Abstract
This study was designed to evaluate the impact of upper tract urothelial carcinoma (UTUC) history on prognosis in patients with non-muscle-invasive bladder cancer (NMIBC) receiving intravesical chemotherapy. We conducted a single center, retrospective, cohort study of 444 NMIBC patients who received intravesical chemotherapy after transurethral resection of the bladder cancer (TURBT) at Peking University People’s Hospital from 2000 to 2015. Patients were divided into UTUC-NMIBC group (with UTUC history) and primary NMIBC group (without UTUC history) by presence of previous UTUC. Demographic, clinical and pathologic factors were analyzed. Kaplan–Meier curves and the log-rank test were used to depict and compare recurrence-free survival (RFS) and progression-free survival (PFS) between the two groups. Multivariable Cox regression models were constructed to determine the variables associated with RFS and PFS. Compared to the primary NMIBC group (n = 410), the UTUC-NMIBC group (n = 34) had an older median age [72.0 (65.0–81.0) vs. 66.0 (58–75) years; P = 0.007], a higher incidence of multiple tumors (52.9% vs. 33.9%; P = 0.026) and a higher recurrence rate (52.9% vs. 30.7%; P = 0.008) and worse RFS (P < 0.001). In multivariate analysis, UTUC history was an independent risk factor for recurrence (hazard ratio = 2.242; P = 0.001), but not for progression. Interestingly, subgroup analysis indicated patients with recent UTUC history (≤ 24 months between UTUC and NMIBC diagnoses) were associated with increased recurrence rates (73.7% vs. 26.7%; P = 0.014). Presence of UTUC history was an independent risk factor for recurrence in patients with NMIBC who received intravesical chemotherapy, especially in those with a shorter interval between UTUC and NMIBC diagnoses.
Similar content being viewed by others
Introduction
Bladder cancer (BC), as one of the most prevalent genitourinary malignancies, accounts for an estimated 500 000 new cases and 200 000 deaths annually worldwide1. About 75% patients are initially diagnosed as non-muscle invasive bladder cancer (NMIBC) with mucosal or submucosal confined disease2. In spite of undergoing transurethral resection of the bladder tumor (TURBT) and adjuvant intravesical therapy including chemotherapy or immunotherapy, disease recurrence and progression remain prevalent, which aggravate the burden of health care system and prompt researchers’ widespread attention3.
Various predictive models have been established to stratify recurrence and progression risk, among which the European Organisation for Research and Treatment of Cancer (EORTC) and Club Urologico Espanol de Tratamiento Oncologico(CUETO) scoring systems are widely adopted, taking into account risk factors including tumor size, tumor multiplicity, age, carcinoma in situ (CIS), tumor grade, tumor stage, and previous recurrence3,4,5,6.
Upper tract urothelial carcinoma (UTUC) stands as a relatively uncommon malignancy, encompassing roughly 5–10% of urothelial carcinomas7. Patients with UTUC history exhibit an augmented risk of intravesical recurrence(IVR), with subsequent BCa occurring in 15–50% of patients who undergo radical nephroureterectomy8. However, the impact of UTUC history on the prognosis of NMIBC remains unclear and overlooked in most predictive models. As a result, its treatment strategy is still based on the guidelines of primary bladder cancer.
While Bacillus Calmette-Guérin (BCG) is the gold standard treatment in high-risk patients, its application is limited by severe toxicity, high price and persistent shortage, especially in developing countries9,10,11. Intravesical chemotherapy (IC) is considered as a reasonable alternative in such scenario. Due to aforementioned reasons, IC remains the most adopted adjuvant intravesical instillation strategy during past decades in our institution.
The goal of our research was to evaluate the impact of UTUC history on the prognosis of primary NMIBC patients receiving adjuvant IC. Through our study, we hope to improve current postoperative treatment strategy in NMIBC patients with prior UTUC.
Patients and methods
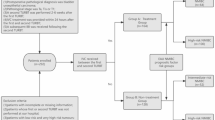
After obtaining approval from the Ethics Committee, a retrospective analysis was conducted on the medical records of patients who underwent TURBT at Peking University People’s Hospital between 2000 and 2015. Patients with primary NMIBC who underwent ‘adequate’ IC were selected. ‘Adequate’ IC was defined as at least weekly induction for the first 8 weeks and then monthly up to 6 months. The follow-up for patients after TURBT was usually every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Follow-up protocols were devised and executed in accordance with the risk stratification conducted by the EAU guidelines.
Patients were divided into two groups: those with primary NMIBC after UTUC (UTUC-NMIBC), and those without prior UTUC. Patients’ clinical characteristics and pathological features were collected: age, sex, body mass index, smoking history, hypertension, diabetes, pathological grade, tumor stage, tumor number and tumor size. Recurrence was characterized as reoccurrence of bladder tumor irrespective of stage or grade. Progression was defined as defined as progression at tumor stage or grade. Patients with previous UTUC that didn’t undergo curative treatment or suffered UTUC recurrence were excluded.
Continuous variables were reported as the median and interquartile range (IQR), while categorical variables were described as proportion of events. The clinicopathological characteristics were compared using Student’s t-test, the Wilcoxon rank test and the chi-squared test as appropriate. Kaplan-Meier survival analysis was utilized for all survival analyses. The associations between clinicopathological parameters and oncologic outcomes were evaluated through univariate and multivariate Cox proportional hazard regression analysis. Multivariate analysis was performed only for parameters with P < 0.1 in univariate analysis. Statistical significance was defined at P ≤ 0.05. IBM SPSS Statistics version 25.0 (IBM, Armonk, NY, USA) was used for all statistical analysis.
Results
Baseline characteristics
The final cohort included 444 patients of NMIBC who received sufficient IC treatment. Among them, 34 individuals had a history of UTUC, with a median interval of 24.0 months (interquartile range [IQR]: 9.0-58.3months) between UTUC and NMIBC diagnoses. The clinicopathological characteristics of these patients are detailed in Table 1. Compared to the primary NMIBC, UTUC-NMIBC group are older [median age, 72.0 (65.0–81.0) vs. 66.0 (58–75) years; P = 0.007] and less likely to have smoking history. Additionally, the UTUC-NMIBC group had a higher incidence of multiple tumors (52.9% vs. 33.9%; P = 0.026). The pathological characteristics of prior UTUC are detailed in Table 2.
Entire cohort survival analysis
Throughout the follow-up period, the UTUC-NMIBC group exhibited a higher rate of recurrences (52.9% vs. 30.7%; P = 0.008) and any-grade/stage progressions, although the latter was on the verge of statistical significance (26.5% vs. 14.6%; P = 0.067). Importantly, the UTUC-NMIBC group was associated with significantly worse recurrence-free survival (median survival, 29.91 vs. 84.00 months, P < 0.001). However, there was no discernible difference in PFS between the two groups (P = 0.117), as depicted in Fig. 1.
Univariate Cox regression analysis revealed that smoking status, tumor grade, pathological stage, multiplicity, and UTUC history were all associated with poorer recurrence-free survival. Further multivariate Cox analysis demonstrated that T1 stage, multifocal tumors, and UTUC history were still statistically significant. The presence of UTUC history was linked to a higher risk of recurrence (HR, 2.257; 95% CI, 1.368–3.725; P = 0.001). T1 stage (HR, 2.232; 95% CI, 1.405–3.545; P = 0.001) and multifocal tumors (HR, 1.564; 95% CI, 1.113–2.197; P = 0.01) were also associated with increased recurrence, with hazard ratio of 2.23 and 1.56, respectively (Table 3). However, UTUC history was not associated with an increased risk of progression in univariate and multivariate regression analysis.
Subgroup analysis in patients with NMIBC
Among patients with UTUC history, stratified by median interval between UTUC and NMIBC diagnoses, 19 individuals had shorter interval (≤ 24 months), while 15 had longer interval (> 24 months). Kaplan-Meier curve revealed a significant difference in recurrence free survival (P = 0.023; Fig. 2), with 73.7% of patients in the short interval group undergoing recurrence versus 26.7% of those in long interval (P = 0.014). Additionally, rates of progression, radical cystectomy and upgrading at cystectomy were not increased in the longer interval group compared with the longer interval group, showed in Table 4.
Discussion
Extensive attention has focused on identifying risk factors that can forecast recurrence, progression, and treatment response in NMIBC patients. Various nomograms have been created to assist healthcare providers in stratifying patient risk, with the EORTC and CUETO scoring systems being the most prevalent3,4,5,6. However, it’s noteworthy that most models excluded patients with UTUC from their analyses. The incidence of NMIBC following UTUC, also defined as intravesical recurrence(IVR), is well-documented, with 15–50% of patients undergoing radical nephroureterectomy experiencing intravesical recurrence8.
Just as two mechanisms exist behind intravesical recurrence after transurethral resection of bladder cancer12, so are both behind intravesical recurrence after prior UTUC, i.e., tumor cells intracavity transplantation following UTUC surgery and field change cancerization effect.
UTUC has a proclivity to reoccur in proximity to the cystostomy tube wall or within the bladder neck, particularly in cases where the urethral duct has suffered damage13. This observation backs the theory that cancer cells, suspended within the bladder, predominantly adhere to the compromised urethra and subsequently manifest as recurring instances through intraluminal inoculation. Notably, Habuchi and colleagues unearthed a consistent pattern of the same distinct p53 mutation in the upper urinary tract and bladder tumors of the same patient14. In a comprehensive analysis of pertinent literature, Doeveren and collaborators systematically reviewed the potential clonal connection between UTUC and BC. Their findings indicated that approximately 94% of primary UTUC cases and intravesical recurrences (IVR) share a clonal relationship15. Field change cancerization effect refers to independent tumor origin, indicating a pan-urinary tract pre-cancerous state, as UTUC and bladder cancer share many risk factors, including smoking, dye exposure and gender.
Currently, the management of patients with IVR is based on the primary bladder tumor guideline. Meanwhile, with shortage and expensiveness of BCG limiting its accessibility to lots of patients, intravesical chemotherapy is still the standard of care in many circumstances. It is thus imperative to evaluate the ramification of previous UTUC on NMIBC prognosis to improve treatment strategy.
The current study revealed that patients with UTUC history exhibited poorer response to intravesical chemotherapy for NMIBC in contrast to those without prior UTUC. These findings are in line with prior studies, which concluded presence of UTUC prior to diagnosis of NMIBC was associated with poor prognosis after BCG therapy16,17. Interestingly, the influence of prior UTUC on muscle invasive bladder cancer (MIBC) seems quite different. Shigeta found MIBC developed after radical nephroureterectomy for UTUC was different from bladder primary MIBC, characterized by higher FGFR3 expression, a protective prognostic factor18. Further tissue sample analysis revealed that molecular expression in the bladder tumor specimen changed from CK20 high, CK5/6 low expression to CK20 low CK5/6 high expression as NMIBC following UTUC progressed to MIBC, indicating a luminal-basal shift19.
A possible explanation of poor IC response is that IVR represent a special subset of NMIBC lesions, with recent data suggesting they primarily exhibit luminal characteristics and are depleted in T-cells, possibly diminishing chemotherapy-induced anti-tumor immune response20.
The current study does come with certain limitations. Firstly, the utilization of a retrospective cohort introduces the potential of unaccounted-for confounding variables. Secondly, given the infrequent occurrence of UTUC, our analysis is constrained by the relatively limited number of UTUC patients in the cohort. Thirdly, treatment variations across different surgeons were not factored in, including differences in UTUC management, the administration of re-Turbt. Lastly, our analysis was confined to patients who received a relatively sufficient amount of intravesical chemotherapy to ensure a consistently treated cohort. However, this could potentially lead to selection bias.
In summary, among patients with history of UTUC, the biological and clinical trajectory of NMIBC may substantially diverge from those with primary NMIBC. This observation seems particularly significant for those facilities troubled by BCG shortage. Under such circumstances, BCG, widely considered to be more efficacious than chemotherapy drugs, should be administered priorly to those with previous UTUC. Other options, such as immune checkpoints inhibitors or even preemptive cystectomy, should also be taken into account. In addition, considering the high recurrence follow intravesical therapy, patients with previous UTUC should undergo more intensive follow-up to detect recurrent lesion at an early stage.
Conclusion
The presence of prior UTUC correlated with a significant increase in recurrence rates following intravesical chemotherapy. UTUC history should be considered when counselling patients and designing cohorts for clinical trials. Future prospective trials are warranted to optimize interventions strategy for this high-risk subgroup.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Richters, A., Aben, K. K. H. & Kiemeney, L. The global burden of urinary bladder cancer: an update. World J. Urol. 38(8), 1895–1904 (2020).
Kamat, A. M. et al. Bladder cancer Lancet 388(10061), 2796–2810 (2016).
Sylvester, R. J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 49(3), 466–465 (2006). discussion 75 – 7.
Lammers, R. J. et al. Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer, including external validation. World J. Urol. 34(2), 173–180 (2016).
Fernandez-Gomez, J. et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J. Urol. 182(5), 2195–2203 (2009).
Cambier, S. et al. EORTC nomograms and Risk groups for Predicting recurrence, progression, and Disease-specific and overall survival in Non-muscle-invasive Stage Ta-T1 urothelial bladder Cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guerin. Eur. Urol. 69(1), 60–69 (2016).
Rouprêt, M. et al. European Association of Urology Guidelines on Upper urinary tract Urothelial Carcinoma: 2023 update. Eur. Urol. 84(1), 49–64 (2023).
Azémar, M. D., Comperat, E., Richard, F., Cussenot, O. & Rouprêt, M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol. Oncol. 29(2), 130–136 (2011).
Harvey, M. et al. Critical shortage in BCG immunotherapy: how did we get here and where will it take us? Urol. Oncol. 40(1), 1–3 (2022).
Lamm, D. L. et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J. Urol. 147(3), 596–600 (1992).
Larsen, E. S., Nordholm, A. C., Lillebaek, T., Holden, I. K. & Johansen, I. S. The epidemiology of Bacille Calmette-Guerin infections after bladder instillation from 2002 through 2017: a nationwide retrospective cohort study. BJU Int. 124(6), 910–916 (2019).
Teoh, J. Y. et al. Recurrence mechanisms of non-muscle-invasive bladder cancer - a clinical perspective. Nat. Rev. Urol. 19(5), 280–294 (2022).
Ito, A. et al. Intravesical seeding of upper urinary tract urothelial carcinoma cells during nephroureterectomy: an exploratory analysis from the THPMG trial. Jpn J. Clin. Oncol. 43(11), 1139–1144 (2013).
Habuchi, T. Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int. J. Urol. 12(8), 709–716 (2005).
van Doeveren, T. et al. Synchronous and metachronous urothelial carcinoma of the upper urinary tract and the bladder: are they clonally related? A systematic review. Urol. Oncol. 38(6), 590–598 (2020).
Bree, K. K. et al. Impact of upper tract urothelial carcinoma on response to BCG in patients with non-muscle-invasive bladder cancer. BJU Int. 128(5), 568–574 (2021).
Miyake, M. et al. Outcomes of subsequent non-muscle-invasive bladder cancer treated with intravesical Bacillus Calmette-Guerin after radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. 121(5), 764–773 (2018).
Shigeta, K. et al. The clinicopathological characteristics of muscle-invasive bladder recurrence in upper tract urothelial carcinoma. Cancer Sci. 112(3), 1084–1094 (2021).
Shigeta, K. et al. Profiling the Biological characteristics and transitions through Upper Tract Tumor Origin, bladder recurrence, and muscle-invasive bladder progression in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 23(9) (2022).
Robinson, B. D. et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat. Commun. 10(1), 2977 (2019).
Funding
This work was supported by Beijing Natural Science Foundation(L248068).
Author information
Authors and Affiliations
Contributions
Conception and design: Jiaxiang Ji and Hao Hu; Data collection, statistical analysis: Fei wang, Chin-hui Lai, Mingrui Wang and Haopu Huwriting: Jiaxiang Ji and Fei Wang, Supervision and review: Kexin Xu, Tao Xu and Hao Hu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
The study was approved by the institutional review board of Peking University People’s Hospital. The data are anonymous, and the requirement of informed consent was therefore waived. However, informed consent from subjects was still obtained from patient whenever possible.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ji, J., Wang, F., Lai, CH. et al. Impact of upper tract urothelial carcinoma history on patients with non-muscle invasive bladder cancer undergoing intravesical chemotherapy. Sci Rep 15, 5977 (2025). https://doi.org/10.1038/s41598-025-89525-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89525-9