Abstract
The myodural bridge (MDB) was described as a dense fibrous tissue connecting the suboccipital musculature with the spinal dura mater. Now, the concept of the MDB was perceived as an exact anatomical structure likely essential for cerebrospinal fluid (CSF) circulation. The MDB has been shown to be universal across mammals, reptiles, and birds. To determine the existence of the MDB in other vertebrates on morphological study, representatives in amphibians and bony fishes were examined. It was found that the dense fibrous tissue connected the interarcuales muscle (IAR) and the spinal dura mater in the Xenopus laevis. In four examined fish species, somatic muscle fibers were directly anchored to the vertebral canal membrane. This observation led to the hypothesis that, during movement, these muscles may exert a pulling force on the membrane, generating negative pressure. It is speculated that this may serve as the driving force for CSF circulation. Thus, this connection suggests a functional similarity to the MDB observed in other vertebrate species. Based on this finding, the study proposes the MDB as a functionally analogous structure with a universal existence in amphibians and bony fishes.
Similar content being viewed by others
Introduction
The myodural bridge (MDB) was first described by Gary D. Hack in 1995 through an anatomical study1. The MDB consists of a bundle of connective tissue that originates from the ventral side of the rectus capitis posterior minor (RCPmi) and extends through the atlanto-occipital interspace to the spinal dura mater, where it forms a bridge-like connection1,2,3,4. Subsequent gross anatomical, histological, and imaging studies have shown that this connective tissue, MDB, also exists in the atlanto-axial interspace in humans, connecting the rectus capitis posterior major muscle (RCPma), the obliquus capitis inferior (OCI), and the nuchal ligament (NL) with the spinal dura mater2,5,6,7,8,9,10,11,12,13,14.
Many researchers have speculated that the MDB may have important physiological functions, such as preventing dural infolding and monitoring dural tension1,2,8,12. The MDB has been thought to be related to chronic cervicogenic headaches and possibly involved in regulating cerebrospinal fluid circulation1,7,10,13,15. Sui et al. proposed that the MDB might be one of the factors influencing the dynamic circulation of the CSF16. Zheng et al. proposed the concept of the “myodural bridge complex” (MDBC) in 2020, stating that MDB fibers originate from different sources in the suboccipital region and exist simultaneously, interacting with each other to form a structural and functional complex along with the RCPmi, RCPma, OCI, and NL10. It is suggested that, during movement, the MDBC may exert a pulling force on the spinal dura mater, widening the space beneath the vertebral canal and creating negative pressure. This negative pressure is speculated to be the driving force for CSF circulation. The above studies have demonstrated that the MDB is an important anatomical structure in the human suboccipital region.
By examining seven mammalian species, including Macaca mulatta, Canis familiaris, Felis catus, Oryctolagus cuniculus, Rattus norvegicus, Cavia porcellus and Neophocaena phocaenoides, Zheng et al. found that the dense connective tissue in the atlanto-occipital interspace closely resembled the MDB previously described in humans. They concluded that the MDB is a universal anatomical structure across mammalian species17. Subsequent studies reported the presence of the MDB in marine mammals (Neophocaena phocaenoides and sperm whale) as tight connective tissue18,19,20 as well as in horses21. These findings further support the conclusions of Zheng et al.
In subsequent studies, researchers observed that the MDB existed in birds (Columba livia, Gallus domesticus, and Gentoo penguins)22,23,24 and reptiles (Trachemys scripta elegans, Siamese crocodile, American alligator, and Gloydius shedaoensis)25,26,27,28. The MDB in birds was located in both the atlanto-occipital and atlanto-axial interspaces22,23,24. The MDB of Trachemys scripta elegans existed in the dorsal atlanto-occipital, atlanto-axial, and second to third cervical (C2-C3) intervertebral spaces25. In crocodiles, although the proatlas was present in the atlanto-occipital interspace, the MDB extended from the level of the proatlas to the anterior border of the C2, where trabecular-like structures ran from the posterior wall of the spinal canal to the dural sac26,27. It was noteworthy that Li et al. reported that the MDB in snakes was observed between every two adjacent vertebrae along the spine, including the atlanto-occipital interspace, the atlanto-axial interspace, and all interspaces between the second and sixth vertebra (V2-V6)28. These findings reveal that the MDB is an evolutionarily conserved anatomical structure and suggest that it may have evolved alongside the general evolution of species.
Vertebrates, a subphylum of chordates, are animals with a head and vertebrae. This subphylum includes fish, amphibians, reptiles, birds, and mammals29. Vertebrates generally possess relatively well-developed sensory organs, locomotor organs, and a highly differentiated nervous system29,30,31,32. Previous studies have investigated the existence of the MDB in mammals, birds, and reptiles17,18,19,20,21,22,23,24,25,26,27,28. Comparative morphological analyses of the MDB from these studies suggest that it is a conserved and significant anatomical structure. Moreover, these studies have shown that the presence of the MDB influences CSF circulation9,10,15,33,34. Therefore, the MDB is thought to have an important physiological function.
It is well-established that evolutionary changes and essential functions have been preserved in vertebrates30. One of the main features of vertebrate development is the formation of the vertebral canal, which contains the spinal cord, CSF, and blood vessels. However, variations exist in their structures, such as flexible head and neck movements in mammals, reptiles, and birds; restricted head and neck movements in Xenopus; and the absence of a neck in fish. Investigating whether the anatomical structure of the MDB is universal across vertebrates remains of great interest. Therefore, proving the occurrence of the MDB in amphibians and fishes is essential. This study aims to explore the histological significance of MDB structures in vertebrate evolution and the form of MDB connections in amphibians and bony fishes. These findings could provide valuable insights into the role of the MDB as a conserved anatomical structure with analogous functions across various vertebrate species.
Results
Dense fibrous connections between the IAR and the spinal dura mater were shown by Masson staining in adult Xenopus laevis
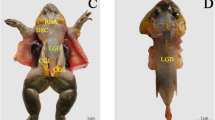
Sequential sections of Xenopus laevis were stained with Masson’s trichrome stain, highlighting muscle fibers in red and collagen fibers in green. In both sagittal and transverse sections, the muscle bundles between the longissimus dorsi muscle (LGD) and the interarcuales muscle (IAR) appeared thickened and reticulated. These bundles connected to the dorsal atlanto-occipital membrane (DAOM) and dura mater at various ventral angles (Fig. 1A and A’, B, B’). The DAOM adhered tightly to the dura, with minimal intervening connective tissue (Fig. 1A and A’, B, B’). In the coronal sections, IAR muscle fibers were arranged longitudinally, from the cranial to the caudal side, forming large mesh-like fiber bundles (Fig. 1C and C’).
Masson stained slides showing the dense fibrous connections between the IAR and the dura mater in Xenopus laevis. The dense fibrous bundles from the IAR connected with the DAOM tightly or passed through the DAOM and then fused with the spinal dura mater (arrowhead) in sagittal (A, A’), transverse (B, B’) and coronal (C, C’). The DAOM fused with the spinal dura mater tightly in all the slices.
LGD: the longissimus dorsi muscle; IAR: the interarcuales muscle; EO: exoccipital; V1: the first vertebra; SC: spinal cord; DAOM: the dorsal atlanto-occipital membrane; arrowhead: the dura mater; arrow: the MDB connections.
Dense fibrous connections between the muscle and the vertebral canal membrane were shown by Masson staining in Dicentrarchus labrax, Scophthalmus maximus, Takifugu rubripes, and Danio rerio
Sequential sections of four bony fish species were stained with Masson’s trichrome stain to visualize muscle fibers (red) and connective tissue (green). This staining was observed throughout the vertebral canal in sagittal sections, where the somatic muscles of the four species were directly attached to the vertebral canal membrane (Fig. 2). Particularly in Takifugu Rubripes, the dorsal fin muscle was associated with the vertebral canal membrane (Fig. 2C, C’). In transverse sections, both sides of the somatic muscles of the four bony fish species were connected to the vertebral canal membrane (Fig. 3). In coronal sections, the somatic muscles on both sides of the vertebral canal were closely connected to the vertebral canal membrane in all four species and were attached at an obtuse angle from rostral to caudal (Fig. 4). Some fibrous tissue originating from the myocomma was connected to the vertebral canal membrane (Figs. 2, 3 and 4).
Masson stained slides showing the dense fibrous connections between the muscles and the vertebral canal membrane in sagittal. The dense fibrous (arrow) from the muscles tightly connected with the vertebral canal membrane (arrowhead) in Dicentrarchus labrax (A, A’), Scophthalmus maximus (B, B’), Takifugu rubripes (C, C’), and Danio rerio (D, D’). m: muscle; mc: myocommata; SC: spinal cord; en: epineurals; V: vertebra; arrowhead: the vertebral canal membrane; half ring: the MDB-like connections.
Masson stained slides showing the dense fibrous connections between the muscles and the vertebral canal membrane in transverse. The dense fibrous (arrow) from the muscles tightly connected with the vertebral canal membrane (arrowhead) iin Dicentrarchus labrax (A, A’), Scophthalmus maximus (B, B’), Takifugu rubripes (C, C’), and Danio rerio (D, D’). m: muscle; mc: myocommata; SC: spinal cord; en: epineurals; V: vertebra; arrowhead: the vertebral canal membrane; half ring: the MDB-like connections.
Masson stained slides showing the dense fibrous connections between the muscles and the vertebral canal membrane in coronal. The dense fibrous (arrow) from the muscles tightly connected with the vertebral canal membrane (arrowhead) in Dicentrarchus labrax (A, A’), Scophthalmus maximus (B, B’), Takifugu rubripes (C, C’), and Danio rerio (D, D’). m: muscle; mc: myocommata; SC: spinal cord; en: epineurals; V: vertebra; arrowhead: the vertebral canal membrane; half ring: the MDB-like connections.
Discussion
The MDB in amphibians
This study demonstrated the presence of the MDB in the Xenopus laevis atlanto-occipital interspace. The LDG and IAR muscle bundles were thickened and anchored to the DAOM and dura mater, with the DAOM firmly adhering to the dura mater and some intervening connective tissue. The atlanto-occipital interspace in Xenopus laevis is narrow, and the occipital-cervical joint is rigid35,36,37. These findings confirm that the MDB forms a tight connection between the suboccipital musculature and the dura mater.
The MDB was reticulately anchored to the DAOM and dura mater, consistent with the anatomical features of Xenopus laevis. It is proposed that contraction of the dorsal occipital muscle might pull on the MDB, the DAOM, and the dura mater, inducing changes in the subdural space and creating local negative pressure, thereby exerting a pumping effect on CSF circulation.
The MDB-like structure in bony fishes
Four species of bony fish (Dicentrarchus labrax, Scophthalmus maximus, Takifugu rubripes, Danio rerio) were investigated in this study. Serial histological sections were used to examine the total vertebrae of the fish. Observations were first made at the head-to-trunk junction, where the atlanto-occipital interspace was absent38,39,40. The results revealed that the MDB was not present at the head-to-trunk junction.
However, in coronal and transverse sections, the somatic muscles were regularly arranged on both sides of the vertebrae and directly anchored to the vertebral canal membrane. Additionally, the myocomma was embedded in the vertebral canal membrane. Particularly in Takifugu rubripes, two muscle types were associated with the vertebral canal membrane on the dorsal and lateral sides: the dorsal fin muscle and the somatic muscles on both sides of the spine.
In this study, the somatic muscle was directly anchored to the vertebral canal membrane. It is hypothesized that muscle contraction could create localized negative pressure by pulling on the membrane, inducing changes in the vertebral canal interspace, thereby influencing CSF flow. This connection in fish is proposed to exert a similar effect to that of the MDB on CSF. Therefore, it is described as an MDB-like structure.
Additionally, MDB-like structures were confirmed in all adjacent vertebral interspaces of fish, exhibiting characteristics consistent with a bilateral oscillatory movement mode. It is speculated that these MDB-like structures may influence CSF flow within the vertebral canal during fish movement. Two origins of MDB-like structures in fish were identified: fibers originating from the somatic muscles on both sides of the vertebral canal, directly connected to the vertebral canal membrane, and fibrous tissue extending from the myocomma into the vertebral canal membrane. These structures likely facilitate and support the fish’s movement dynamics and CSF flow modulation.
In this study, the MDB was defined based on three components, with references to initial definitions for humans1,10,16, mammals17,18,19,20,21, reptiles25,26,27,28, and birds22,23,24. The three components are: (1) fibers originating from the suboccipital muscles; (2) fibers passing through the posterior atlanto-occipital or posterior atlanto-axial interspaces, penetrating the corresponding membranes; and (3) fibers anchored to the dura mater. Given the anatomical location between the somatic muscles and the vertebral canal membrane, it is speculated that fish possess MDB-like structures, based on previous studies summarizing the anatomical location and connections of the MDB.
The MDB is a universal anatomical structure in vertebrates
Previous studies have confirmed the presence of the MDB in seven terrestrial mammal species (Macaca mulatta, Canis familiaris, Felis catus, Oryctolagus cuniculus, Rattus norvegicus, Cavia porcellus, and horses)17,21, two marine mammal species, including the finless porpoise (Neophocaena phocaenoides) and sperm whale18,19,20, four reptilian species (Trachemys scripta elegan, Siamese crocodile, Alligator mississippiensis, Gloydius shedaoensis)25,26,27,28, and three bird species (Columba livia, Gallus domesticus, and Gentoo penguins)22,23,24 .
This study demonstrates that dense fibrous tissue was anchored to the DAOM, and the dura mater was observed in the atlanto-occipital interspace of Xenopus laevis. The study also showed the existence of the MDB in amphibians, similar to that in other vertebrates. Vertebrates include bony fishes, amphibians, reptiles, birds, and mammals. Amphibians developed a neck and began to adapt to terrestrial environments, while fish, the earliest vertebrates in the class Pisces, lacked a neck and lived in water. This raises the question: as the earliest bony vertebrates, do fish possess an MDB?
In this study, MDB-like structures were observed on both sides of the vertebral canal in fish, forming a tight connection between the somatic muscles and the vertebral canal membrane. When the somatic muscles contract, the MDB-like structures pull on the vertebral canal membrane, thus impacting CSF circulation. The MDB-like structures, as analogous organs, are speculated to play a similar role in CSF circulation as the MDB. Therefore, it is suggested that the MDB is a universal and conserved structure in vertebrates.
Functions of the MDB
Over the past decade, several studies have focused on the physiological functions of the MDB. It has been proposed that the MDB may prevent dural infolding, thereby maintaining normal cerebrospinal fluid flow within the cisterna magna or cerebellomedullaris cistern1,2,8,12. Other reports suggest that MDB dysfunction may occur under pathological conditions affecting the RCPmi muscle, potentially leading to cervicogenic headaches and other craniofacial disorders7,13,15.
Sui et al. proposed that the MDB may play an important role in CSF circulation in 201316. Xu et al. demonstrated that CSF flow rates change after head rotations, as shown by cine phase-contrast MR imaging33. The results of the present study support the view that CSF flow is impacted by head movement and that the MDB may be involved in this mechanism33. Additionally, Ma et al. demonstrated the effects of stimulated contractions of the suboccipital and atlanto-occipital muscles and passive movements of the OCI joints on CSF pressure34. The CSF pressure significantly increased when the OCI muscles were electrically stimulated, an effect that was eliminated by cutting the MDB34. Subsequently, Zheng et al. proposed the concept of the MDBC, in which the suboccipital muscles and dense connective tissue work in a coordinated pattern10. The MDB is hypothesized to play a critical role in the dynamic circulation of CSF by transmitting movement forces from the head to the spinal dura mater10,16,33,34.
It is well known that, from an evolutionary perspective, structures without function degrade over time41. In birds, the tail aids in balancing the body while flying and standing. Monkeys use their tails to balance when jumping and climbing trees42,43. In contrast, humans retain only the caudal vertebrae, which have no significant function43. The whale’s hind limbs have degraded because swimming is powered primarily by the tail fin, reducing the need for functional hind limbs. In contrast, this study found that vertebrates, including amphibians, reptiles, birds, and mammals, possess the MDB, while fish have an MDB-like structure.
Given evolutionary constraints, it appears that function has been constrained while structure has adapted during the transition from water to land. Despite morphological changes, the MDB-like structure in fish remains functionally similar to that in other vertebrates. The variability in the existence of the MDB reflects different survival and evolutionary scenarios, as well as the diversity in MDB morphology. Furthermore, this high degree of conservation suggests that MDB and MDB-like structures play important physiological roles.
The MDB transmits contractile forces from the muscles to the dura mater, influencing CSF circulation. In Neophocaena phocaenoides, the MDB is an independent muscle that originates from the caudal border of the occiput, passes through the posterior atlanto-occipital interspace, and attaches to the cervical spinal dura materr20. However, fish lack a cervical region and an atlanto-occipital interspace. This study observed tight connections between somatic muscles and the vertebral canal membrane in Danio rerio throughout the body. Similar structures were found in Dicentrarchus labrax, Scophthalmus maximus, and Takifugu rubripes. Based on this shared anatomy, the study proposes that MDB-like structures serve as analogous organs in fish, impacting CSF circulation. The discovery of these MDB-like structures suggests the widespread presence of the MDB and its potential role in CSF flow as a crucial physiological function.
Environmental changes drive alterations in both behavior and anatomy. Following the transition of vertebrates to land, locomotion shifted from side-to-side swimming to back-and-forth movement, accompanied by anatomical adaptation. The MDB-like connection between muscle fibers and the vertebral canal membrane in fish evolved into the MDB connection between the posterior occipital muscle and the dura mater in terrestrial vertebrates. This modification is considered a key adaptation for CSF circulation in terrestrial environments. Variations in MDB-like and MDB structures reflect adaptations to the distinct needs of aquatic and terrestrial life.
This study aims to analyze whether the MDB is an evolutionarily conserved anatomical structure common to vertebrates, focusing on its morphology. It is hypothesized that the MDB or MDB-like structures play a significant role in CSF circulation by transmitting forces from the head to the spinal dura mater. However, the species selection in this study is limited. Future research would benefit from including transitional species. Further functional analysis of fish is needed to determine whether MDB-like structures function as the MDB does in other vertebrates.
Conclusions
This study found that in Xenopus laevis, the MDB exists between the LDG, IAR, and the dura mater. Four fish species possess MDB-like structures between their somatic musculature and the vertebral canal membrane (Dicentrarchus labrax, Scophthalmus maximus, Takifugu rubripes, and Danio rerio). MDB-like structures may perform a function similar to that of the MDB, suggesting that MDB-like and MDB are analogous organs. Furthermore, we propose that the MDB is a common and conserved anatomical feature among vertebrates.
Materials and methods
Ethics statement
All the animal experiments were conducted in compliance with the protocol which was reviewed by the Institutional Animal Care and Use Committee and approved by the Dalian Medical University (Permit Number: 2024 No. 005).
Materials
Nine Dicentrarchus labrax and nine Takifugu rubripes were collected from Dalian Ocean University. Four Scophthalmus maximus were collected from the Lushun seafood market in Dalian. Five Xenopus laevis were purchased from the Xenopus Resource Center. Nine Danio rerio were purchased from the Shanghai FishBio Co., Ltd. In this study, all animals were adults. The experiments were carried out in accordance with the Dalian Medical University guidelines.
Methods
Histological slices and staining
Fish and Xenopus laevis were euthanized in 0.02% Tricaine. Subsequently, the samples were fixed in 10% formalin (3 days) and then decalcified with the decalcifying solution until the bones of the tissues could be easily pierced with a needle (5–8 days). The samples were then rinsed with running water overnight, dehydrated by a graded alcohol series (30%, 50%, 70%, 80%, 90%, 95%, and 100%), and then treated with xylene before being embedded in melted paraffin. The embedded specimens were sectioned (thickness 10 μm) using a rotary microtome (Leica Micro HM450; Leica Microsystems GmbH, Wetzlar, Germany). Subsequently, the sections were stained with Masson trichrome (Masson) and observed under a light microscope. All the sections were stained concurrently using the same staining protocol. All stained sections were photographed using a NIKON research light microscope.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Hack, G. D., Koritzer, R. T., Robinson, W. L., Hallgren, R. C. & Greenman, P. E. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine 20, 2484–2486. https://doi.org/10.1097/00007632-199512000-00003 (1995).
Humphreys, B. K., Kenin, S., Hubbard, B. B. & Cramer, G. D. Investigation of connective tissue attachments to the cervical spinal dura mater. Clin. Anat. N Y N. 16, 152–159. https://doi.org/10.1002/ca.10109 (2003).
Nash, L., Nicholson, H., Lee, A. S. J., Johnson, G. M. & Zhang, M. Configuration of the connective tissue in the posterior atlanto-occipital interspace: a sheet plastination and Confocal Microscopy Study. Spine 30, 1359. https://doi.org/10.1097/01.brs.0000166159.31329.92 (2005).
Zumpano, M. P., Hartwell, S. & Jagos, C. S. Soft tissue connection between rectus capitus posterior minor and the posterior atlanto-occipital membrane: a cadaveric study. Clin. Anat. N Y N 19, 522–527. https://doi.org/10.1002/ca.20220 (2006).
Scali, F., Marsili, E. S. & Pontell, M. E. Anatomical connection between the rectus capitis posterior major and the dura mater. Spine 36, E1612–1614. https://doi.org/10.1097/BRS.0b013e31821129df (2011).
Scali, F., Pontell, M. E., Enix, D. E. & Marshall, E. Histological analysis of the rectus capitis posterior major’s myodural bridge. Spine J. Off J. North. Am. Spine Soc. 13, 558–563. https://doi.org/10.1016/j.spinee.2013.01.015 (2013).
Zheng, N. et al. Definition of the to be named ligament and vertebrodural ligament and their possible effects on the circulation of CSF. PloS One 9, e103451. https://doi.org/10.1371/journal.pone.0103451 (2014).
Scali, F., Pontell, M. E., Nash, L. G. & Enix, D. E. Investigation of meningomyovertebral structures within the upper cervical epidural space: a sheet plastination study with clinical implications. Spine J. Off J. North. Am. Spine Soc. 15, 2417–2424. https://doi.org/10.1016/j.spinee.2015.07.438 (2015).
Zheng, N. et al. Orientation and property of fibers of the myodural bridge in humans. Spine J. Off J. North. Am. Spine Soc. 18, 1081–1087. https://doi.org/10.1016/j.spinee.2018.02.006 (2018).
Zheng, N. et al. The myodural bridge complex defined as a new functional structure. Surg. Radiol. Anat. SRA 42, 143–153. https://doi.org/10.1007/s00276-019-02340-6 (2020).
Pontell, M. E., Scali, F., Enix, D. E., Battaglia, P. J. & Marshall, E. Histological examination of the human obliquus capitis inferior myodural bridge. Ann. Anat. Anat. Anz off Organ. Anat. Ges. 195, 522–526. https://doi.org/10.1016/j.aanat.2013.04.013 (2013).
Pontell, M. E., Scali, F., Marshall, E. & Enix, D. The obliquus capitis inferior myodural bridge. Clin. Anat. N Y N 26, 450–454. https://doi.org/10.1002/ca.22134 (2013).
Dean, N. A. & Mitchell, B. S. Anatomic relation between the nuchal ligament (ligamentum nuchae) and the spinal dura mater in the craniocervical region. Clin. Anat. N Y N 15, 182–185. https://doi.org/10.1002/ca.10001 (2002).
Mitchell, B. S., Humphreys, B. K. & O’Sullivan, E. Attachments of the ligamentum nuchae to cervical posterior spinal dura and the lateral part of the occipital bone. J. Manipulative Physiol. Ther. 21, 145–148 (1998).
Yuan, X. Y. et al. Patterns of attachment of the myodural bridge by the rectus capitis posterior minor muscle. Anat. Sci. Int. 91, 175–179. https://doi.org/10.1007/s12565-015-0282-1 (2016).
Sui, H. J. et al. Anatomical study on the connections between the suboccipital structures and the spinal dura mater. Chin. J. Clin. Anat. 31, 489490 (2013).
Zheng, N. et al. The universal existence of myodural bridge in mammals: an indication of a necessary function. Sci. Rep. 7, 859. https://doi.org/10.1038/s41598-017-06863-z (2017).
Liu, P. et al. The myodural bridge existing in the Nephocaena phocaenoides. PloS One. 12, e0173630. https://doi.org/10.1371/journal.pone.0173630 (2017).
Liu, P. et al. The myodural bridges’ existence in the sperm whale. PloS One 13, e0200260. https://doi.org/10.1371/journal.pone.0200260 (2018).
Zhang, Z. X. et al. A specialized myodural bridge named occipital-dural muscle in the narrow-ridged finless porpoise (Neophocaena asiaeorientalis). Sci. Rep. 11, 15485. https://doi.org/10.1038/s41598-021-95070-y (2021).
McElroy, A. et al. Evaluation of the structure of Myodural bridges in an equine model of Ehlers-Danlos syndromes. Sci. Rep. 9, 9978. https://doi.org/10.1038/s41598-019-46444-w (2019).
Okoye, C. S., Zheng, N., Yu, S. B. & Sui, H. J. The myodural bridge in the common rock pigeon (Columbia Livia): morphology and possible physiological implications. J. Morphol. 279, 1524–1531. https://doi.org/10.1002/jmor.20890 (2018).
Dou, Y. R. et al. Existence and features of the myodural bridge in Gallus Domesticus: indication of its important physiological function. Anat. Sci. Int. 94, 184–191. https://doi.org/10.1007/s12565-018-00470-2 (2019).
Chen, C. et al. Existence and features of the myodural bridge in Gentoo penguins: a morphological study. PloS One 16, e0244774. https://doi.org/10.1371/journal.pone.0244774 (2021).
Huangfu, Z. et al. Existence of Myodural Bridge in the Trachemys scripta elegans: indication of its important physiological function. Int. J. Morphol. 37, 1353–1360. https://doi.org/10.4067/S0717-95022019000401353 (2019).
Zhang, J. H. et al. Connection of the Posterior Occipital Muscle and Dura Mater of the Siamese Crocodile. Anat. Rec. Hoboken NJ 2007 299, 1402–1408. (2016). https://doi.org/10.1002/ar.23445
Young, B. A. et al. The myodural bridge of the American alligator (Alligator mississippiensis) alters CSF flow. J. Exp. Biol. 223, jeb230896. https://doi.org/10.1242/jeb.230896 (2020).
Li, C. et al. Identification of the Myodural Bridge in a venomous snake, the Gloydius shedaoensis: what is the functional significance? Int. J. Morphol. 40, 304–313. https://doi.org/10.4067/S0717-95022022000200304 (2022).
Hall, B. K. Evolutionary Developmental Biology 2 (Chapman & Hall, 1998).
Shimeld, S. M. & Holland, P. W. H. Vertebrate innovations. Proc. Natl. Acad. Sci. U. S. A. 97, 4449–4452 (2000).
Hollway, G. & Currie, P. Vertebrate myotome development. Birth Defects Res. Part. C Embryo Today Rev. 75, 172–179. https://doi.org/10.1002/bdrc.20046 (2005).
Grillner, S. Evolution of the vertebrate motor system—from forebrain to spinal cord. Curr. Opin. Neurobiol. 71, 11–18. https://doi.org/10.1016/j.conb.2021.07.016 (2021).
Xu, Q. et al. Head movement, an important contributor to human cerebrospinal fluid circulation. Sci. Rep. 6, 31787. https://doi.org/10.1038/srep31787 (2016).
Ma, Y. et al. The morphology, biomechanics, and physiological function of the suboccipital myodural connections. Sci. Rep. 11, 8064. https://doi.org/10.1038/s41598-021-86934-4 (2021).
Porro, L. B. & Richards, C. T. Digital dissection of the model organism Xenopus laevis using contrast-enhanced computed tomography. J. Anat. 231, 169–191. https://doi.org/10.1111/joa.12625 (2017).
Stewart, M. M. Biology of amphibians. William E. Duellman, Linda Trueb. Q. Rev. Biol. 69, 532–532. https://doi.org/10.1086/418798 (1994).
Sefton, E. M., Bhullar, B. A. S., Mohaddes, Z. & Hanken, J. Evolution of the head-trunk interface in tetrapod vertebrates. eLife 5, e09972. https://doi.org/10.7554/eLife.09972 (2024).
Minicozzi, M., Kimball, D., Finden, A., Friedman, S. & Gibb, A. C. Are extreme anatomical modifications required for fish to move effectively on land? Comparative anatomy of the posterior axial skeleton in the cyprinodontiformes. Anat. Rec. Hoboken N. J. 303, 53–64. https://doi.org/10.1002/ar.24117 (2020).
Dietrich, K. et al. Skeletal biology and disease modeling in zebrafish. J. Bone Min. Res. Off J. Am. Soc. Bone Min. Res. 36, 436–458. https://doi.org/10.1002/jbmr.4256 (2021).
Morin-Kensicki, E. M., Melancon, E. & Eisen, J. S. Segmental relationship between somites and vertebral column in zebrafish. Dev. Camb. Engl. 129, 3851–3860. https://doi.org/10.1242/dev.129.16.3851 (2002).
Shubin, N., Tabin, C. & Carroll, S. Deep homology and the origins of evolutionary novelty. Nature 457, 818–823. https://doi.org/10.1038/nature07891 (2009).
Larson, S. G. & Stern, J. T. Maintenance of above-branch balance during primate arboreal quadrupedalism: coordinated use of forearm rotators and tail motion. Am. J. Phys. Anthropol. 129, 71–81. https://doi.org/10.1002/ajpa.20236 (2006).
Williams, S. A., Middleton, E. R., Villamil, C. I. & Shattuck, M. R. Vertebral numbers and human evolution. Am. J. Phys. Anthropol. 159, 19–36. https://doi.org/10.1002/ajpa.22901 (2016).
Acknowledgements
We thank Prof. Liu Ying of Dalian Ocean University for the provision of a specimen; Dalian Hoffen Plastination Institute for valuable discussions; as well as Xiao-Yi Liu and Ming-He Wang of Dalian Medical University for their help in photographing histological sections.
Author information
Authors and Affiliations
Contributions
H-J.S., C.C conceived the project, designed the experiments, analyzed the data, and wrote the manuscript. C.C., H.Y., Y.S., Y-L.D., J.Z., T.Q. and Y-Y.X. designed and performed most of the experiments. M.A.A.S., J-F.Z., N.Z. and S-B.Y. assisted with data analysis and interpretation and critically read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All of the described experiments were approved by the Animal Experimental Center of Dalian Medical University. All methods were carried out in accordance with relevant guidelines and regulations. The present study was designed, performed, and reported according to the principles of the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, C., Yang, H., Song, Y. et al. A new analogous organ in bony fishes and amphibians: an anatomical structure related with the cerebrospinal fluid circulation. Sci Rep 15, 5646 (2025). https://doi.org/10.1038/s41598-025-89599-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89599-5