Abstract
Epicardial adipose tissue (EAT) is associated with microvascular obstruction (MVO). However, its association with new-onset atrial arrhythmias after percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI) is unclear. We investigated the correlation of cardiac magnetic resonance (CMR)-measured EAT with MVO and its effect on new-onset atrial arrhythmias after PCI in patients with STEMI. This study employed a single-centre retrospective design. Patients diagnosed with STEMI who underwent CMR after PCI between January 2019 and January 2023 were consecutively included and followed-up regularly. Participants were categorised based on whether they developed new-onset atrial arrhythmia after PCI. In comparison to the non-arrhythmia group, the atrial arrhythmia group exhibited higher values for age, heart rate, peak hs-TnT, peak NT-proBNP, EATV, LAES, LAED, and MVO, alongside reduced LVEF. A positive association was identified between EATV and MVO. Univariate analysis using logistic regression revealed that age, heart rate, hs-TnT level, NT-proBNP level, LVEF, EATV, LAES, LAED, and MVO were significant risk factors for atrial arrhythmia. Multivariate logistic regression analysis further identified age, LAES, EATV, and MVO as independent predictors of atrial arrhythmia. ROC curve analysis produced AUC values of 0.690 for age, 0.584 for LAES, 0.607 for MVO, and 0.769 for EATV. The EATV demonstrated a strong positive relationship with MVO after PCI in patients with STEMI. Age, LAES, EATV, and MVO were independent predictors of new-onset atrial arrhythmias and exhibited substantial prognostic significance.
Similar content being viewed by others
Introduction
Acute coronary syndrome (ACS) includes ST-segment elevation myocardial infarction (STEMI) and non-ST-segment ACS (NST-ACS), which refers to a sudden decrease in blood supply to the heart. STEMI accounts for approximately 30% of all ACS cases and is associated with high morbidity and mortality1. Atrial fibrillation (AF) is the most common non-physiological arrhythmia in humans with a high incidence and poor prognosis, which affects the survival time and quality of life of patients2. Inflammation and oxidative stress play important roles in the pathogenesis of AF. Elevated serum uric acid levels are associated with endothelial dysfunction and an elevated inflammatory response. Studies have shown that the uric acid/albumin ratio is an independent predictor of new-onset AF (NOAF) development in patients with STEMI3, and many studies continue to explore atrial arrhythmias. In the new population of STEMI patients with posterior atrial arrhythmia after percutaneous coronary intervention (PCI), we will face many challenges in the study and treatment, including heart rate changes, the use of arrhythmia medications, and the risk of blood clots or bleeding. There has been an increased interest in epicardial adipose tissue (EAT), which is metabolically active between the myocardium and the epicardium that can destroy the myocardium by paracrine or vascular secretion of proactive and inflammatory factors and also promote myocardial fibrosis through direct infiltration of the myocardium4. The ‘gold standard’ for EAT in patients with atrial fibrillation is cardiac magnetic resonance (CMR) imaging, which regulates the electrophysiology of atrial myocytes and alters ion flow, leading to atrial arrhythmia5. Myocardial reperfusion can induce cardiomyocyte death, known as ‘myocardial reperfusion injury’ (RI), with irreversible consequences, including microvascular obstruction (MVO)6. AF has direct haemodynamic consequences on the coronary arteries, leading to a reduction in microvascular coronary blood flow, which can lead to microcirculatory disturbances7. Furthermore, EAT affects the development of coronary microcirculatory dysfunction (CMD) through inflammatory pathways8. EAT seems related to MVO, but its relationship with new-onset atrial arrhythmias after PCI in patients with STEMI is unclear. Our study focused on the correlation between CMR-measured EAT and MVO and its effect on new-onset atrial arrhythmias after PCI in patients with STEMI.
Methods
Study population
This retrospective, single-centre study recruited 700 consecutive patients with STEMI who underwent CMR after PCI between January 2019 and January 2023. All participants met the inclusion criteria and required PCI within 12 h of symptom onset. The discharged patients were monitored at regular intervals of 1, 3, 6, 9, and 12 months using Soss ambulatory cardiac monitoring. For symptomatic patients, arrhythmias were evaluated over three to seven days of monitoring.
Exclusion criteria comprised patients with incomplete or unclear CMR images (n = 25), significant valvular abnormalities (n = 2), moderate to severe pericardial effusion, congenital cardiac anomalies, prior myocardial infarction (n = 20), severe hepatic or renal dysfunction (n = 1), systemic inflammatory disorders, hyperthyroidism or subclinical hyperthyroidism (n = 2), severe anaemia, prior use of antiarrhythmic medications (n = 20), or a history of atrial arrhythmias (n = 20). Based on these criteria, 90 patients were excluded, and the final cohort consisted of 610 patients categorised into two groups: those with atrial arrhythmias (n = 117, including 39 with atrial fibrillation and 78 with atrial tachycardia) and those without atrial arrhythmias (n = 493) (Fig. 1).
Ethical approval was obtained from the local ethics committee. Owing to the low-risk nature of the study, the requirement for written informed consent was waived in accordance with relevant institutional guidelines.
General clinical data
Documented patient characteristics included name, body mass index (BMI), age, smoking history, presence of hypertension, diagnosis of diabetes mellitus, and medication use prior to the onset of atrial arrhythmias. Additional clinical parameters recorded included systolic blood pressure (SBP) and diastolic blood pressure (DBP) at admission, heart rate, and high-sensitivity troponin T (peak hs-TnT) and N-terminal pro-B-type natriuretic peptide (peak NT-proBNP) levels. Lipid profiles, including triglycerides (TG) and total cholesterol (TC), were assessed along with the glomerular filtration rate (eGFR), glycated haemoglobin (HbA1c), and left ventricular ejection fraction (LVEF).
CMR image analysis
The imaging department utilised a 3.0 T MRI scanner (Ingenia 3.0 T, Philips, The Netherlands) for cardiac magnetic resonance (CMR) imaging. Patients underwent CMR scanning at a median of 4 days (interquartile range, 3–6 days) after hospitalisation. Sequential imaging included short-axis cine sequences comprising 10 to 20 slices at various cross-sectional levels, long-axis cine sequences for four-chamber heart views, and late gadolinium enhancement (LGE) images for contrast-enhanced visualisation. The CMR data were analysed using Cvi42 software (v5.13.5, Circle V, Vascular Imaging, Canada). The left atrial systolic volume (LAES) and diastolic volume (LAED) were determined using the long-axis module. Within the tissue intensity module, both inner and outer cardiac chamber boundaries were manually outlined in the LGE-sequenced two-chamber heart images. Epicardial adipose tissue volume (EATV) was calculated as the area between the inner and outer chambers. Additionally, the left ventricular region was delineated to determine the no-reflow or microvascular obstruction (MVO) volume (Fig. 2).
All measurements were manually performed by two attending cardiologists with over three years of experience in CMR imaging analysis.
Statistical analysis
SPSS software (version 25.0) was used to analyse the gathered patient data. The normality of the data distribution was assessed using the Kolmogorov-Smirnov (K-S) test. Variables conforming to a normal distribution were represented as mean ± standard deviation (SD) and compared using independent samples t-tests. For categorical variables, comparisons were performed using the chi-square (χ²) test. Given the large values of TnT and BNP, these variables were recategorised according to established guidelines9,10 to facilitate meaningful comparisons based on indicator abnormalities, and ordinal data were evaluated using the rank-sum test. Indicators showing significant differences between the groups in the univariate logistic regression analysis were subsequently incorporated into a multivariate logistic stepwise regression model to identify independent risk factors for atrial arrhythmia. The identified independent predictors were further examined using ROC curve analysis to determine their predictive value for atrial arrhythmia, and the correlation between EAT and MVO volumes was calculated using Pearson’s correlation coefficient, with statistical significance defined as p < 0.05.
Results
Comparison of general data between the two groups
Compared with the non-atrial arrhythmia group, the atrial arrhythmia group had age (64.49 ± 12.70 vs. 56.24 ± 12.19) (years), heart rate (81.31 ± 17.99 vs. 77.66 ± 12.41) (beats/min), peak hs-TnT (6.8, 93.2 vs. 14.6, 85.4) (ng/L), peak NT-proBNP (31.6,68.4 vs. 44.6,55.4) (pg/mL), EATV (146.17 ± 11.52 vs. 116.30 ± 34.64) (mL), LAES (42.85 ± 21.51 vs. 35.85 ± 13.78) (mL), LAED (67.88 ± 25.45 vs. 62.81 ± 19.06) (mL), MVO (7.49 ± 3.56 vs. 6.00 ± 2.13) was high, and LVEF (49.26 ± 6.24 vs. 54.25 ± 5.27) (%) was low (p < 0.05) (Table 1; Fig. 3).
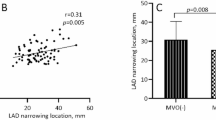
The correlation between EATV and MVO
There was a significant positive correlation between EATV and MVO volume (r = 0.433, P < 0.01) (Table 2).
Logistic regression analysis
Variables that differed significantly between the two groups were included as independent variables, with atrial arrhythmia as the dependent variable. Univariate logistic regression analysis identified the following risk factors for atrial arrhythmia: age, heart rate, peak hs-TnT level, peak NT-proBNP level, LVEF, EATV, LAES, LAED, and MVO (p < 0.05). Variables with statistical significance in univariate analysis were further incorporated into a multivariate logistic stepwise regression model to explore their independent predictive value for atrial arrhythmia. This analysis revealed that age (OR = 1.060, 95% CI: 1.038–1.082), MVO (OR = 1.098, 95% CI: 1.008–1.195), LAES (OR = 1.020, 95% CI: 1.007–1.034), and EATV (OR = 1.046, 95% CI: 1.032–1.059) were significant independent risk factors for atrial arrhythmia (p < 0.05) (Table 3).
ROC analysis
Age, MVO, EATV, and LAES were identified as independent risk factors for atrial arrhythmia. Receiver operating characteristic (ROC) curve analysis was conducted to determine the area under the curve (AUC) for these variables. The analysis yielded an AUC of 0.690 for age with a threshold of 62.5 years, an AUC of 0.584 for LAES with a cut-off of 43.53 mL, an AUC of 0.607 for MVO with a threshold of 7.825 mL, and an AUC of 0.769 for EATV with a cut-off value of 126.9 mL (Fig. 4).
Discussion
To our knowledge, this is the first study to examine the correlation between EAT and MVO and its effect on new-onset atrial arrhythmias in STEMI patients after PCI. The main findings: (1) atrial arrhythmia group versus non-atrial arrhythmia group: age, heart rate, peak hs-TnT, PeakNT-proBNP, EATV, LAED, MVO LAED, MVO was high and LVEF was low; (2) EAT was positively correlated with MVO volume; (3) age, LAES, EATV, and MVO were independent risk factors for atrial arrhythmia and had strong predictive value.
ACS is a challenging clinical disease, and STEMI patients with high morbidity and mortality require attention11. Atrial arrhythmia is a common type of arrhythmia that has been a popular topic in clinical research. Its prognosis is poor and researchers have raised different questions regarding its treatment12. This study focused on patients with STEMI and new-onset atrial arrhythmia after PCI.
Epicardial adipose tissue (EAT) exhibits both endocrine and paracrine properties, releasing bioactive molecules such as adipokines, cytokines, and inflammatory mediators. Among these, pro-inflammatory factors can infiltrate myocardial vessels, contribute to myocardial fibrosis, and ultimately result in myocardial injury13. EAT also facilitates the irregular growth of plaques, increasing the likelihood of rupture, and consequently, myocardial infarction14. Research has shown that substances secreted by the EAT influence the electrophysiology and ionic currents of atrial myocytes, significantly increasing the risk of atrial arrhythmias15. In a study involving patients with ankylosing spondylitis (AS) patients, EAT thickness was notably higher, indicating a greater predisposition to coronary artery disease and atrial fibrillation in these patients16. Recent investigations have explored the relationship between atrial fibrillation and EAT, highlighting the potential of EAT as a predictive marker for atrial fibrillation. Our findings reveal that EAT volume (EATV) is elevated in patients with atrial arrhythmias, serves as an independent risk factor for atrial arrhythmias, and offers substantial predictive value, as demonstrated in related studies17.
CMR is considered the ‘gold standard’ for accurately assessing coronary microvascular status after PCI18. The assessment of MVO using CMR in the present study agreed with the standard, which is an important factor contributing to left ventricular remodelling and death after STEMI19. It has also been shown that EAT-induced elevated levels of paracrine mediators may activate platelet aggregation and thus increase MVO and Fis. et al. noted that high EAT was associated with more MVO and greater ST deviation20, and the correlation between EAT and MVO was also confirmed in our study, with the difference that we were a retrospective study and a prospective study. Mah. et al. demonstrated that EAT mainly influences the development of CMD through the inflammatory pathway affecting the development of CMD8, which is in line with our study. Interestingly, they chose transoesophageal echocardiography to look at epicardial fat; we used CMR, we chose patients who had only had coronary angiography, and we chose patients who had PCI after STEMI; the modality and the population were different. Atrial fibrillation can have direct haemodynamic consequences in the coronary arteries, leading to a reduction in microvascular coronary blood flow and causing microcirculatory disturbances11. Our study showed that MVO is an independent risk factor and has a good predictive value in the population of new-onset atrial arrhythmias in patients with STEMI after PCI, which aligns with its viewpoint. We differed from most of the studies in this study. This study differs from most other studies, which mainly focus on the population.
In addition, age is an independent risk factor for atrial arrhythmia and has a predictive value consistent with most studies’ findings21,22. For example, an interesting study pointed out that the occurrence of atrial fibrillation after double-cavity PPM implantation is an important factor affecting the prognosis of elderly patients23. Some researchers also showed that age is a predictor of PAF in a study that first confirmed a significant relationship between the DII lead P-wave peak time and paroxysmal atrial fibrillation in patients with acute ischaemic stroke24. Left atrial volume predicts surgical success in patients with atrial fibrillation25, LAV, and LAV index can be used to detect people at high risk of malignant ventricular arrhythmias26. LAES is another manifestation of atrial remodelling and was an independent risk factor for atrial arrhythmias in our study. It had a good predictive value, which aligns with their studies and seems reasonable.
This study had several limitations. First, as a retrospective investigation, it involved a relatively small sample size and all participants were recruited from a single centre. Considering only the scarcity of STEMI patients with cardiac MRI, this study combined CMR to investigate the correlation between EAT and MVO and their effects on new atrial arrhythmias after PCI. To validate these findings and explore their broader applicability, future multicentre prospective studies focusing specifically on STEMI patients with new-onset atrial arrhythmias after PCI are warranted. Second, the arrhythmias analysed in this study were not entirely homogeneous, which may have introduced variability into the results. Third, the findings are specific to patients with STEMI with new-onset atrial arrhythmias following PCI and may not be generalisable to other patient populations. Finally, owing to the retrospective nature of this study, we were unable to monitor patients with preexisting arrhythmias, which represents a significant limitation.
Conclusion
EAT is positively correlated with MVO volume after PCI in patients with STEMI. Patient age, LAES, EATV, and MVO are independent risk factors for new-onset atrial arrhythmias and have good predictive values.
Data availability
The datasets generated during and/or analyzed during the current study are available by request fromthe corresponding author (Xyfyduanyang0125@163.com).
References
Bhatt, D. L., Lopes, R. D. & Harrington, R. A. Diagnosis and treatment of acute coronary syndromes: A review. JAMA 327, 662–675 (2022).
Pedro, B., Fontes-Sousa, A. P. & Gelzer, A. R. Canine atrial fibrillation: Pathophysiology, epidemiology and classification. Vet. J. 265, 105548 (2020).
Selçuk, M. et al. Predictive value of uric acid/albumin ratio for the prediction of new-onset atrial fibrillation in patients with ST-Elevation myocardial infarction. Rev. Invest. Clin. 74, 156–164 (2022).
Goudis, C. A., Vasileiadis, I. E. & Liu, T. Epicardial adipose tissue and atrial fibrillation: Pathophysiological mechanisms, clinical implications, and potential therapies. Curr. Med. Res. Opin. 34, 1933–1943 (2018).
Wong, C. X., Ganesan, A. N. & Selvanayagam, J. B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 38, 1294–1302 (2017).
Fröhlich, G. M., Meier, P., White, S. K., Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury: Looking beyond primary PCI. Eur. Heart J. 34, 1714–1722 (2013).
Saglietto, A. et al. A computational analysis of atrial fibrillation effects on coronary perfusion across the different myocardial layers. Sci. Rep. 12, 841 (2022).
Mahmoud, I. et al. Epicardial adipose tissue differentiates in patients with and without coronary microvascular dysfunction. Int. J. Obes. 45, 2058–2063 (2021).
Collet, J. et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42(14), 1289–1367 (2021).
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726 (2021).
Jneid, H. Insights and opportunities in STEMI care in China. JAMA Cardiol. 7, 492–493 (2022).
Huber, M. P. et al. Left atrial strain and the risk of atrial arrhythmias from extended ambulatory cardiac monitoring: MESA. J. Am. Heart Assoc. 11, e026875 (2022).
Guglielmo, M. et al. Epicardial fat and coronary artery disease: Role of cardiac imaging. Atherosclerosis 321, 30–38 (2021).
Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466 (2003).
Lin, Y. K., Chen, Y. C., Chen, J. H., Chen, S. A. & Chen, Y. J. Adipocytes modulate the electrophysiology of atrial myocytes: Implications in obesity-induced atrial fibrillation. Basic. Res. Cardiol. 107, 293 (2012).
Öz, A. et al. Evaluation of atrial conduction times and epicardial adipose tissue thickness in patients with ankylosing spondylitis. Istanbul Med. J. 21, 430–435 (2020).
Goldenberg, G. R. et al. Epicardial fat and the risk of atrial tachy-arrhythmia recurrence post pulmonary vein isolation: A computed tomography study. Int. J. Cardiovasc. Imaging. 37, 2785–2790 (2021).
De Maria, G. L. et al. Index of microcirculatory resistance as a tool to characterize microvascular obstruction and to predict infarct size regression in patients with STEMI undergoing primary PCI. JACC Cardiovasc. Imaging. 12, 837–848 (2019).
Bonfig, N. L. et al. Circadian dependence of microvascular obstruction during ST-segment elevation myocardial infarction. Int. J. Cardiol. 366, 25–29 (2022).
Fisser, C. et al. The impact of epicardial adipose tissue in patients with acute myocardial infarction. Clin. Res. Cardiol. 110, 1637–1646 (2021).
Duong, P. et al. Atrial arrhythmia after transcatheter closure of secundum atrial septal defects in patients ≥ 40 years of age. Europace 19, 1322–1326 (2017).
Krause, U. Age matters: Atrial arrhythmias in adult patients with atrioventricular septal defect. JACC Clin. Electrophysiol. 8, 341–342 (2022).
Hayıroğlu, M. İ. et al. Cardiac variables associated with atrial fibrillation occurrence and mortality in octogenarians implanted with dual chamber permanent pacemakers. Aging Clin. Exp. Res. 34, 2533–2539 (2022).
Öz, A. et al. Novel electrocardiography parameter for paroxysmal atrial fibrillation in acute ischaemic stroke patients: P wave peak time. Postgrad. Med. J. 96, 584–588 (2020).
Albano, J. Left atrial volume index predicts arrhythmia-free survival in patients with persistent atrial fibrillation undergoing cryoballoon ablation. J. Atr. Fibrillation 12, 2192 (2019).
Kaplan, A., Gurdal, A., Akdeniz, C., Kiraslan, O. & Bilge, A. K. The relationship between left atrial volume and ventricular arrhythmias in the patients with dilated cardiomyopathy. Int. Cardiovasc. Res. J. 8, 18–23 (2014).
Acknowledgements
The authors express their gratitude to the manufacturers of the offline software used for the analysis, and to the scientists for their valuable guidance.
Funding
This study was funded by the Key Research and Development Plan (Social Development) of the Science and Technology Project of Xuzhou City, Jiangsu Province (Grant No. KC23274), and the Affiliated Hospital of Xuzhou Medical University (Grant No.2022ZL.01).
Author information
Authors and Affiliations
Contributions
Q.Y. and H.L. performed the experiments and analyzed the data. S.H., Y.Y., Y.R. and Q.C. were involved in data collection. H.L. wrote the manuscript. Y.D. directed the entire research work and corrected the articles. All authors read and approve the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work is appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent
This study was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (approval no. XYFY2024-KL042-01) and conducted in compliance with the principles outlined in the Declaration of Helsinki. Owing to its retrospective design, the ethics committee waived the requirement for written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, H., Yin, Q., Han, S. et al. Correlation of epicardial adipose tissue with microvascular obstruction and its effect on new-onset atrial arrhythmias after PCI in STEMI patients. Sci Rep 15, 5052 (2025). https://doi.org/10.1038/s41598-025-89568-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89568-y