in the ‘Pathological grading of IgAN’ section. Additionally, taking renal biopsy as the starting point of treatment, collect data on the patient’s medications, including prednisone, renin-angiotensin system inhibitors (RASI), immunosuppressants, iron supplements, and erythropoietin. Among them, RASI refers to medications such as angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs). Immunosuppressants include drugs like cyclophosphamide, cyclosporine, tacrolimus, and mycophenolate mofetil.
Statistical analysis
All analyses were conducted using SPSS 29.0 (IBM) and Empower (R) (http://www.empowerstats.com, X and Y solutions, Inc, Boston, MA, USA). Descriptive statistics summarized the demographic and clinical characteristics of the study population. Continuous data are reported as mean ± standard deviation (SD) if they meet the normality criterion (Kolmogorov-Smirnov test); otherwise, they are reported as medians and interquartile range (IQR). Categorical variables are presented as counts and percentages. Variables between two groups were compared using an unpaired t-test if normally distributed; otherwise, the Mann-Whitney U test was used for non-normally distributed continuous variables. The Pearson’s chi-square test was used to compare categorical variables. Respective glomerular disease groups were compared to assess changes in incidence rates before and during the COVID-19 pandemic, using Bonferroni correction for multiple comparisons. Binary Logistic regression models were used to evaluate the association between prespecified factors and the COVID-19 pandemic. In the initial model selection for the multivariate analysis, covariates with P-values < 0.1 from univariate analyses, or those deemed important, were included, with age and sex treated as fixed factors. The final model was determined by retaining covariates with P-values < 0.05. All P-values were two-sided, and significance was set at < 0.05.
Results
Demographics and characteristics of the study population
Between 1 January 2016 to 5 May 2023, 2,450 kidney biopsy patients from two medical institutions. After excluding 14 cases of repeat kidney biopsy and 25 cases of patients lacking renal histopathological diagnosis, a total of 2,411 kidney biopsy patients were finally included in the observational study (Supplementary Fig. 1). During the early phase of the COVID-19 pandemic (January to February 2020), the number of kidney biopsies sharply declined to zero (Fig. 1). Starting in March 2020, the number of kidney biopsies gradually recovered and reached pre-pandemic levels by the end of the year. Between 2021 and 2022, the monthly number of biopsies remained stable and above pre-pandemic baseline levels, averaging 30–40 biopsies per month. In early 2023, during the later phase of the COVID-19 pandemic, the number of kidney biopsies showed a relatively significant upward trend. Table 1 presents the demographic characteristics of the study population and the distribution of various glomerular diseases. During the Pre-COVID-19 period, 1,161 kidney biopsies were conducted, while 1,250 biopsies were performed during the COVID-19 period. Out of the total cases diagnosed with glomerular diseases (MCD, FSGS, MN, IgAN, LN, DN), 2074 cases were recorded, 991 during the Pre-COVID-19 period and 1,083 during the COVID-19 period. There were no statistically significant differences in patient age and gender before and during the pandemic. Among the glomerular diseases observed, the incidence rates of MCD, FSGS, LN, and DN remained stable. However, significant changes in the incidence rates of MN and IgAN were observed (P = 0.026, P = 0.001).
Trends in incidence of MN and IgAN
The annual incidence rates of MN and IgAN, as well as the SARS-CoV-2 infection rates in Shenzhen (Fig. 2). Prior to the onset of COVID-19, the annual incidence rates of MN and IgAN fluctuated slightly but remained stable. After the emergence of COVID-19 in 2019, the annual incidence rate of MN decreased, while the incidence rate of IgAN increased compared to the pre-pandemic period. Following the first reported case of COVID-19 in Shenzhen on January 19, 2020, the SARS-CoV-2 infection rate has increased progressively each year. In 2021, the infection rate increased slightly (from 0.0038 to 0.0043%) compared to 2020, and then rose sharply in 2022, reaching 0.0552%. A temporal association exists between the SARS-CoV-2 infection rate and the incidence of IgAN, as demonstrated more clearly in the monthly analysis (Fig. 3). During the pandemic, the incidence rate of MN decreased by 3.6% compared to the pre-pandemic period (P = 0.026, Fig. 4). However, after the Bonferroni correction, the change in incidence rate did not reach a significant p-value (P > 0.05/2). There was a significant increase in the incidence rate of IgAN (P < 0.05/2), but there was no significant change in the overall incidence rate of glomerular diseases (P = 0.364). Multivariate analysis employing binary Logistic regression reveals a significant association between the COVID-19 pandemic and an increased incidence of IgAN (P = 0.033, Supplementary Fig. 2). In contrast, no significant association was observed between the COVID-19 pandemic and MN.
Annual incidence trends of MN, IgAN, and SARS-CoV-2 infection. The trends of MN and IgAN incidence from 2016 to 2022 are presented, with data for 2023 excluded due to the study period ending on May 5, 2023. The data on SARS-CoV-2 infections were sourced from the Shenzhen Municipal Health Commission, which reported the first confirmed case on January 19, 2020, and ceased daily updates on the pandemic on December 18, 2022. IgAN, IgA nephropathy; MN, membranous nephropathy; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Monthly incidence of IgAN before and during the COVID-19 pandemic. The trend of IgAN monthly incidence from 2020 to 2023 is presented, with data for May 2023 excluded due to the study period ending on May 5, 2023. Pre-pandemic monthly incidence of IgAN is the average of the preceding years. Avg., average; IgAN, IgA nephropathy; Mo., month.
Incidence of MN and IgAN before and during the COVID-19 pandemic. Inter-group comparisons were conducted using Pearson’s chi-square test, and pairwise comparisons were performed with the Bonferroni method. GD, glomerular diseases; IgAN, IgA nephropathy; MN, membranous nephropathy; *P < 0.05; **P < 0.01; NS, not significant.
The COVID-19 pandemic was divided into two phases: pre-vaccination and post-vaccination (Supplementary Fig. 3). During the vaccination period, the incidence of IgAN significantly increased (from 42.0 to 48.5%, P < 0.05), while the incidence of other glomerular diseases, including MCD, FSGS, MN, LN, and DN, remained stable before and after vaccination (P > 0.05). The incidence of IgAN gradually increased during the pre-COVID-19, pre-vaccination, and post-vaccination periods (Fig. 5). Although the increase from pre-COVID-19 to pre-vaccination was not statistically significant (P = 0.462), a significant increase was observed in the post-vaccination period compared to the pre-vaccination period (P = 0.030). The overall increase from pre-COVID-19 to post-vaccination was highly significant (P < 0.001).
Clinical and pathological features of IgAN patients
Out of 1,042 IgAN patients from two treatment centers, clinical and pathological data were available for 746. We compared the clinical and pathological characteristics of these patients before and during the COVID-19 pandemic. There were no significant changes in the age and sex distribution of patients before and during the pandemic (Table 2). Common clinical manifestations, such as hypertension, edema, recurrent macroscopic hematuria, microscopic hematuria, nephrotic syndrome, acute kidney injury, and chronic renal failure, remained consistent before and during the pandemic. Additionally, laboratory indicators reflecting disease activity, such as hemoglobin, serum albumin, serum creatinine, eGFR, and proteinuria, showed no significant changes.
Regarding pathological parameters, the Oxford classification revealed significantly higher proportions of M1 (97.9% vs. 73.3%; P < 0.001), E1 (25.0% vs. 16.6%; P = 0.006), and S1 (44.5% vs. 35.0%; P = 0.009) in patients during the pandemic compared to those before the pandemic (Table 3). There were no significant differences in T1-2 and C1-2 between the two groups (P > 0.05). Immunofluorescence analysis revealed significant changes in C3 staining intensity during the COVID-19 pandemic. Compared to pre-pandemic levels, the proportion of positive C3 staining decreased significantly (24.8% vs. 40.2%; P < 0.001), while the proportion of strongly positive C3 staining increased significantly (55.5% vs. 44.8%; P = 0.004). Additionally, those during the pandemic exhibited more significant changes in thickening of the vessel wall (80.2% vs. 69.9%; P = 0.003), hyalinization (25.0% vs. 10.1%; P < 0.001), and foam cell formation (3.0% vs. 0.9%; P = 0.047). In terms of treatment, patients during the pandemic had significantly higher usage rates of prednisone (40.0% vs. 28.2%; P = 0.003), RASI (83.1% vs. 69.9%; P < 0.001), and immunosuppressants (25.5% vs. 17.2%; P = 0.020) compared to those before the pandemic. In contrast, the usage rates of iron supplements (P = 0.428) and erythropoietin (P = 0.224) did not differ significantly.
Covariates in the binary Logistic regression models (via multivariate analyses) were associated with the COVID-19 pandemic (Fig. 6). We adjusted for measures of significance (M1, E1, S1, C3 staining, thickening of the vessel wall, hyalinization, foam cell formation, prednisone, RASI, immunosuppressants) and borderline significance (microscopic hematuria, acute renal failure, Scr, T1-2, C1-2) in the univariate analysis, as well as basic patient characteristics, including age and sex. The results indicate that pathological parameters M1 (P < 0.001), E1 (P = 0.014), strong positive C3 staining (P = 0.018), and hyalinization (P < 0.001) remain significantly associated with the COVID-19 pandemic, whereas S1, thickening of the vessel wall, and foam cell formation are no longer significant (P > 0.05). Regarding treatment medications, the use of RASI (P = 0.017) and prednisone (P = 0.006) has significantly increased during the COVID-19 pandemic compared to the period before the pandemic. However, the use of immunosuppressants shows no significant difference (P > 0.05).
Effects of the COVID-19 pandemic on renal biopsy pathology and therapy in IgAN patients analyzed by multivariate Logistic regression. In the initial model selection for the multivariate analysis, covariates with P-values < 0.1 from univariate analyses, or those deemed important, were included, with age and sex treated as fixed factors. The final model was determined by retaining covariates with P-values < 0.05. C, crescent formations; C3, complement C3; E, endocapillary hypercellularity; M, mesangial hypercellularity; RASI, renin-angiotensin system inhibitors; Ref, reference group; S, segmental sclerosis; T, interstitial fibrosis/tubular atrophy.
Discussion
To date, numerous cases of kidney diseases associated with COVID-19 have been reported17,18,19,20,21. However, given the broad scope of diagnoses, it remains unclear which specific diseases have an increased diagnostic frequency based on small series and case reports. One of the studies predicted that IgAN may be a serious complication of COVID-19 and COVID-19mRNA vaccines, and that the incidence of IgAN may increase in the future, however, conclusive evidence to support this hypothesis is lacking9. We formed a collaborative project across two centers to identify which kidney diseases have shown increased diagnostic frequencies by comparing renal biopsy cohorts from during and before the COVID-19 pandemic. This study represents the first preliminary assessment of the pandemic’s impact on kidney disease incidence. Our findings indicate an increase in the diagnosis of IgAN cases since the onset of the COVID-19 pandemic, with a notably higher incidence compared to the pre-pandemic period, especially during the vaccination phase. The predominant factors influencing the population during the COVID-19 era were COVID-19 infection and vaccination. Consequently, COVID-19 infection and/or vaccination may serve as potential triggers for IgAN during this period. So far, the pathogenesis of COVID-19-related IgAN remains unclear. Elevated levels of galactose-deficient IgA1 (Gd-IgA1) have been suggested to contribute to the pathogenesis of IgAN22. The COVID-19 virus primarily affects the respiratory tract, resulting in increased production of IgA1, including Gd-IgA11. Gd-IgA1 may recognize specific structures on certain microorganisms and form cyclic complexes with them, which could enhance antigen recognition. However, our data show that serum IgA levels in patients did not significantly increase during the pandemic. One possible explanation for this discrepancy is that total serum IgA levels may not accurately reflect local mucosal immune responses or the production of specific IgA1, particularly Gd-IgA1, which plays a critical role in the pathogenesis of IgAN. Additionally, renal biopsy is typically performed 3–7 days after patient admission at our center, with this timeframe potentially being shorter during the pandemic. As the IgA peak in the immune response occurs later (the median time is 23 days)23, it may not have been captured during biopsy, potentially missing peak changes during acute infection. The observed increase in IgAN incidence during the COVID-19 era may also be influenced by other factors, such as COVID-19 vaccines and related medications24. Mass vaccination against SARS-CoV-2 has become a key strategy in combating the COVID-19 pandemic. However, with the global administration of COVID-19 vaccines, there has been a growing number of reports describing the onset of IgAN24,25. In a systematic review of renal diseases associated with COVID-19 vaccination, IgAN was reported as the second most common renal disorder after minimal change disease26. Nevertheless, a study by Diebold et al. indicated that SARS-CoV-2 was not associated with new-onset glomerulonephritis, and that most temporal associations between SARS-CoV-2 vaccination and glomerulonephritis were likely coincidental27. The differences in these perspectives still require further investigation to be resolved. Other related medications, such as antimalarial agents (chloroquine and hydroxychloroquine), azithromycin, favipiravir, ritonavir, lopinavir, and rapamycin, have been reported to cause kidney damage28,29,30,31,32. Additionally, nonsteroidal anti-inflammatory drugs (NSAIDs), widely used for their anti-inflammatory and antipyretic effects during the COVID-19 pandemic, have been associated with kidney damage33.
Since 2009, the Oxford Classification (MEST)34 has been widely adopted in clinical practice because it predicts the risk of progression in IgAN more accurately. It has largely replaced previously popular, less evidence-based classifications. In 2016, Haas et al.35 confirmed through a study of 3,096 IgAN patients that C formation is an independent risk factor for poor prognosis. Patients with glomerular Cs ≥ 25% have a significantly higher risk of renal progression compared to those with Cs < 25%. In 2017, the IgA Nephropathy Classification Working Group recommended adding C to the MEST score14. The revised Oxford Classification (MEST-C) has since become the standard accepted by most clinicians and researchers worldwide. Using the revised Oxford Classification (MEST-C) to assess IgAN pathological changes, our study found that patients diagnosed with IgAN during the COVID-19 pandemic had a higher risk of renal damage compared to those diagnosed before the pandemic. This was evidenced by significantly increased proportions of M1 (OR = 11.764, P < 0.001), E1 (OR = 1.718, P = 0.014), and strong positive C3 staining (OR = 2.157, P = 0.018). Sensitivity analysis confirmed the robustness of these findings. The high intensity C3 deposition observed during the COVID-19 pandemic may be associated with abnormal activation of the complement system in patients following SARS-CoV-2 infection36,37. The significant increase in M1 ratio is likely closely related to this C3 deposition. This is consistent with previous studies, which have reported that patients with stronger C3 deposition are more prone to M1 lesions38. Additionally, the increase in E1 may be related to the deposition of complexes within the glomerular capillaries, triggering local inflammation. This may represent an active lesion, and E lesions are reversible with timely immunosuppressive therapy39,40. Published cohort studies provide strong and consistent evidence that M lesions can reliably provide prognostic information through univariate analysis34,41,42. Two studies that did not include patients receiving corticosteroid/cytotoxic treatment reported that E1 is independently associated with faster loss of renal function and poorer renal survival41,43. The severity of kidney pathology grading has consistently been independently associated with poor prognosis, with severe IgAN carrying a higher risk of progression to end-stage renal disease (ESRD)44. To minimize the risk of IgAN progressing to ESRD during the COVID-19 pandemic, it is crucial to ensure timely treatment and nephrology care when clinically indicated.
Evidence from randomized withdrawal trials indicates that existing RASI therapy does not need to be discontinued in non-severe COVID-19 patients if prescribed for significant indications45,46. The increased use of RASI during the pandemic may be associated with worsening renal pathological damage. Given the established renoprotective effects of RAS blockade in IgAN47, clinicians may have been more inclined to prescribe RASI in response to more severe pathological changes. The increased use of glucocorticoids in our study population likely reflects a higher proportion of patients with active lesions (particularly E1) during the pandemic, as these findings typically necessitate immunosuppressive therapy according to current guidelines14. It is noteworthy that although we observed a significant increase in the proportion of M1 and E1 lesions in IgAN patients during the COVID-19 pandemic, clinical parameters such as proteinuria and hematuria remained stable. One possible explanation is that during the pandemic, increased health awareness and more timely therapeutic interventions, particularly the use of RASI and prednisone, effectively controlled clinical symptoms, despite more severe pathological lesions. Additionally, there may be a time lag between the development of pathological changes and clinical manifestations. This suggests that inflammatory lesions could represent early changes not yet translated into clinical deterioration. The observed decline in the diagnosis of MN during the COVID-19 pandemic warrants cautious interpretation. The availability of non-invasive diagnostic methods for MN, especially anti-PLA2R antibody testing48,49, may have reduced the need for kidney biopsies in suspected cases. This may have resulted in an underestimation of the true incidence of MN during the pandemic, as only cases requiring histological confirmation underwent biopsy.
This study has several limitations. Firstly, it includes clinical and pathological data from kidney biopsies collected during the COVID-19 pandemic, specifically from March 11, 2020, to May 5, 2023. However, it lacks longitudinal clinical follow-up, which prevents us from comparing diagnostic data with patient outcomes. Secondly, This retrospective study describes fluctuations in the incidence of IgAN and changes in its pathological features during the COVID-19 pandemic, and suggests to some extent that COVID-19 infection and/or vaccination may be contributing factors to these changes, although definitive confirmation is still pending.We intend to carry out other studies to investigate the associated factors.Furthermore, our findings are restricted to individuals newly diagnosed with IgAN and do not provide insight into whether COVID-19 triggers relapse or worsening of previously diagnosed IgAN. This is because clinical recurrence can be determined by evaluating urinary protein, blood creatinine, and other factors, without the necessity of conducting multiple kidney biopsies.
In conclusion, our study observed a significant rise in the incidence of IgAN during the COVID-19 pandemic, along with worsened renal damage and an increase in the proportions of Oxford classification M1 and E1. Further research is needed to gain a better understanding of the epidemiology, pathology, and long-term clinical outcomes of IgAN in the context of COVID-19.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Farooq, H. et al. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: A systematic review. J. Taibah Univ. Med. Sci. 17(1), 1–13. https://doi.org/10.1016/j.jtumed.2021.08.012 (2022).
Leow, E. H. et al. IgA nephropathy in children: Before and after the start of COVID-19. Pediatr Nephrol. 39(4), 1161–1167. https://doi.org/10.1007/s00467-023-06196-2 (2024).
Elgardt, I., Carmi, O. & Levy, Y. IgA nephropathy (Henoch-Schönlein Purpura) associated with recent COVID-19 infection. Isr. Med. Assoc. J. 24(11), 697–698 (2022).
Göre, B. et al. IGA nephropathy and spinal epidural abscess after COVID-19 infection: a case report. Future Virol. 17, 611–615. https://doi.org/10.2217/fvl-2021-0314 (2022).
Wyatt, R. J. & Julian, B. A. IgA nephropathy. N. Engl. J. Med. 368(25), 2402–2414. https://doi.org/10.1056/NEJMra1206793 (2013).
Barratt, J. & Feehally, J. IgA nephropathy. J. Am. Soc. Nephrol. 16(7), 2088–2097. https://doi.org/10.1681/ASN.2005020134 (2005).
Pattrapornpisut, P., Avila-Casado, C. & Reich, H. N. IgA nephropathy: Core curriculum 2021. Am J Kidney Dis. 78(3), 429–441. https://doi.org/10.1053/j.ajkd.2021.01.024 (2021).
Jarrick, S. et al. Mortality in IgA nephropathy: A nationwide population-based cohort study. J. Am. Soc. Nephrol. 30(5), 866–876. https://doi.org/10.1681/ASN.2018101017 (2019).
Abdi Saed, Y. et al. As signals from the Kawasaki-like illness during the COVID-19 pandemic: Is it possible that the incidence of IgA nephropathy may increase in the future. Front. Med. (Lausanne). 2(8), 737692. https://doi.org/10.3389/fmed.2021.737692 (2021).
Floege, J. & Feehally, J. The mucosa-kidney axis in IgA nephropathy. Nat. Rev. Nephrol. 12(3), 147–156. https://doi.org/10.1038/nrneph.2015.208 (2016Mar).
Yu, H. Q. et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 56(2), 2001526. https://doi.org/10.1183/13993003.01526-2020 (2020).
Batlle, D. et al. Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J. Am. Soc. Nephrol. 31(7), 1380–1383. https://doi.org/10.1681/ASN.2020040419 (2020).
Kudose, S. et al. Kidney biopsy findings in patients with COVID-19. J. Am. Soc. Nephrol. 31(9), 1959–1968. https://doi.org/10.1681/ASN.2020060802 (2020).
Trimarchi, H. et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 91(5), 1014–1021. https://doi.org/10.1016/j.kint.2017.02.003 (2017).
Churg, J., & Sobin, L.H. Renal Disease: Classification and Atlas of Glomerular Diseases. Igaku-Shoin Medical (1982).
Churg, J., Bernstein, J., & Glassock, R.J. Renal Disease: Classification and Atlas of Glomerular Diseases Igaku-Shoin Medical (1995).
Sharma, P. et al. COVID-19-associated kidney injury: A case series of kidney biopsy findings. J. Am. Soc. Nephrol. 31(9), 1948–1958. https://doi.org/10.1681/ASN.2020050699 (2020).
Gupta, R. K. et al. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: A report of 2 cases. BMC Nephrol. 21(1), 326. https://doi.org/10.1186/s12882-020-01970-y (2020).
Huang, Y. et al. Clinical and pathological findings of SARS-CoV-2 infection and concurrent IgA nephropathy: A case report. BMC Nephrol. 21(1), 504. https://doi.org/10.1186/s12882-020-02163-3 (2020).
Suso, A. S. et al. IgA vasculitis with nephritis (Henoch-Schönlein Purpura) in a COVID-19 patient. Kidney Int. Rep. 5(11), 2074–2078. https://doi.org/10.1016/j.ekir.2020.08.016 (2020).
Li, N. L. et al. Immunoglobulin-A vasculitis with renal involvement in a patient with COVID-19: A case report and review of acute kidney injury related to SARS-CoV-2. Can. J. Kidney Health Dis. 5(8), 2054358121991684. https://doi.org/10.1177/2054358121991684 (2021).
Stamellou, E. et al. IgA nephropathy. Nat. Rev. Dis. Primers. 9(1), 67. https://doi.org/10.1038/s41572-023-00476-9 (2023).
Xu, J. et al. Detection methods and dynamic characteristics of specific antibodies in patients with COVID-19: A review of the early literature. Heliyon. 10(3), e24580. https://doi.org/10.1016/j.heliyon.2024.e24580 (2024).
Mima, A. & Lee, S. IgA nephropathy after COVID-19 vaccination and analysis of reported cases. Heliyon. 9(6), e17206. https://doi.org/10.1016/j.heliyon.2023.e17206 (2023).
Ma, Y. & Xu, G. New-onset IgA nephropathy following COVID-19 vaccination. QJM. 116(1), 26–39. https://doi.org/10.1093/qjmed/hcac185 (2023).
Zhang, J., Cao, J. & Ye, Q. Renal side effects of COVID-19 vaccination. Vaccines (Basel). 10(11), 1783. https://doi.org/10.3390/vaccines10111783 (2022).
Diebold, M. et al. Incidence of new onset glomerulonephritis after SARS-CoV-2 mRNA vaccination is not increased. Kidney Int. 102(6), 1409–1419. https://doi.org/10.1016/j.kint.2022.08.021 (2022).
Wiwanitkit, V. Antimalarial drug and renal toxicity. J. Nephropharmacol. 5(1), 11–12 (2015).
Mansoor, G. A., Panner, B. J. & Ornt, D. B. Azithromycin-induced acute interstitial nephritis. Ann. Intern. Med. 119(7 Pt 1), 636–637. https://doi.org/10.7326/0003-4819-119-7_part_1-199310010-00027 (1993).
Sissoko, D. et al. Experimental treatment with Favipiravir for Ebola virus disease (the JIKI Trial): A historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 13(3), e1001967. https://doi.org/10.1371/journal.pmed.1001967 (2016).
Hussain, S. et al. Nephrotoxicity in a child with perinatal HIV on tenofovir, didanosine and lopinavir/ritonavir. Pediatr Nephrol. 21(7), 1034–1036. https://doi.org/10.1007/s00467-006-0109-3 (2006).
Deray, G. et al. Néphrotoxicité du ritonavir [Nephrotoxicity of ritonavir]. Presse Med. 27(35), 1801–1803 (1998).
See, Y. P. et al. Risk factors for development of acute kidney injury in COVID-19 patients: A retrospective observational cohort study. Nephron. 145(3), 256–264. https://doi.org/10.1159/000514064 (2021).
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 76(5), 534–545. https://doi.org/10.1038/ki.2009.243 (2009).
Haas, M. et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J. Am. Soc. Nephrol. 28(2), 691–701. https://doi.org/10.1681/ASN.2016040433 (2017).
Ge, X. et al. Complement and complement regulatory proteins are upregulated in lungs of COVID-19 patients. Pathol. Res. Pract. 247, 154519. https://doi.org/10.1016/j.prp.2023.154519 (2023).
Guo, W. Y. et al. Complement system is overactivated in patients with IgA nephropathy after COVID-19. Clin. Immunol. 263, 110232. https://doi.org/10.1016/j.clim.2024.110232 (2024).
Kang, Y. et al. Mesangial C3 deposition, complement-associated variant, and disease progression in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 18(12), 1583–1591. https://doi.org/10.2215/CJN.0000000000000290 (2023).
Shen, X. H. et al. Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: A repeat-biopsy based observation. J. Nephrol. 28(4), 441–449. https://doi.org/10.1007/s40620-014-0165-x (2015).
Markowitz, G. Glomerular disease: Updated Oxford Classification of IgA nephropathy: A new MEST-C score. Nat. Rev. Nephrol. 13(7), 385–386. https://doi.org/10.1038/nrneph.2017.67 (2017Jul).
El Karoui, K. et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J. Am. Soc. Nephrol. 23(1), 137–148. https://doi.org/10.1681/ASN.2010111130 (2012).
Zeng, C. H. et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am. J. Kidney Dis. 60(5), 812–820. https://doi.org/10.1053/j.ajkd.2012.06.011 (2012).
Chakera, A. et al. Prognostic value of endocapillary hypercellularity in IgA nephropathy patients with no immunosuppression. J. Nephrol. 29(3), 367–375. https://doi.org/10.1007/s40620-015-0227-8 (2016).
Kim, J. K. et al. Clinical features and outcomes of IgA nephropathy with nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 7(3), 427–436. https://doi.org/10.2215/CJN.04820511 (2012).
Writing Committee for the REMAP-CAP Investigators et al. Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: A randomized clinical trial. JAMA. 329(14), 1183–1196. https://doi.org/10.1001/jama.2023.4480 (2023).
Self, W. H. et al. Renin-Angiotensin system modulation with synthetic angiotensin (1–7) and angiotensin II type 1 receptor-biased ligand in adults with COVID-19: Two randomized clinical trials. JAMA. 329(14), 1170–1182. https://doi.org/10.1001/jama.2023.3546 (2023).
Xie, X. et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A Bayesian network meta-analysis of randomized clinical trials. Am. J. Kidney Dis. 67(5), 728–741. https://doi.org/10.1053/j.ajkd.2015.10.011 (2016).
Bobart, S. A. et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 95(2), 429–438. https://doi.org/10.1016/j.kint.2018.10.021 (2019).
Bobart, S. A. et al. Noninvasive diagnosis of PLA2R-associated membranous nephropathy: A validation study. Clin. J. Am. Soc. Nephrol. 16(12), 1833–1839. https://doi.org/10.2215/CJN.05480421 (2021).
Funding
This research was funded by Shenzhen Key Medical Discipline Construction Fund (SZXK009) and Shenzhen medical and health three project (SZSM202211013).
Author information
Authors and Affiliations
Contributions
Conceptualization, funding acquisition, writing—review and editing, Q.W.; methodology, software, validation, writing—original draft preparation, W.L.; data curation, project administration, writing—review and editing, R.X.; data curation, visualization, supervision, Z.S.; data curation, investigation, resources, D.W.; data curation, supervision, project administration Y.C.; data curation, project administration, software, H.H.; data curation, investigation, writing—review and editing, X.Z. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, W., Xu, R., Wu, D. et al. Incidence and pathological features of IgA nephropathy before and during the COVID-19 pandemic. Sci Rep 15, 4656 (2025). https://doi.org/10.1038/s41598-025-89170-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89170-2