Abstract
The occurrence of atherosclerotic lesions in the coronary bifurcation tends to progress confined to specific areas depending on blood flow patterns. We conducted a numerical investigation into the three-dimensional bifurcation flow of the coronary artery, focusing on a side branch vessel branching off at a specified bifurcation angle. This study examined the impact of the bifurcation angle on flow characteristics around the bifurcation region, including wall shear stress, static pressure, the size of the reverse flow zone, and the flow rate distribution to the side branch vessel. Unsteady pulsatile flow has been considered at Re = 300 assuming blood as a Newtonian fluid. Our numerical results indicate that as the bifurcation angle increases, the pressure drop between the inlet and outlet increases, while the flow rate to the side branch vessel decreases. Additionally, in our study, a reverse flow zone near the outer wall of the side branch was observed, and it was found that the reverse flow becomes stronger as the bifurcation angle increases due to the lower momentum of blood near the outer wall and the central part of the side branch vessel. The height of reverse flow zone of the side branch rapidly increases as the bifurcation angle increases up to about 60 degrees and then mildly saturates as the bifurcation angle increases. Flow stagnation in the reverse flow zone of the side branch may promote platelet aggregation and lipid deposition, accelerating the formation of atherosclerotic plaques.
Similar content being viewed by others
Introduction
Hemodynamic factors have been used to explain the progression of atherosclerosis in the coronary artery. Caro et al. showed that areas with low endothelial wall shear stress (WSS) are associated with the occurrence and localization of atherosclerosis through a coronary artery model based on autopsy and a computational fluid dynamics study of carotid bifurcation1,2,3. Other studies suggest that high oscillatory shear stress occurs at local sites during the systolic phase, which may be a flow condition that promotes the atherosclerotic process at those sites4. These local hemodynamic stress distributions change most dynamically with geometric morphology in the coronary bifurcation region5. Several previous studies have aimed to create simulation models to uncover BA-related mechanisms involved in the progression of stenosis at bifurcation sites. Early numerical studies investigating the impact of BA on the hemodynamics of branch vessels focused primarily on carotid artery bifurcations, with subsequent research increasingly addressing coronary artery bifurcations incorporating the pulsatile characteristics of coronary arteries4,6. A numerical investigation of the effect of arterial bifurcation angle on the formation of atherosclerotic plaques showed that WSS distribution of a side branch vessel (SB) played an important role in the formation of atherosclerosis5,7. Larger angles of the main vessel (MV) and SB decrease the WSS on the wall opposite the carina, and significantly increase the oscillatory shear stress around the carina, which promotes the proliferation of plaques in the bifurcation region8,9. Previous studies have shown that larger MV-SB angles are independently related to the initial atherosclerotic changes in the bifurcation region10,11. Clinical data also have shown that SB of the coronary arteries with large angles of MV-SB have been reported to have more severe SB outer wall (opposite to the carina side) disease than smaller angles12. In particular, the distribution of low endothelial WSS in these regions has been demonstrated through experiments13,14. This trend continues with a model of coronary arteries covered with implanted stents. No matter what technique is used to insert a stent at the bifurcation, it is known that in-stent restenosis (ISR) is more likely to occur at the SB outer wall15,16. Even in the case of bifurcation lesions treated with stents, clinical results have been reported to be poor with a large bifurcation angle (BA)17. However, this remains controversial due to existing clinical studies’ lack of statistical power to determine the effect of BA on stenosis. Additionally, setting a specific cutoff angle to assess risk is challenging.
Investigating the hemodynamics of coronary artery bifurcation flows using computational fluid dynamics (CFD) is more challenging than simulating general fluid flow due to the unique flow characteristics of coronary circulation. However, recent studies have attempted to apply models that more realistically simulate coronary blood flow dynamics. Pulsatile flow simulations for symmetric and asymmetric bifurcating vessels of artery were conducted to examine the effect of BA on the flow fields in SBs18. The effect of BA on the pressure distribution of SBs was numerically investigated to show that pressure boundary condition was effective to reduce the simulation time used by velocity boundary conditions19. Recently, Chu et al.20 examined the effect of non-Newtonian model of blood flow on the hemodynamics of bifurcated vessels and reported that the WSS of daughter branch predicted by non-Newtonian model is greater than that by Newtonian model. As the extension of Murray’s law to pulsatile flows, a general scaling law has been recently proposed validating its performance by using numerical simulations7.
However, most numerical studies investigating the impact of BA on the hemodynamics of branch vessels have been limited to examining velocity fields and WSS distributions in SB of bifurcating flows for a few selected BAs; no correlation formulae have been proposed between BA and hemodynamic quantities such as flow distribution, size of reverse flow and WSS, etc13,14,21. In this study, we have comprehensively investigated BA’s effect on the hemodynamics of coronary bifurcating flow by simulating unsteady equations of the flow field for ten BAs. From the present numerical simulations, some correlations for the local maximum WSS and the size of the reverse flow zone of a SB have been proposed as a function of BA and the dependency of other hemodynamic quantities such as flow distribution, pressure drop on BA of coronary bifurcating flow also examined.
Materials and methods
Problem description
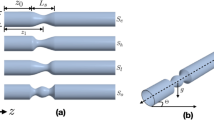
We have conducted numerical simulations of the bifurcating flow shown in Fig. 1a for ten BAs. Pulsatile inlet flow of uniform profile is used as inlet boundary condition based on the study of Liu et al.22, where the effect of the uniform profile was found to be reduced much when the length of MV(LMV of Fig. 1b) is long enough to be the seven times of the inlet diameter and zero pressure is imposed at the two outlets and the definitions of BA(α), length and height of reverse flow zone(Lr and Hr), inner and outer wall of SB and distal-MV are provided in Fig. 1a. It should be noted that the inlet area of branch vessel increases as BA decreases because a branching vessel of the same diameter as that of the MV is bifurcated from the straight MV. From the present numerical results, the flow on the outer wall side of the bifurcating branch vessel is mostly examined because the possibility of plaque generation is high around that region.
Numerical method
ANSYS 2020R2 solved the present problems by using a polyhedron mesh shown in Fig. 1c. SIMPLE algorithm has been employed both for transient simulations23. Second-order upwind scheme was used for the spatial discretization of momentum equation and the second order Crank-Nicolson method employed for the temporal discretization. Both momentum and continuity equations were iteratively solved until the residuals were less than 10− 5.
The effect of bifurcating angle (BA) on the flow characteristics of the bifurcating flow in a coronary artery is discussed based on the numerical results of unsteady flow simulations with various BAs. The Reynolds number is 300, which is based on the inlet mean velocity (0.318 m/s), inlet diameter (0.003 m) and the kinematic viscosity (\(\:3.18\times\:{10}^{-6}{\text{m}}^{2}/\text{s}\)) of blood. Figure 1d shows the evolution of inlet flow rate of a coronary artery used in the present unsteady simulation and a dashed line of Fig. 1d denotes the average inlet velocity. \(\:{\text{u}}_{0}\) denotes the average velocity of the pulsatile inlet flow. Although there may be the pressure difference between the two outlets which increases as BA increases19, the two outlets are assumed to have the same gage pressure of 0 Pa18,24. It should be noted that the pressure differences for various BAs could be obtained by CFD after assuming the flow rate to the SB by a scaling law19.
Grid and time-step independence test
We have examined the effect of grid resolution on the time-averaged velocity profile component parallel to the branch vessel near the bifurcation region, with a transient inlet flow, for eighty degrees of BA. Supplementary Fig. 1a compares the profiles for various grid resolutions along a vertical line to the outer wall, which is located 1 mm downstream from the bifurcating point B. The effect of time-step on the evolution of WSS at point A is shown in Supplementary Fig. 1b by using the grid resolution of D/40. It shows that the time-step of 0.001s needs to be used for unsteady simulations. A further validation of grid-resolution and time-step is given for 90 degrees of BA in Supplementary Fig. 1c. We have confirmed that the evolutions of WSS at point A with BA = 90o are indistinguishable for the grid-resolutions of D/30 and D/40 when time-step of 0.001s is used. The mesh consists of 5 boundary layers, with the size of the first mesh layer is \(\:1.5\times\:{10}^{-5}\)(m) (D/200, D is diameter of inlet) and the growth rate of the boundary layer is 1.2. Grid resolution D/40 is the surface element’s size. The volume mesh size is grown from the surface mesh with a growth rate of 1.2. Following the result of Supplementary Fig. 1, the grid resolution of D/30 with time-step of 0.001s has been employed for all the simulations of the present study.
Results
Effect of BA on flow rate distribution and pressure drop
Figure 2a plots the ratio (β) of the flow rate into the side-branch vessel to the inlet flow rate for various BAs. It shows that the ratio decreases as BA increases and the trend with time-averaged flow is similar to that at the maximum inlet velocity. It also shows that β decreases mildly as BA increases up to a certain BA (~ 50 o), but the decreasing rate is very small for BA > 50o. It needs to be noted again that the inlet area of side-branch vessel of Fig. 1 decreases as BA increases. Therefore, it is conjectured that the bigger inlet area of branch vessel at low BAs may also attribute to the higher flow rate into side-branch vessel. Figure 2b shows that the pressure drop between inlet and the two outlets increases as BA increases implying that the head loss of the bifurcating flow increases with the increase of BA. It also shows that the magnitude of pressure drop slightly increases as BA increases up to a certain BA(~ 50 o) and then, it is nearly constant beyond the BA.
Figure 3a compares the contours of time-averaged VZ’ (velocity parallel to SB) at the cross-section of SB inlet for BA = 30 and 90 degrees. It is noted that A/B of line A-B denotes a point on the outer/inner wall and line C-D is orthogonal to line A-B. Figure 3a shows that VZ’ along line A-B is higher(lower) near the inner(outer) wall side resulting in a higher(lower) WSS whereas VZ’ near the walls of point(C) and point(D) is higher than that near the wall of point(A). It is also noted that VZ’ profile along the line C-D is symmetric about the line A-B. To quantify the relative magnitude of flowrate to the inner/outer wall side, VZ’ profiles are plotted along the lines A-B and C-D for various BAs in Fig. 3b by using the time-averaged data and the data at the maximum inlet velocity. The left column of Fig. 3b confirms that VZ’ is quite lower in the region of outer wall side regardless of BA while VZ’ is much higher near the inner wall side with being rapidly decreased as BA increases. Therefore, the average velocity along the line A-B decreases with the increase of BA. On the other hand, the right column of Fig. 3b reveals that VZ’ profiles along the line C-D have two local maxima near the wall with one local minimum located at the center of the cross section and that WSS magnitudes on the both ends of line C-D are higher compared to the outer wall region of point(A) regardless of BA. Therefore, it can be said that the wall region of point(C) and point(D) have a lower possibility of flow separation because it is likely to occur on a wall region of lower fluid velocity25, which is marked by a dashed circle in the left column of Fig. 3b. It is also noted that the flow rate along the line C-D is bigger with the increase of BA because of the increasing two local maximum velocities. This fact explains why the flowrates to SB of BA = 30 and 90 degrees differs only by 30 percents although the flowrate to the inner wall side with BA = 30 degree is more than two times of that with BA = 90. As a result, it can be said that Fig. 3b is consistent with the result of Fig. 2a that the flow rate to SB mildly decreases with the increase of BA; beta of 30 degrees is only about 1.3 times larger than that of 90 degrees. Figure 4 plots the time-averaged pressure coefficient along the inner/outer wall for three BAs. The regions of dashed circles reveal that the upstream pressure is lower than the downstream pressure along the outer wall of SB or the three BAs except for a small portion near the bifurcating region of BA = 30, and vice versa. Considering that the momentum of blood near the outer-wall side, which is under adverse pressure gradient, is quite small compared to other parts of SB, it can be said that a reverse flow is highly possible to occur along the outer wall side of the bifurcating region and that the lager BA is, the stronger the reverse flow is because of the lower momentum near the outer wall side and the center part of the cross-sectional area (See Fig. 3). The contours of y-velocity component for three BAs are shown in Fig. 5a. Figure 5b shows vorticity (\(\:{{\upomega\:}}_{\text{x}}=\partial\:\text{w}/\partial\:\text{y}-\partial\:\text{v}/\partial\:\text{z}\)) contours in the region of adverse flow for the three cases. It shows that a stronger vortex with a shorter length of reverse flow zone is generated as BA increases qualitatively confirming the results of the preceding figures. Figure 5c and d plot the normalized lengths and heights of reverse flow zones defined in Fig. 1 for various BAs. It reveals that the correlations obtained between the length and BA by neglecting a few data at low BAs can be approximated well by a straight line. The correlations are computed as follows:
Those correlations were derived by least-square fitting formular calculated by Tecplot (Tecplot 360 Build 11.0-4-084, Tecplot Inc.). The normalized height tends to rapidly increase with the increase of BA up to around 50o ~ 60 o and approaches the asymptotic value of 0.53 as BA increases more. Therefore, it can be said that the larger BA is, the narrower the flow passage of side-branch is. This is clearly demonstrated by the two animations that show path lines generated by continuously seeding particles at inlet of the computational domain for the case of two BAs of 30 and 90 degrees. We used EnSight to visualize the pathlines and vector fields of the unsteady pulsatile flows for BA = 30 o (Fig. 6a) and BA = 90 o (Fig. 6b). This analysis demonstrated that low flow rate areas and stagnation zones form on the outer wall of the SB (mainly near the ostium) and the outer wall of the MV (relatively distal to the ostium).
Interpretation of the effect of branch angle on blood flow patterns by visualizing the pathlines and vector fields. (a) Numerical simulations of the bifurcating flow model with a 30-degree bifurcation angle. The reverse flow zone is observed along the outer wall side of the side branch. The length of the reverse flow zone is longer, and the height of the reverse flow zone is lower (narrower) than 90-degree model (Supplementary movie 1). (b) Numerical simulations of the bifurcating flow model with a 90-degree bifurcation angle. The reverse flow zone is observed along the outer wall side of the side branch. The length of the reverse flow zone is shorter, and the height of the reverse flow zone is higher (wider) than 30-degree model (Supplementary movie 2).
Effect of BA on WSS distribution
To investigate the effect of BA on WSS distribution, WSS related to the reverse flow zone shown in the animations of the preceding section are examined. Figure 7a shows WSS contours on the reverse flow zone of SB vessel for three selected BAs. It shows that the larger BA is, the stronger/wider the reverse flow zone is. It also reveals that WSS has two local maximum values when BA > 50o with the local maximum (P2) near the bifurcating region being greater than the local maximum (P1) located near the center of the reverse flow zone. Figure 7b plots the values of the local maximum WSS in the reverse flow zone of SB vessel for various BAs. It shows that WSS increases as BA increases for BA > 10o and more importantly, the correlation can be approximated well by a straight line. The correlations are given as follows:
Nondimensionalized WSS distributions along the outer/inner walls of side-branch and distal main-branch are displayed in Fig. 8. The WSS on the outer wall of the SB was observed to be smaller than that on the corresponding inner wall, and the WSS of the SB decreased as the BA increased. This trend was observed not only in the SB but also in the MV. Therefore, it can be said that the WSS in the reverse flow zone of the SB increases with the increase of BA, whereas beyond the reverse flow zone, the WSS in most regions of the SB tends to decrease as BA increases.
Discussion
We conducted a numerical study to investigate the effect of BA on the hemodynamics of branched vessels in cardiovascular flow. We simulated the unsteady equations of the flow field for ten BAs to comprehensively investigate the effect of BA on the flow distribution, pressure drop and reverse flow zone distribution in the coronary bifurcation region. What we found are as follows. (1) Numerical analysis showed that as the BA increased, the pressure drops between the inlet and outlet of the SB increased while the flow rate of the SB decreased. (2) The WSS of the outer wall of the SB was lower than that of the corresponding inner wall, and the WSS at the outer wall of the MV distal part was lower than that of the other area. (3) As the BA increased, the flow passage in the side branch became narrower. (4) We observed a reverse flow zone along the outer wall side of the side branch. The local maximum WSS in the reverse flow zone increased with the increase of BA (Fig. 6). (5) The height of the reverse flow zone rapidly increased with the increase of BA up to 50 degrees approaching the asymptotic value of 0.53. On the other hand, the length of the zone increased rapidly with the increase of BA up to about 20 degrees and then linearly decreased as BA increased more.
Atherosclerotic lesions tend to form in specific areas of the coronary arteries, particularly in regions with low WSS and disrupted blood flow26. Areas with low WSS are thought to promote endothelial cell dysfunction, leading to increased lipoprotein uptake, upregulation of leukocyte adhesion molecules, and endothelial transmigration of leukocytes, all of which contribute to the development and progression of atherosclerosis27,28. Because dramatic hemodynamic changes occur at arterial branch points, coronary bifurcations represent a typical environment that influences the progression of atherosclerosis29,30. Autopsy and various imaging studies report that atherosclerosis in coronary bifurcations most commonly occurs on the outer walls of the MV and SB, while it is less frequent in the carina (flow divider) region9,10,31,32. According to a clinical study by van der Giessen et al. conducted on old-aged individuals with angina, atherosclerosis first develops along the outer wall of a bifurcation33. As the plaque grows, it expands circumferentially from the outer wall toward the carina. Additionally, in the post-stenotic region along the outer wall, blood flow becomes slower and more turbulent, leading to oscillatory endothelial wall shear stress due to the pulsatile flow34. This oscillatory endothelial WSS further exacerbates the localized atherosclerotic environment, promoting the progression of the lesion. In our study, we also found that WSS is lowest on the outer wall of the SB, and the WSS at the outer wall of the MV distal part was lower than that of the other area, making it a vulnerable area for plaque formation. The unique strength of our findings is that even without plaque formation, the SB in the bifurcation shows a reverse flow zone, and its size and WSS intensity change consistently with the BA. This is clearly demonstrated by the two animation images (Fig. 6a and b) that show path lines generated by continuously seeding particles at inlet of the computational domain for the case of two BAs of 30 and 90 degrees. This analysis demonstrated that low flow rate areas and stagnation zones form on the outer wall of the SB (mainly near the ostium). This reverse flow zone may lead to flow stagnation, creating conditions that are conducive to atherosclerosis through mechanisms such as platelet aggregation and lipid deposition.
Coronary bifurcation lesions account for up to 20% of all percutaneous coronary interventions (PCI). Bifurcation lesions are among the most challenging clinical types due to the complexity of the intervention process, frequent ISR, and late stent thrombosis. Zhang et al. studied 1,200 patients with 1,171 bifurcation lesions and examined the impact of BA on SB occlusion after MV stenting during coronary bifurcation intervention15. This study found that the higher the BA, the higher the SB occlusion rate. Another previous study used pulsatile flow simulations on symmetrical and asymmetrical bifurcation vessels to investigate the impact of arterial branch angles on the flow field of branch vessels35. Although this study used a different vessel geometry from our present research, it showed that as the BA increased, the flow rate into the SB vessel decreased. Our experimental results also showed a similar trend, with increasing branch angles resulting in higher pressure drops between the inlet and outlet, along with a reduction in flow rate within the bifurcated vessel. The increased pressure drops and reduced flow rate on the SB side due to larger BA create a favorable environment for atherosclerotic plaque formation, especially in some regions of the vessel, such as the ostium and the outer wall opposite the carina.
The importance of the BA in initiating atherosclerosis in native coronary bifurcation vessels is undisputed. However, there is still debate over how significant BA is in predicting the prognosis of bifurcation vessels with coronary stents in clinical settings. Girasis et al. investigated the impact of BA on 5-year clinical outcomes in patients who underwent PCI for left main coronary artery (LM) as part of the SYNTAX trial. Among the 266 participants, when the distal branch angle before dilation was divided into three groups (< 82°, 82°-106°, ≥ 107°), the rate of major adverse cardiac and cerebrovascular events (MACCE) were numerically higher in larger BA group, however, there were no statistically significant differences (p = 0.99)17. Ki et al. used clinical data from the COBIS registry on coronary bifurcation treatments. They divided 462 patients undergoing LM bifurcation PCI into low LM to left anterior descending coronary artery (LAD) angle and high LM-LAD angle groups using a median branch angle of 152°, then compared target lesion failure over a median 2.87-year follow-up period36. In patients treated with the crush 2 stent technique, a significantly higher target lesion failure rate, mostly driven by revascularization, was observed in the high LM-LAD angle group compared with the low LM-LAD angle group (35.7% vs. 14.6%; adjusted hazard ratio 3.476; 95% confidence interval 1.612–7.492). On the other hand, another clinical data from the COBIS registry which divided 1,432 patients into low-angle and high-angle groups using a median branch angle of 50°, the two groups had no significant difference in the rates of MACE or revascularization of target lesions during 21-month follow-up (6.6% vs. 6.9%, p = 0.856, and 4.6% vs. 5.7%, p = 0.375, respectively)37. This study was retrospective, and the cutoff may not have been appropriate, with the added limitation of only analyzing non-LM bifurcations. In clinical data of bifurcation PCI, factors other than BA could play more significant role, which can dilute the independent impact of BA. In addition, setting a specific cutoff angle to assess risk is challenging.
Future perspectives and clinical implication
With the currently available data, it is challenging to establish an appropriate cutoff value for high-risk bifurcation angles or to provide simple and clear recommendations based on such criteria. However, there are several therapeutic approaches that clinicians can consider. A deeper understanding of the interaction between local flow conditions and the formation and rapid progression of plaques at bifurcation points can improve the ability to prospectively identify areas most likely to develop plaques in high-risk patients. For example, if plaque progression is observed in the reverse flow zone of an SB, even if it does not meet the criteria for direct invasive revascularization, we might shorten the intervals for follow-up imaging or functional testing and apply more aggressive lipid-lowering therapy. In addition, from a treatment strategy perspective, bifurcation lesions are challenging to stent precisely at the orifice, and even if the stent is properly placed, stent thrombosis and ISR can occur due to the influence of the reverse flow zone identified in our study. Therefore, drug-coated balloon (DCB) therapy, which leaves no struts behind, could be selectively applied for greater benefit to the patient. As DCB technology has evolved, even in two-stent strategies with a high metal burden, the SB DCB treatment is now being considered as a treatment option. These strategies require further research through randomized controlled trials to verify clinical outcome improvement or non-inferiority compared to conventional stent-based approaches. However, given the local hemodynamics aspect demonstrated in our study, it is theoretically reasonable to apply a strut-free strategy in the SB reverse flow zone, which has low endothelial WSS and high oscillatory shear stress.
Limitation
At first, in this experiment, we used a 3D vascular model where the MV extends straight, and the SB originates from the side. This typically corresponds to non-LM bifurcations in actual coronary arteries. For non-LM bifurcations, the bifurcation angle is developmentally less than or equal to 90 degrees. Therefore, in our study, the range of bifurcation angles in the model was set between 10 and 90 degrees. In patients’ coronary arteries, LM bifurcations often don’t have a straight structure between the LM and the left anterior descending artery, so our results cannot be directly applied or interpreted for those vessel configurations. Second, although we applied unsteady pulsatile waveforms in this experiment, the 3D vessel shape used a rigid pipe model, so it did not incorporate the elasticity or cyclic strain characteristics of actual coronary arteries. Further studies using fluid-structure interaction could address these limitations. Third, WSS predicted by the non-Newtonian model has been reported to be higher than that by the Newtonian model and therefore, the non-Newtonian model needs to be considered for a more accurate prediction of the hemodynamics of coronary artery.
Conclusions
A three-dimensional flow of a side branch vessel (SB) bifurcating with a given angle from a main straight vessel was numerically examined. Unsteady pulsatile flow was considered at Re = 300, which is based on the inlet mean velocity, diameter of the coronary vessel and the kinematic viscosity of blood, which was assumed to be a Newtonian fluid. The bifurcating flow had a bigger pressure drop with a smaller flow rate to the side branch vessel as the bifurcation angle (BA) increased. The reverse flow of SB began to appear with the BA bigger than 10 degrees because blood has a lower momentum as BA increases near the outer wall that is subject to adverse pressure gradient. The presence of a reverse flow zone indicates that blood flow is likely to become stagnant. In addition, the height/strength of reverse flow zone was found to increase with the increase of BA implying that the larger BA is, the narrower the flow passage of SB is.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Caro, C. G., Fitz-Gerald, J. M. & Schroter, R. C. Arterial wall shear and distribution of early atheroma in man. Nature 223, 1159–1160. https://doi.org/10.1038/2231159a0 (1969).
Grøttum, P., Svindland, A. & Walløe, L. Localization of atherosclerotic lesions in the bifurcation of the main left coronary artery. Atherosclerosis 47, 55–62. https://doi.org/10.1016/0021-9150(83)90071-0 (1983).
Markl, M. et al. In vivo wall shear stress distribution in the carotid artery: effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ. Cardiovasc. Imaging. 3, 647–655. https://doi.org/10.1161/circimaging.110.958504 (2010).
Ku, D. N., Giddens, D. P., Zarins, C. K. & Glagov, S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5, 293–302. https://doi.org/10.1161/01.atv.5.3.293 (1985).
Arjmandi Tash, O. & Razavi, S. E. Numerical investigation of pulsatile blood flow in a bifurcation model with a non-planar branch: the effect of different bifurcation angles and non-planar branch. Bioimpacts 2, 195–205. https://doi.org/10.5681/bi.2012.023 (2012).
Perktold, K., Peter, R. O., Resch, M. & Langs, G. Pulsatile non-newtonian blood flow in three-dimensional carotid bifurcation models: a numerical study of flow phenomena under different bifurcation angles. J. Biomed. Eng. 13, 507–515. https://doi.org/10.1016/0141-5425(91)90100-L (1991).
Shumal, M., Saghafian, M., Shirani, E. & Nili-Ahmadabadi, M. A. General Scaling Law of Vascular Tree: Optimal Principle of bifurcations in Pulsatile Flow. J. Appl. Fluid Mech. 17, 2203–2214 (2024).
Kimura, B. J. et al. Atheroma morphology and distribution in proximal left anterior descending coronary artery: in vivo observations. J. Am. Coll. Cardiol. 27, 825–831. https://doi.org/10.1016/0735-1097(95)00551-x (1996).
Kaazempur-Mofrad, M. R. et al. Characterization of the atherosclerotic carotid bifurcation using MRI, finite element modeling, and histology. Ann. Biomed. Eng. 32, 932–946. https://doi.org/10.1023/b:abme.0000032456.16097.e0 (2004).
Sun, Z. & Cao, Y. Multislice CT angiography assessment of left coronary artery: correlation between bifurcation angle and dimensions and development of coronary artery disease. Eur. J. Radiol. 79, e90–95. https://doi.org/10.1016/j.ejrad.2011.04.015 (2011).
Huo, Y. et al. Which diameter and angle rule provides optimal flow patterns in a coronary bifurcation? J. Biomech. 45, 1273–1279. https://doi.org/10.1016/j.jbiomech.2012.01.033 (2012).
Rodriguez-Granillo, G. A., Rosales, M. A., Degrossi, E., Durbano, I. & Rodriguez, A. E. Multislice CT coronary angiography for the detection of burden, morphology and distribution of atherosclerotic plaques in the left main bifurcation. Int. J. Cardiovasc. Imaging. 23, 389–392. https://doi.org/10.1007/s10554-006-9144-1 (2007).
Finet, G. et al. Structure-function relation in the coronary artery tree: from fluid dynamics to arterial bifurcations. EuroIntervention https://doi.org/10.4244/eijv6supja3 (2010). 6 Suppl J, J10-15.
Giannoglou, G. D., Antoniadis, A. P., Koskinas, K. C. & Chatzizisis, Y. S. Flow and atherosclerosis in coronary bifurcations. EuroIntervention 6, 16–23. https://doi.org/10.4244/eijv6supja4 (2010).
Zhang, D. et al. How bifurcation angle impacts the fate of side branch after main vessel stenting: a retrospective analysis of 1,200 consecutive bifurcation lesions in a single center. Catheter Cardiovasc. Interv. 85 (Suppl 1), 706–715. https://doi.org/10.1002/ccd.25858 (2015).
Lee, C. H. et al. 5-Year outcomes according to FFR of Left Circumflex Coronary Artery after Left main crossover stenting. JACC Cardiovasc. Interv. 12, 847–855. https://doi.org/10.1016/j.jcin.2019.02.037 (2019).
Girasis, C. et al. Impact of 3-dimensional bifurcation angle on 5-year outcome of patients after percutaneous coronary intervention for left main coronary artery disease: a substudy of the SYNTAX trial (synergy between percutaneous coronary intervention with taxus and cardiac surgery). JACC Cardiovasc. Interv. 6, 1250–1260. https://doi.org/10.1016/j.jcin.2013.08.009 (2013).
Nagargoje, M. S., Mishra, D. K. & Gupta, R. Pulsatile flow dynamics in symmetric and asymmetric bifurcating vessels. Phys. Fluids 33 (2021).
Jiang, B., Poon, E., Illingworth, S., Barlis, P. & Ooi, A. Pressure boundary condition investigation for side branch in coronary bifurcation simulations. In 21st Australasian Fluid Mechanics Conference (2018).
Chu, J. et al. Simulation of Non-newtonian Blood Flow in diverging bifurcated vessels. J. Appl. Fluid Mech. 17, 1204–1216 (2024).
Otero-Cacho, A. & P, M. The geometry of coronary artery bifurcations and its role in plaque formation. Clin. Cardiol. Cardiovasc. Med. 24–30. https://doi.org/10.33805/2639.6807.131 (2022).
Liu, Y. et al. Pulsatile flow simulation in arterial vascular segments with intravascular ultrasound images. Med. Eng. Phys. 23, 583–595 (2001).
Patankar, S. Numerical heat Transfer and Fluid flow (CRC, 2018).
Rabbi, M. F., Laboni, F. S. & Arafat, M. T. Computational analysis of the coronary artery hemodynamics with different anatomical variations. Inf. Med. Unlocked. 19, 100314 (2020).
Schlichting, H. & Gersten, K. Boundary-Layer Theory (Springer, 2016).
Friedman, M. H., Bargeron, C. B., Deters, O. J., Hutchins, G. M. & Mark, F. F. Correlation between wall shear and intimal thickness at a coronary artery branch. Atherosclerosis 68, 27–33. https://doi.org/10.1016/0021-9150(87)90090-6 (1987).
Nakazawa, G. et al. Pathological findings at bifurcation lesions: the impact of flow distribution on atherosclerosis and arterial healing after stent implantation. J. Am. Coll. Cardiol. 55, 1679–1687. https://doi.org/10.1016/j.jacc.2010.01.021 (2010).
Traub, O. & Berk, B. C. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc Biol. 18, 677–685. https://doi.org/10.1161/01.atv.18.5.677 (1998).
Soulis, J. V. et al. Spatial and phasic oscillation of non-newtonian wall shear stress in human left coronary artery bifurcation: an insight to atherogenesis. Coron. Artery Dis. 17, 351–358. https://doi.org/10.1097/00019501-200606000-00005 (2006).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. Jama 282, 2035–2042. https://doi.org/10.1001/jama.282.21.2035 (1999).
Toggweiler, S., Urbanek, N., Schoenenberger, A. W. & Erne, P. Analysis of coronary bifurcations by intravascular ultrasound and virtual histology. Atherosclerosis 212, 524–527. https://doi.org/10.1016/j.atherosclerosis.2010.06.045 (2010).
Oviedo, C. et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ. Cardiovasc. Interv. 3, 105–112. https://doi.org/10.1161/circinterventions.109.906016 (2010).
van der Giessen, A. G. et al. Plaque and shear stress distribution in human coronary bifurcations: a multislice computed tomography study. EuroIntervention 4, 654–661. https://doi.org/10.4244/eijv4i5a109 (2009).
Koskinas, K. C. et al. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation 121, 2092–2101. https://doi.org/10.1161/circulationaha.109.901678 (2010).
Gharahi, H. et al. A multiscale framework for defining homeostasis in distal vascular trees: applications to the pulmonary circulation. Biomech. Model. Mechanobiol. 22, 971–986. https://doi.org/10.1007/s10237-023-01693-7 (2023).
Ki, Y. J. et al. Clinical implications of bifurcation angles in left main bifurcation intervention using a two-stent technique. J. Interv. Cardiol. 2475930 (2020). https://doi.org/10.1155/2020/2475930 (2020).
Yang, J. H. et al. Impact of coronary bifurcation angle on clinical outcomes after percutaneous coronary intervention in real-world practice: results from the COBIS registry. Cardiology 122, 216–224. https://doi.org/10.1159/000338817 (2012).
Funding
This work was supported by the Specialized Academic Activities 2024 of the National Academy of Sciences, Republic of Korea and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2020R1C1C1010316) and the Catholic Medical Center Research Foundation made in the program year of 2023.
Author information
Authors and Affiliations
Contributions
K.Y.L. and H.G.C. analyzed and interpreted the data and wrote the drafts of the manuscript. S.T.H. contributed to the data curation, funding acquisition, formal analysis and investigation. K.Y.L. contributed to the conceptualization, resources, funding acquisition, and project administration. D.H.V. contributed to the methodology, visualization and writing the original draft. J.Y.Y. contributed to the supervision and investigation. H.G.C. contributed to the funding acquisition, writing review and editing. K.Y.L. is the guarantor of this work, and as such, has full access to all the data, gave final approval of the manuscript submitted. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Video 1.
Supplementary Video 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, H.G., Vo, D.H., Yoo, J.Y. et al. The influence of reverse flow within side branches on plaque formation relative to coronary bifurcation angles. Sci Rep 15, 11977 (2025). https://doi.org/10.1038/s41598-025-89427-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89427-w