Abstract
To explore the association between serum high-sensitivity C-reactive protein (HSCRP) levels and osteoarthritis (OA) in adults, providing new evidence for the diagnosis of adult OA. We selected data from the 2015–2018 National Health and Nutrition Examination Survey (NHANES) and conducted a cross-sectional study. Serum HSCRP levels were extracted from laboratory data, and OA patients were identified through questionnaire responses. Participants under the age of 20 and those with incomplete data were excluded. We used multivariable logistic regression models, restricted cubic spline (RCS) functions, and stratified analyses to study the association between serum HSCRP levels and osteoarthritis in adults. After screening, a total of 9,948 participants were included, among whom 1,196 were osteoarthritis patients, representing a prevalence rate of 12.02%. Multivariable logistic regression analysis, along with three adjusted models, showed a positive correlation between serum HSCRP levels and the occurrence of osteoarthritis in adults. Compared to the lowest HSCRP quartile, the highest quartile showed a 1.86-fold higher prevalence of OA (95% confidence interval: 1.55 ~ 2.23, P < 0.001). The restricted cubic spline analysis showed a significant increase in OA incidence with rising serum HSCRP levels (P < 0.05). Subgroup and forest plot analyses indicated a positive correlation between HSCRP levels and osteoarthritis across different subgroups, such as age, gender, hypertension status, activity status, drinking status, and Smoke status (P < 0.05). There is a positive correlation between serum HSCRP levels and the occurrence of osteoarthritis in adults. When a patient’s serum HSCRP level is elevated, the possibility of osteoarthritis should be considered.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is the most common degenerative joint disease worldwide. According to Safiri et al.1, its global prevalence exceeds 20%, especially affecting older adults. The pathological features of OA include progressive degeneration of joint cartilage, subchondral bone sclerosis, osteophyte formation, and synovitis2. These changes lead to joint pain, stiffness, and reduced mobility, severely impacting patients’ quality of life3. OA is one of the leading causes of disability, particularly among the elderly, and the societal and economic burden of OA is increasing due to population aging and rising obesity rates—both major risk factors for the disease4,5.
The etiology of OA is multifactorial, including mechanical stress, genetic susceptibility, metabolic abnormalities, and inflammation6. Traditionally, OA has been considered a “wear-and-tear” disease, but increasing evidence suggests that systemic inflammation plays a crucial role in its development7,8. Therefore, systemic biomarkers that can predict disease risk and progression have become a key focus of OA research8,9.
One of the most studied systemic inflammatory markers is high-sensitivity C-reactive protein (HSCRP), a protein produced by the liver during inflammatory responses10. Elevated serum HSCRP levels are typically associated with various inflammatory conditions, including cardiovascular disease, diabetes, and rheumatoid arthritis10,11,12. Increasing evidence suggests that elevated HSCRP levels are also linked to OA, indicating that systemic inflammation may exacerbate disease progression13,14. Specifically, elevated HSCRP levels have been associated with increased pain and functional decline in OA patients, as well as the presence of synovitis, a marker of intra-articular inflammation14. However, the association between HSCRP levels and OA remains controversial, with some studies reporting a positive correlation between elevated HSCRP levels and OA prevalence13,15,16, while others find no association17,18. Discrepancies in study results may be due to differences in study populations, sample sizes, and methods. Moreover, many existing studies have small sample sizes or lack adjustments for confounding factors such as age, gender, and comorbidities, leading to biased statistical results and lower levels of evidence for the conclusions.
To address the limitations of previous studies, we used data from the 2015–2018 National Health and Nutrition Examination Survey (NHANES) to investigate the association between serum HSCRP levels and OA prevalence. NHANES provides comprehensive health data from a nationally representative sample of the U.S. population. Since 2015, the NHANES database has included HSCRP data, making it an ideal platform for studying the role of HSCRP in OA. By using advanced statistical techniques such as multivariable logistic regression and restricted cubic splines, we aimed to provide reliable evidence for the association between serum HSCRP levels and OA prevalence, while accounting for potential confounders. The study results could deepen our understanding of OA pathogenesis and help identify individuals at high risk for the disease, facilitating early diagnosis and intervention.
Participants and methods
Study Population
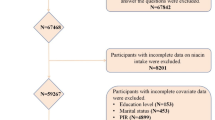
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), is a nationally representative health and nutrition survey. It uses a complex probability sampling method to collect information from standardized interviews, physical examinations, and laboratory tests to assess the health and nutritional status of the U.S. population. The study protocol was approved by the NCHS Ethics Review Board, and all participants provided informed consent. All NHANES data are publicly available on the NHANES website. For details on survey response rates and participant recruitment methods, please refer to the NHANES official website. This study used data from the 2015–2018 NHANES cycles. Due to the COVID-19 pandemic, NHANES data collection was paused after 2018. Therefore, this study included data from 2015 to 2018. This was a cross-sectional study. Participants under 20 years old and those with missing data on key variables such as OA diagnosis, HSCRP levels, vitamin D, white blood cell count, smoke status, activity status, hypertension, and drinking status were excluded. After exclusions, 9,948 participants were included in the study. (Fig. 1)
Study variables
Exposure and outcomes
The exposure in this study was HSCRP levels. Serum samples were processed, stored, and sent to the University of Minnesota’s Advanced Research and Diagnostic Laboratory in Minneapolis for HSCRP analysis. Detailed procedures are provided in the HSCRP laboratory manual on the NHANES website.
The outcome was the presence of osteoarthritis (OA). OA data were obtained through survey responses. Participants answered the question: “Has a doctor or other health professional ever told you that you had arthritis?” If the participant responded “yes,” they were then asked, “What type of arthritis?”. Those who reported osteoarthritis were classified as part of the OA group, while others were considered non-OA.
Covariates
We analyzed potential covariates that might influence the association between HSCRP levels and OA, including age, gender, race, hypertension, activity status, Smoke status, drinking status, serum vitamin D levels, and white blood cell count. Age, gender, race, drinking status, activity status, and Smoke status data were obtained from survey responses. Race was categorized as Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian, and Other Race - Including Multi-Racial. Smoking were classified as “yes” or “no” based on the survey results. Activity status was categorized into high, moderate, low, and none, with high activity defined as having either vigorous work or vigorous recreational activity, moderate activity as having either moderate work or moderate recreational activity, low activity as walking or cycling, and no activity as being sedentary. Hypertension was determined based on the average of four blood pressure measurements, with hypertension defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg. Drinking status was categorized into heavy (women: ≥2 drinks/day or men: ≥3 drinks/day), moderate (women: 1 drink/day or men: 1–2 drinks/day), and none, based on responses to the question: “During the past 12 months, on days when {you/SP} drank alcoholic beverages, on average, how many drinks did {you/he/she} have? A drink refers to a 12 oz. beer, a 5 oz. glass of wine, or 1.5 ounces of liquor”. Serum vitamin D and white blood cell levels were obtained from laboratory test results. The serum vitamin D levels were measured as 25OHD2 + 25OHD3.
Statistical analysis
For continuous variables following a normal distribution, means and standard deviations were reported, and t-tests were used for group comparisons. Differences between categorical variables were analyzed using chi-square tests. We used multivariable logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI) to evaluate the association between HSCRP and osteoarthritis. Four models were built to adjust for potential covariates: Model 1: Unadjusted, Model 2: Adjusted for gender, age, and race, Model 3: Further adjusted for vitamin D and white blood cell count. Model 4: Additionally adjusted for hypertension, activity status, smoking, and drinking status.
Restricted cubic spline (RCS) analysis was performed to assess non-linear associations between HSCRP and OA. Subgroup analyses and forest plots were used to evaluate the association between HSCRP and OA in various subgroups. All analyses were conducted using SPSS 24.0 and R software (R4.2.0), with statistical significance set at P < 0.05.
Results
Participant characteristics
A total of 9,948 participants met the inclusion criteria. Their characteristics are summarized in Table 1. Of these, 1,196 (12.0%) had osteoarthritis, and 8,752 (88.0%) did not. Significant differences were observed between the OA and non-OA groups in terms of age, gender, race, HSCRP levels, hypertension status, activity status, smoke status, drinking status, and serum vitamin D levels (P < 0.01). No significant differences were found in white blood cell counts between the two groups. (Table 1 )
Correlation between serum HSCRP levels and OA
Multivariable logistic regression analysis showed a positive association between serum HSCRP levels and OA across all four models. As HSCRP quartiles increased (Q1 to Q4), the risk of OA also increased. In Model 1, participants in the highest HSCRP quartile had a 1.86-fold higher risk of OA compared to those in the lowest quartile (95% CI: 1.55–2.23, P < 0.001). The results were similar across the other three models. (Table 2)
Restricted cubic spline analysis
RCS analysis revealed a non-linear association between serum HSCRP levels and OA after adjusting for all covariates (Model 4). The risk of OA increased as HSCRP levels rose (P < 0.05). (Figure 2)
Subgroup analysis
To further analyze the association between serum HSCRP levels and osteoarthritis (OA) in different demographic groups, we conducted subgroup analyses. Subgroups included age, gender, race, hypertension status, activity status, smoking, and Drinking status. Results showed a positive correlation between HSCRP levels and OA in subgroups defined by gender, hypertension, Drinking status, and smoking status. In the age groups > 20 ≤ 40 years and > 40 ≤ 60 years, and in the race subgroups Mexican American, Non-Hispanic White, and Non-Hispanic Black, there was also a positive correlation between HSCRP levels and OA, except for the insufficient activity group. (Fig. 3)
Discussion
This study aimed to investigate the association between serum HSCRP levels and OA prevalence among U.S. adults, using data from the 2015–2018 National Health and Nutrition Examination Survey (NHANES). Our results demonstrate a positive association between HSCRP levels and OA prevalence, even after adjusting for potential confounders such as age, gender, race, activity status, smoking, and Drinking status. The restricted cubic spline (RCS) results confirmed this association. Subgroup analyses also revealed that the association between HSCRP levels and OA prevalence was significant across various demographic groups.
Comparison with previous research
Our findings align with several studies that reported a significant positive correlation between elevated HSCRP levels and OA13,19,20. For example, Pearle et al.13 found a significant correlation between the degree of inflammatory infiltration in OA patients and HSCRP levels. Stannus et al.19 conducted a prospective study showing that HSCRP levels were positively correlated with changes in knee pain associated with osteoarthritis. These studies, together with our findings, support the hypothesis that systemic inflammation, as measured by HSCRP, is a key factor in OA pathogenesis.
However, some studies have reported inconsistent results regarding the role of HSCRP in OA17,21. For instance, VLAD et al.21, in a large cohort study of 1,235 participants, found no evidence linking HSCRP or any other inflammatory markers to radiographic OA. These discrepancies may be due to differences in study design, sample size, and methods of OA assessment. Some studies focus on radiographic OA diagnosis, while others rely on self-reported OA diagnoses, which could contribute to varied results. Moreover, the inflammatory response in OA may vary depending on disease stage and severity, with HSCRP potentially being more closely associated with advanced or symptomatic OA16,22.
Potential mechanisms linking HSCRP and OA
The observed positive correlation between HSCRP levels and OA suggests that systemic inflammation may play a role in OA development and progression. Although OA has traditionally been considered a “wear-and-tear” disease, studies indicate that low-grade systemic inflammation may lead to cartilage degradation, synovial inflammation, and subchondral bone remodeling, contributing to the onset of arthritis7,23,24. Recent research by Zhou et al.25 demonstrated that systemic inflammatory biomarkers are new predictors of all-cause and cardiovascular mortality in OA patients, with higher systemic immune-inflammatory indices being significantly associated with increased mortality risk.
HSCRP is an acute-phase reactant produced by the liver in response to pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), and is a recognized marker of systemic inflammation26. Several mechanisms may explain the link between elevated HSCRP levels and OA. First, chronic inflammation may directly cause joint damage by promoting the release of matrix metalloproteinases (MMPs) and other catabolic enzymes that degrade cartilage, as well as reactive oxygen species (ROS) and superoxide dismutases27,28. Second, systemic inflammation may activate intra-articular pro-inflammatory pathways, exacerbating local inflammation and accelerating OA progression29,30. Finally, systemic inflammation may lead to metabolic dysfunction, such as insulin resistance and dyslipidemia31,32, both known risk factors for OA33,34.
Clinical implications
Identifying HSCRP as a potential biomarker for OA has important clinical implications. First, HSCRP can be used as a screening tool to identify individuals at high risk of developing OA, particularly among populations with known risk factors such as obesity, advanced age, and sedentary lifestyles. Early identification of high-risk individuals could promote timely interventions, such as weight management, physical therapy, and anti-inflammatory treatments, to slow OA progression and improve patient outcomes. Moreover, targeting systemic inflammation may represent a novel therapeutic approach for OA. Although current OA treatments focus primarily on symptom management and joint protection35, interventions aimed at reducing systemic inflammation, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or biologics targeting inflammatory cytokines, may help address the underlying disease process36,37,38,39.
Study strengths and limitations
One of the main strengths of this study is the use of NHANES data, which provides a large, nationally representative dataset with high-quality, standardized health information across diverse populations. The large sample size enhances the reliability of the statistical analysis and the generalizability of the study findings. Additionally, the use of multivariable logistic regression models and restricted cubic spline analysis allowed us to adjust for potential confounders and explore the non-linear association between HSCRP levels and OA.
However, several limitations should be considered. First, the cross-sectional design of this study prevents us from establishing a causal association between elevated HSCRP levels and OA. Longitudinal studies are needed to determine whether elevated HSCRP levels precede OA development or are a consequence of the disease. Second, our analysis relied on self-reported OA diagnoses, which may be subject to recall bias and misclassification. Although NHANES uses standardized data collection methods, further studies using radiographic or clinical OA diagnoses are needed to confirm these findings. Third, while we adjusted for several potential confounders, residual confounding by unmeasured variables (e.g., dietary factors, obesity, diabetes mellitus and medication use) cannot be ruled out.
Future research directions
Our study highlights the need for further research to elucidate the role of systemic inflammation in OA. Future studies should focus on longitudinal designs to establish the temporal association between HSCRP levels and OA development. Additionally, studies are needed to explore the effects of anti-inflammatory therapies on OA progression. Identifying subgroups of OA patients who may benefit from targeted anti-inflammatory treatments could lead to more personalized therapeutic strategies. And future research should aim to identify other systemic inflammatory biomarkers that may contribute to OA pathogenesis, such as IL-640, TNF-α39, and adipokines41. These biomarkers, combined with HSCRP, could provide a more comprehensive understanding of the inflammatory processes underlying OA and help guide the development of new therapeutic approaches. Finally, It is recommended that variables such as BMI, obesity, diabetes, and diet be included in future studies, and that NHANES data be combined with other databases containing more comprehensive clinical measures to further explore the relationship between HSCRP and OA.
Conclusion
This study demonstrates a significant positive association between elevated serum HSCRP levels and osteoarthritis (OA) prevalence in adults. Higher HSCRP levels correlate with an increased risk of OA, even after adjusting for confounders. HSCRP could serve as a potential biomarker for early OA detection and risk assessment, supporting targeted interventions to manage inflammation and slow disease progression. Further longitudinal research is necessary to clarify the causal association and assess the therapeutic potential of addressing systemic inflammation in OA management.
Data availability
Publicly available datasets were analyzed in this study. All data are available in the NHANES database (www.cdc.gov/nchs/nhanes).
Abbreviations
- HSCRP:
-
high-sensitivity C-reactive protein
- OA:
-
osteoarthritis
- NHANES:
-
National Health and Nutrition Examination Survey
- RCS:
-
restricted cubic spline
- OR:
-
odds ratios
- CI:
-
confidence intervals
References
Safiri, S. et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of Disease Study 2017. Ann. Rheum. Dis. 79 (6), 819 (2020).
Abramoff, B., Caldera, F. E. & Osteoarthritis Pathology, diagnosis, and Treatment options. Med. Clin. 104 (2), 293–311 (2020).
Moseng, T. et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 83 (6), 730 (2024).
Hunter, D. J., March, L. & Chew, M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet 396 (10264), 1711–1712 (2020).
Steinmetz, J. D. et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet Rheumatol. 5 (9), e508–e522 (2023).
Kulkarni, P., Martson, A., Vidya, R., Chitnavis, S. & Harsulkar, A. Chapter Two - Pathophysiological landscape of osteoarthritis. In: Advances in Clinical Chemistry. Edited by Makowski GS, vol. 100: Elsevier; : 37–90. (2021).
van den Bosch, M. H. J., Blom, A. B. & van der Kraan, P. M. Inflammation in osteoarthritis: our view on its presence and involvement in disease development over the years. Osteoarthr. Cartil. 32 (4), 355–364 (2024).
van den Bosch, M. H. J., van Lent, P. L. E. M. & van der Kraan, P. M. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 28 (5), 532–543 (2020).
Koh, S. M. et al. Elevated plasma and synovial fluid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis. Knee 27 (1), 26–35 (2020).
Dupuy, A. M. et al. [Is C-reactive protein a marker of inflammation?]. Nephrologie 24 (7), 337–341 (2003).
Ma, X. et al. The effect of hsCRP on TyG index-associated cardiovascular risk in patients with acute coronary syndrome undergoing PCI. Sci. Rep-Uk. 14 (1), 18083 (2024).
Fang, Y. et al. Exploring the relations of NLR, hsCRP and MCP-1 with type 2 diabetic kidney disease: a cross-sectional study. Sci. Rep-Uk. 14 (1), 3211 (2024).
Pearle, A. D. et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr. Cartil. 15 (5), 516–523 (2007).
Mobasheri, A. et al. Biomarkers for osteoarthritis: current status and future prospects. Best Pract. Res. Clin. Rheumatol. 37 (2), 101852 (2023).
He, K. & Huang, H. The significant role of Alcohol in the relationship between C-Reactive protein and self-reported osteoarthritis. J. Nutr. 154 (2), 600–609 (2024).
Zhu, Z. et al. SAT0004 cross-sectional and longitudinal associations between serum levels of HS-CRP, resistin and knee bone marrow lesions in patients with knee osteoarthritis. Ann. Rheum. Dis. 74 (Suppl 2), 651 (2015).
Kerkhof HJM, Bierma-Zeinstra SMA, Castano-Betancourt MC, de Maat MP, Hofman A, Pols HAP, Rivadeneira F, Witteman JC, Uitterlinden AG, van Meurs JBJ. Serum C reactive protein levels and genetic variation in the CRP gene are not associated with the prevalence, incidence or progression of osteoarthritis independent of body mass index. Ann Rheum Dis. 2010; 69(11):1976.
Loures, F. B. C-reactive protein has no correlation with the severity of knee osteoarthritis. A cross-sectional, observational study. Osteoarthr. Cartil. 26, S196 (2018).
Stannus, O. P., Jones, G., Blizzard, L., Cicuttini, F. M. & Ding, C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann. Rheum. Dis. 72 (4), 535–540 (2013).
Singh, M. et al. Multifactorial Landscape parses to reveal a predictive model for knee osteoarthritis. Int. J. Env Res. Pub He ; 18(11). (2021).
Vlad, S. C., Neogi, T., Aliabadi, P., Fontes, J. D. T. & Felson, D. T. No Association between Markers of Inflammation and osteoarthritis of the hands and knees. J. Rhuematol. 38 (8), 1665 (2011).
Jin, X. et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 74 (4), 703 (2015).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64 (6), 1697–1707 (2012).
Daghestani, H. N. & Kraus, V. B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 23 (11), 1890–1896 (2015).
Zhou, E., Wu, J., Zhou, X. & Yin, Y. Systemic inflammatory biomarkers are novel predictors of all-cause and cardiovascular mortality in individuals with osteoarthritis: a prospective cohort study using data from the NHANES. Bmc Public. Health. 24 (1), 1586 (2024).
Black, S., Kushner, I. & Samols, D. C-reactive protein *. J. Biol. Chem. 279 (47), 48487–48490 (2004).
Afonso, V., Champy, R., Mitrovic, D., Collin, P. & Lomri, A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 74 (4), 324–329 (2007).
Lin, H. et al. Targeting G6PD to mitigate cartilage inflammation in TMJOA: the NOX4-ROS-MAPK axis as a therapeutic avenue. Int. Immunopharmacol. 139, 112688 (2024).
De Roover, A., Escribano-Núñez, A., Monteagudo, S. & Lories, R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthr. Cartil. 31 (10), 1303–1311 (2023).
Wojdasiewicz, P., Poniatowski, A. A. & Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of Osteoarthritis. Mediat Inflamm. 2014 (1), 561459 (2014).
Sun, J. et al. Puerarin attenuates insulin resistance by inhibiting endoplasmic reticulum stress and suppresses inflammation by modulating the JNK and IKKβ/NF-κB pathways in Epididymal White Adipose tissue of mice on a high-Fat Diet. Mol. Nutr. Food Res. 68 (16), e2400003 (2024).
Meng, Y. et al. Research Progress on the mechanism of Acute Hypertriglyceridemic Pancreatitis. Pancreas 53 (8), e700–e709 (2024).
Mocanu, V., Timofte, D. V., Zară-Dănceanu, C. M., Labusca, L. & Obesity Metabolic syndrome, and Osteoarthritis Require Integrative understanding and management. Biomedicines ; 12(6). (2024).
Binvignat, M., Sellam, J., Berenbaum, F. & Felson, D. T. The role of obesity and adipose tissue dysfunction in osteoarthritis pain. Nat. Rev. Rheumatol. 20 (9), 565–584 (2024).
Richard, M. J., Driban, J. B. & Mcalindon, T. E. Pharmaceutical treatment of osteoarthritis. Osteoarthr. Cartil. 31 (4), 458–466 (2023).
Zhu, R. et al. Inflammation as a therapeutic target for osteoarthritis: a literature review of clinical trials. Clin. Rheumatol. 43 (8), 2417–2433 (2024).
Duong, V., Oo, W. M., Ding, C., Culvenor, A. G. & Hunter, D. J. Evaluation and treatment of knee Pain: a review. Jama-J Am. Med. Assoc. 330 (16), 1568–1580 (2023).
Cai, G. et al. Study protocol for a randomised controlled trial of diacerein versus placebo to treat knee osteoarthritis with effusion-synovitis (DICKENS). Trials 23 (1), 768 (2022).
Zheng, W. et al. Simplified α(2)-macroglobulin as a TNF-α inhibitor for inflammation alleviation in osteoarthritis and myocardial infarction therapy. Biomaterials 301, 122247 (2023).
Negishi, Y. et al. IL-6 reduces spheroid sizes of osteophytic cells derived from osteoarthritis knee joint via induction of apoptosis. Am. J. Pathol. 194 (1), 135–149 (2024).
Farrag, Y. et al. Adipokines as potential pharmacological targets for immune inflammatory rheumatic diseases: focus on rheumatoid arthritis, osteoarthritis, and intervertebral disc degeneration. Pharmacol. Res. 205, 107219 (2024).
Acknowledgements
Thanks to NHANES database participants and staff.
Author information
Authors and Affiliations
Contributions
Tao Gao and Chao Wu analyzed and interpreted the patient data regarding the OA. Tao Gao were major contributors in writing the manuscript. Zhi-Yu Chen, Tao Li, Xu Lin, Hai-Gang Hu and Fan Wu assisted with data collection. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the NCHS Ethics Review Board, and all participants provided informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, T., Chen, ZY., Li, T. et al. Association between serum high-sensitivity C-reactive protein levels and osteoarthritis in adults from NHANES 2015 to 2018. Sci Rep 15, 5579 (2025). https://doi.org/10.1038/s41598-025-89253-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89253-0