Abstract
Species-specific wingbeat frequency of mosquitoes has already been shown to be useful in species identification. However, mosquito identification using their fundamental wingbeat frequency requires proper evaluation along with its morphological and ecological characters. An acoustic study was carried out on four species of laboratory reared mosquitoes Aedes albopictus, Culex quinquefasciatus, Anopheles crawfordi, and Armigeres subalbatus. However, a detailed study on wingbeat frequency and its variation at different points of the adult life stages was conducted for Ae. albopictus and Cx. quinquefasciatus. Recorded wingbeat beat frequency during the different point of adult life stages was analyzed using the Raven Pro 1.6.1 sound analysis software. Result showed that there was a significant difference in the fundamental frequency between four study species (F = 81.62; df = 151; p < 0.001). Wingbeat frequency of Ae. albopictus and Cx. quinquefasciatus observed to be low immediately after emergence from the pupal stage and gradually became peak during the swarming stage which is considered as the species’ fundamental frequency. These change in wingbeat frequency during the early adult stages leads to uncertainty in species identification based on the fundamental frequency. Swarming and pairing activities in Cx. quinquefasciatus and Ae. albopictus exclusively depends on the convergence between male first harmonics (M1) and female second harmonics (F2) of their fundamental frequency and make combined harmonic frequency at 1400–1500 Hz. Interestingly, this study observed that the frequency of adult male and early stages of female did not converge at the M1–F2 harmonics, thereby preventing successful mating. It thus infers that the wingbeat frequency of active male and female have significant role in the selection of potential mates within the species.
Similar content being viewed by others
Introduction
Acoustic signals play a crucial role in insect communication1. Insects produce sound by various means like stridulation, tapping of some body parts on the substrate, and continuous wing flap during the flight. In mosquitoes, wing beat sound constitutes an important mode of communication. Mosquitoes use these wingbeat frequencies for inter and intra species communication2,3 and during mating behaviours1. Johnston’s organ is important part of the mosquitoes’ auditory system located at the base of antenna, which detect auditory signals through antennal vibrations2. Different species of male mosquitoes possesses functionally different Johnston organ at the base of their antenna which helps them to detect the wingbeat frequency of females of the same species2. Flagellar hairs on the antenna of male mosquitoes detect the frequency of female mosquitoes and simulate the Johnston organs to detect their mate3. It is established that each mosquito species produces a specific wingbeat frequency used for communication among the species, and this fundamental frequency is partially overlapped in case of sibling species4,5. Studies have shown that properties of mosquito wingbeat frequency can be used as one of the identifying characters, subject to corroboration with morphological characters in the laboratory6.
In previous studies, analysis of wingbeat frequency of different mosquito species belonging to Culex, Aedes and Anopheles genera have shown that the fundamental wingbeat frequency ranges between 200 and 800 Hz and produce multiple harmonics of higher frequencies7. Studies have validated that the fundamental wingbeat frequency represents the actual wingbeat frequency of the mosquito and is responsible for communication and recognition of their own species in the mixed swarm8,9. Each sound signal produced by a mosquito is composed of several harmonics according to its fundamental frequency, and convergence of these harmonics play a crucial role to detect the mate in the swarming group10. This phenomenon has been observed well in the Anopheles gambiae and Anopheles arabiensis, which commonly perform mixed swarm8,10,11,12. The female mosquitoes produce a specific wingbeat frequency, which is differentiated by their male counterparts within the swarming group by detecting the converging sound harmonics13,14,15.
Studies on wing beat frequency provide an ideal tool to understand the behaviour of different mosquito species3,8 and identifying each wingbeat frequency in correspondence to its behaviour is crucial to develop management strategies. These behavioural manipulation techniques, such as the mosquito hearing disruption and acoustic attraction methods44, can be implemented as species-specific mosquito trapping devices for surveillance and control measures. Mosquito identification through analysis of wingbeat frequency and its application in the development of automated species identification technology has been suggested14,23,24. Mosquito traps based on wingbeat frequency has also been designed and tested17,18,19,20,21. However, detail analysis of wingbeat frequencies at different time points of adult mosquito after emergence from pupa, which can directly affect the performance of these technologies, is not available. The present study includes the documentation of wingbeat frequency changes at the different time points of adult mosquitoes, which will help in development of the species-specific management plan to control the population7,25,26.
Results
Of the four species studied Ae. albopictus has the highest wingbeat frequency (463.83 Hz–541.03 Hz ± 18.06) followed by Cx. quinquefasciatus (366.26 Hz–437.42 Hz ± 14.94), Ar. subalbatus (347.47 Hz–369.64 Hz ± 5.56), and An. crawfordi (295.29 Hz–338.29 Hz ± 10.92). One way ANOVA test showed that there was a significant difference in the typical wingbeat frequency between four study species (F = 81.62; df = 151; p < 0.001) (Supplementary file—Figure S1(a) and Table 1).
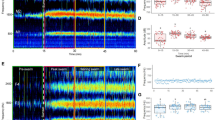
Male and female Ae. albopictus (463.83 Hz–541.03 Hz ± 18.06) and Cx. quinquefasciatus (366.26 Hz–437.42 Hz ± 14.94) mosquitoes produce different sets of wingbeat frequencies and male mosquitoes produce higher wingbeat frequencies (> 500 Hz) as compared to their respective female. Mean wingbeat frequency of male Ae. albopictus was 704.17 ± 50.49SD and in Cx. quinquefasciatus was 533.90 ± 33.98SD, whereas in female Ae. albopictus was 499.28 ± 18.06 SD and female Cx. quinquefasciatus was 414.61 ± 18.24SD (Fig. 1 & Table 2). Interestingly, Ae. albopictus female wingbeat frequency was found to be overlapping with the male frequency of Cx. quinquefasciatus. As compared to female (Aedes-18.6 SD and Culex- 18.24 SD), male mosquito wingbeat frequency has a wider range for both study species (Aedes-50.49 SD and Culex- 33.98 SD). Similar to earlier reports, it was observed that wing flap frequencies change depending on body weight and different stages of maturity. In adult individuals, fundamental frequencies increases after blood meal and slightly decreases after egg laying in both species studied (Figs. 2, 3).
This acoustic study on the adult mosquitoes showed that, the Ae. albopictus and Cx. quinquefasciatus wingbeat frequency varies at different time points after emerging from pupa and depending on the activities of the mosquito. Ae. albopictus shows very low wingbeat frequency when it hatches out (after one hour—314.10 Hz ± 10.39 SD), which gradually increases with time (7 h—389.91 Hz ± 13.09 SD; 24 h—480.87 Hz ± 15.05 SD; 36 h—502.29 Hz ± 6.5 SD) (Table 3). The wingbeat frequency stabilizes in a constant range after 24 h until blood feeding (499.28 Hz18.06 SD). Like Ae. albopictus, the same pattern was also observed in Cx. quinquefasciatus which starts with low wingbeat frequency range during their early adult stage and became constant after 36 h (1 h—244.52 ± 17.58 SD;7 h—297.87 Hz ± 10.39 SD; 24 h—356.38 Hz ± 34.11 SD; 36 h—381.99 Hz ± 25.31 SD) (Table 3).Ae. albopictus and Cx. quinquefasciatus show a very wide range of wingbeat frequencies during their respective adult stage. Ae. albopictus wingbeat frequency ranges between 290 and 696 Hz and Cx. quinquefasciatus frequency between 208 and 444 Hz during different time points of the adult stages depending on the activities. In Ae. albopictus, wingbeat frequencies from 24 h up to swarming were found to fall within the same range, which was considered to be the fundamental frequency for the particular female species. The fundamental frequencies of the four studied species plotted against the wingbeat frequencies of Ae. albopictus and Cx. quinquefasciatus at different time points after emergence from pupa, showed that the wingbeat frequencies overlap with fundamental frequencies of Ar. subalbatus and An. Crawfordi at certain points in their maturity stages (Fig. 4).
Wingbeat frequency convergence between male first harmonic (M1) and female second harmonic (F2) of fundamental frequency make a single combined harmonics, which is essential for mate selection in Cx. quinquefasciatus and Ae. albopictus. In Ae. albopictus convergence of F2-M1 was observed only in the adult individuals (1400–1500 Hz) and lead to precopulatory interactions during swarming. Whereas, the M1 harmonics of the adult male did not converge with the F2 harmonics of the immature female Ae. albopictus species (Fig. 5).
Sound Harmonicof male (Red line) and female (Black lines) wingbeat frequency of Aedes albopictusat different time points after emergence from pupa. Each line represents the harmonics of male (M1, M2, M3) and female (F1, F2, F3) followed by the fundamental frequency (FF). Harmonic convergence (F2–M1) was obsrved after 24 h of female with adult male frequency.
Discussion
Disease vector management and application of new techniques are prerequisites to curtail the risk of vector borne diseases32. Studying distribution pattern, species composition, and ecology of vectors in a given habitat and geographical area is a challenging task as compared to vertebrates. To date, several studies on mosquito vector control and management have been described29,30,31,32. Computational entomology has become a new area of research in the twenty-first century with high relevance of disease vectors’ monitoring and management7,25,26,33,34. Mosquito acoustic character is one of the important components in computational entomology which is mainly use of emerging technologies to understand insect and their ecological interaction. Studying the mosquito wingbeat sound and its properties based on the behavioural pattern may give insight into computational entomology. Species-specific wingbeat frequency has already been shown to be useful in identifying field mosquitoes25,35. However, this wingbeat frequency study revealed the necessity on detailed understanding of the acoustic character in order to use this technique for species identification.
In the present study, as shown in Fig. 3, four species of unfed female mosquitoes were found to have distinct wingbeat frequency. The wingbeat frequency of female Ae. albopictus was higher compared to other study species. The deviation of this typical frequency depends on many variables, such as the weight of the individual mosquito, wing size, maturity stage, and other environmental variables36. In addition, mosquitoes alter their wing flap speed and wing rotation during different activities, which is also reflected as changes in their wingbeat frequency37,38.
The aerodynamic properties of different mosquito species vary depending upon their behavioural pattern36,39. Aerodynamic properties in the mosquitoes include the lift, drag and thrust variations during wing flap, which enable mosquitoes to control their flight36,37. Additionally, the efficiency of wings is also a factor that controls the sound frequency. Previous studies have demonstrated that the aerodynamics buzz in mosquitoes during different behaviours contributes to the fluctuations in wingbeat frequencies26,40. Wingbeat frequency tends to be low right after emergence from the pupa and gradually peak during the transformation to an active adult which is considered as the species fundamental frequency. Wingbeat frequency plays a major role in the mating behaviour of mosquito species. It has been reported that female mosquitoes get attracted to male sound26. Male mosquitoes detect female of the same species by typical swarming loop flights around the female41. Antennal flagella hairs (plumose) in the males are induced by the fundamental frequency of female mosquitoes of the same species3 and these properties of the male antenna help them to find a mating partner even in the mixed swarm of closely related species. During swarming, this is the most active phase of the adult mosquito, the individual male and female mosquito respond to each other by altering and converging their wingbeat frequencies42.The convergence and production of harmonics is essential for mating and is specific only to certain stage of its adult life.
This study has shown that, the harmonic convergence of adult male Ae. albopictus occurs when the female wingbeat frequency corresponds to the approximately 500 Hz. Whereas in Cx. quinquefasciatus male detects their female wingbeat frequency of around 425 Hz. It was also observed that, Ae. albopictus harmonic convergence F2-M1 (1400–1500 Hz) was observed only in the adult active individuals mosquitoes, whereas wingbeat of the active adult male did not make harmonic converge with the wingbeat frequency of those female in their early stage of adult life (Fig. 5). Previous studies have shown that mosquito physical fitness and other behavioural factors also influence the wingbeat frequency level23. As described in the Figs. 2 and 3, the wingbeat frequency gradually increases after a blood meal possibly due to the increased body weight and tents to decreases after oviposition. .
These findings implicate that the wingbeat frequency of mosquito species changes as per their behavioural pattern and different time points during their adult stage. In addition, it was observed that the wingbeat frequency range of male Ae. albopictus vary within a wide range of frequency as compared to its female counterpart. As shown in the Fig. 4 and Table 2, male wingbeat frequency of Ae. albopictus (50.49 SD) and Cx. quinquefasciatus(33.98 SD) were wider in range than observed in the female mosquitoes with 18.06 SD and 18.24 SD respectively. Other previous studies elsewhere also showed that, unlike other species, the females of Ae. Albopictus have lesser accuracy in the acoustic identification of species than the male24.The typical adult wingbeat frequency of Cx. quinquefasciatus, An. crawfordi, and Ar. subalbatus was recorded between these ranges. Cx. quinquefasciatus wingbeat frequency during adult stage was recorded between 366.26 and 444.52 Hz during their adult stage and these frequencies are overlapping with the fundamental wingbeat frequency of An. crawfordi and Ar. subalbatus. The partial overlapping of the fundamental frequency of some species has already been reported4,43. These findings makes it highly doubtful in using wingbeat frequency for accurate species identification as the composition of field mosquito population at any given time will comprise of mixed individuals with different behavioural pattern and at different time points of their adult life.
Although, successful exploitation of the wingbeat frequencies to manipulate their behaviour and trap specific species of mosquitoes has been reported29,40,44, based on the findings of the present study, accurate identification of mosquito species may not be as accurate due to overlapping of wingbeat frequencies among different species of mosquitoes during different behavioural pattern and during different time points of their adult life.
Methodology
In the current study, four different species of laboratory reared mosquitoes were included namely, Aedes albopictus, Culex quinquefasciatus, Anopheles. crawfordi and Armigeres. subalbatus. The mosquito culture were maintained at 27 ± 2 °C temperature, 70–80% relative humidity and all experiments were conducted under the laboratory condition. The mosquito species were confirmed based on morphological characteristics using stage micrometer under the ‘Olympus SZX16’ Stereo zoom microscope (Olympus Corporation, Tokyo, Japan). Unfed adult mosquitoes were used for the recording of typical wing beat. To record the natural wing beat sound, each individual/ or in the group were released freely into a separate net cage (1m3) and then allowed to settle for a few minutes (Supplementary file—Figure S1(b)). The same procedure was repeated for a minimum of 25 individuals of each mosquito species to study the fundamental frequencies of four species.
Cx. quinquefasciatus and Ae. albopictus mosquitoes were used to study the variation in wingbeat frequency at different time points from adult emergence from pupa to post oviposition. Eggs of these two species were reared up to the pupation stage, followed by transfer of the pupae to the special net cage for hatching. After the emergence of the adult mosquitoes, sound data was acquired at 1 h, 7 h, 24 h, 36 h, swarming, pre blood meal and post blood meal of the adult mosquitoes. The wingbeat sounds were recorded as described above. Additionally, wing beat sounds were also recorded among free ranging mosquitoes in the field to understand the frequency range of field mosquitoes with the laboratory reared individuals and confirm the similarity in the frequencies. This study includes only laboratory experiments conducted under the controlled condition, with a temperature of 27 ± 2 oC, 70–80% relative humidity, and minimal sound disturbance to ensure the accuracy of recordings.
The wing beat sounds of mosquitoes’ were recorded by ‘Zoom H1n handy recorder’ (Zoom Coorporation, Japan). Recording was done at the 128 kbps and 80 Hz low cut voice to ensure the best quality sound without unwanted background noise. Individual mosquito of each species was kept separately and sound was recorded in mp3 format. The recorded sound files were analyzed using the Raven Pro 1.6.1 sound analysis software27 (Center for Conservation Bioacoustics 2019). Each spectrogram of the recorded sound showed the Fundamental Frequency (FF) followed by repeated harmonic peaks corresponding to the fundamental frequency of the respective species. Spectrogram of recorded mosquito sound was found to be repeatable overtones of wingbeat frequencies with respect to their fundamental frequencies (Supplementary File S1(c)). Each repeated overtones assigned as consecutive harmonics (in female—F1, F2, F3, etc. and male—M1, M2, M3). The lowest band in the spectrogram was considered as the fundamental frequency and numerical data was extracted from the spectrogram for detailed analysis. Harmonics of each sound note was measured from the spectrogram to demonstrate the mosquitoes’ sexual pairing behaviour during the swarm. Fundamental frequency was considered as the typical wingbeat frequency of the species. Frequency (Hz) and loudness level (dB)) was identified from the spectrogram of each sound note (Supplementary File—Figure S1(d)) and mean wingbeat frequency was calculated from the low and high frequency of individual note. The data was collected and analyzed using MS Excel and R statistical software (version 4.1.0) in RStudio software (version 1.4.1106) with ‘ggplot2″ package28 for the statistical analysis and graphical representation.
Data availability
The authors confirming that the raw data (Microsoft excel) which were used for this study are available from the corresponding author and is ready for submission for review at any time.
References
Drosopoulos, S. & Claridge, M. F. Insect Sounds and Communication: Physiology, Behaviour, Ecology, and Evolution. (1st ed.). CRC Press. https://doi.org/10.1201/9781420039337 (2005)
Johnston, C. Original communications: Auditory apparatus of the Culex mosquito. J. Cell Sci. 1, 97–102 (1855).
Gopfert, M. C., Briegel, H. & Robert, D. Mosquito hearing: sound-induced antennal vibrations in male and female Aedes aegypti. J. Exp Biol. 202, 2727–2738 (1999).
Briscoe, M. S. Aedes aegypti the yellow fever mosquito, its life history, bionomics and structure. J. NatMediAsso. 54, 132 (1962).
Clements, A. N. The Physiology of Mosquitoes.: international series of monographs on pure and applied biology: zoology. 17, (Elsevier, 2013)
Mukundarajan, H., Hol, F. J. H., Castillo, E. A., Newby, C. & Prakash, M. (2017). Using mobile phones as acoustic sensors for high-throughput mosquito surveillance. Elife, 6, e27854. https://doi.org/10.7554/eLife.27854 (2017)
Vasconcelos, D., Nunes, N. J. & Gomes, J. An annotated dataset of bioacoustic sensing and features of mosquitoes. Sci. Data. 7, 382 (2020).
Tripet, F., Dolo, G., Traoré, S. & Lanzaro, G. C. The" wingbeat hypothesis" of reproductive isolation between members of the Anopheles gambiae complex (Diptera: Culicidae) does not fly. J. MediEnto. 41, 375–384 (2004).
Arthur, B. J., Emr, K. S., Wyttenbach, R. A. & Hoy, R. R. Mosquito (Aedesaegypti) flight tones: Frequency, harmonicity, spherical spreading, and phase relationships. J. AcouSoci Am. 135, 933–941 (2014).
Garcia Castillo, S. S., Pritts, K. S., Krishnan, R. S., Harrington, L. C. & League, G. P. Harmonic convergence coordinates swarm mating by enhancing mate detection in the malaria mosquito Anopheles gambiae. Sci. Rep. 11, 1–18 (2021).
Cator, L. J., Ng’Habi, K. R., Hoy, R. R. & Harrington, L. C. Sizing up a mate: variation in production and response to acoustic signals in Anopheles gambiae. Behav. Ecol. 21, 1033–1039 (2010).
Wekesa, J. W., Brogdon, W. G., Hawley, W. A.&Besansky, N. J. Flight tone of field‐collected populations of An. gambiae and An.arabiensis (Diptera: Culicidae). Physiol. Entomol. 23, 289–294 (1998).
Andres, M. et al. Auditory efferent system modulates mosquito hearing. Curr Biol. 26, 2028–2036 (2016).
Raman, D. R., Gerhardt, R. R. & Wilkerson, J. B. Detecting insect flight sounds in the field: Implications for acoustical counting of mosquitoes. Trans. ASABE. 50, 1481–1485 (2007).
Warren, B., Gibson, G. & Russell, I. J. Sex recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr. Biol. 19, 485–491 (2009).
Eisen, L., Bolling, B. G., Blair, C. D., Beaty, B. J. & Moore, C. G. Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the northern Colorado Front Range. J. Mediento. 45, 800–811 (2008).
Balestrino, F. et al. A sound trap for Aedes albopictus (Skuse) male surveillance: response analysis to acoustic and visual stimuli. Acta Tropica. 164, 448–454 (2016).
Johnson, B. J., Rohde, B. B., Zeak, N., Staunton, K. M., Prachar, T. & Ritchie, S. A. A low-cost, battery-powered acoustic trap for surveilling male Aedes aegypti during rear-and-release operations. PLoS One. 13, E0201709. https://doi.org/10.1371/journal.pone.0201 709 (2018).
Rohde, B. B. et al. Waterproof, low-cost, long-battery-life sound trap for surveillance of male Aedesaegypti for rear-and-release mosquito control programmes. Parasit. Vectors. 12, 417 (2019).
Swan, T. et al. The effect of sound lure frequency and habitat type on male Aedes albopictus (Diptera: Culicidae) capture rates with the Male Aedes Sound Trap. J. Med Entomol. 58, 708–716 (2021).
Staunton, K. M. et al. Designing aedes (Diptera: Culicidae) mosquito traps: The evolution of the male aedes sound trap by iterative evaluation. Insects. 12, 388 (2021).
Menda, G. et al. The long and short of hearing in the mosquito Aedes aegypti. Curr Biol. 29, 709–714 (2019).
Offenhauser Jr, W. H.& Kahn, M. C. The sounds of disease‐carrying mosquitoes. J. Acoust. Soc. Am. 21, 259–263 (1949).
Li, Z., Zhou, Z., Shen, Z. & Yao, Q. Automated identification of mosquito (diptera: Culicidae) wingbeat waveform by artificial neural network. Inproceedings of the International Conference on Artificial Intelligence Applications and Innovations. 483–489 (2005).
Wang, J., Zhu, S., Lin, Y., Svanberg, S. & Zhao, G. Mosquito counting system based on optical sensing. Appl. Phys. B 126, 1–10 (2020).
Feugère, L., Gibson, G., Manoukis, N. C. & Roux, O. Mosquito sound communication: are male swarms loud enough to attract females?. J. R. SociInterface. 18, 1–11. https://doi.org/10.1098/rsif.2021.0121(2021).
Center for Conservation Bioacoustics. Raven Pro: Interactive sound analysis software (Version 1.6.1) [computer software]. Ithaca, NY: The Cornell Lab of Ornithology. Available at: http://ravensoundsoftware.com/ (2019).
Wickham, H. Data Analysis. In: ggplot2. Use R!. Springer, Cham. https://doi.org/10.1007/978-3-319-24277-4_9 (2016).
Lees, R. S., Gilles, J. R., Hendrichs, J., Vreysen, M. J. & Bourtzis, K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 10, 156–162 (2015).
Benelli, G. Research in mosquito control: current challenges for a brighter future. Parasitol. Res. 114, 2801–2805 (2015).
Wilke, A. B. B., Nimmo, D. D., St John, O., Kojin, B. B., Capurro, M. L. & Marrelli, M. T. Mini-review: Genetic enhancements to the sterile insect technique to control mosquito populations. Asian Pac. J. Mol. Biol. Biotechnol. 17, 65–74 (2009).
Poulin, B., Lefebvre, G., Muranyi-Kovacs, C. &Hilaire, S. Mosquito traps: an innovative, environmentally friendly technique to control mosquitoes. Int. J. Environ. Res. Public Health 14, 313 (2017).
Silva, D. F., Souza, V. M., Ellis, D. P., Keogh, E. J. & Batista, G. E. Exploring low cost laser sensors to identify flying insect species. J. Intell. Robot. Syst. 80, 313–330 (2015).
Kiskin, I. et al. Mosquito detection with neural networks: the buzz of deep learning. arXiv preprint arXiv:1705.05180. (2017).
Spitzen, J. & Takken, W. Keeping track of mosquitoes: a review of tools to track, record and analyse mosquito flight. Parasit. Vectors. 11, 1–11 (2018).
Shyy, W., Kang, C. K., Chirarattananon, P., Ravi, S. & Liu, H. Aerodynamics, sensing and control of insect-scale flapping-wing flight. In Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 472, 20150712. https://doi.org/10.1098/rspa.2015.0712 (2016).
Miller, L. A. The aerodynamics buzz from mosquitoes. Nature. 544, 40–41 (2017).
Bomphrey, R. et al. Smart wing rotation and trailing-edge vortices enable high frequency mosquito flight. Nature. 544, 92–95. https://doi.org/10.1038/nature21727 (2017).
Cheng, X. & Sun, M. Wing-kinematics measurement and aerodynamics in a small insect in hovering flight. Sci. Rep. 6, 1–12 (2016).
Mankin, R. W. Applications of acoustics in insect pest management. CAB Rev 7, 1–7 (2012).
Ng’habi, K. R. et al. Sexual selection in mosquito swarms: may the best man lose?. Anim. Behav. 76, 105–112 (2008).
Gibson, G. & Russel, J. I. Flying in tune: Sexual recognition in mosquitoes. Curr. Biol. 16, 1311–1316. https://doi.org/10.1016/j.cub.2006.05.053 (2006).
Clements, A. N. The physiology of mosquitoes: international series of monographs on pure and applied biology: Zoology, 17. (Elsevier, 2013).
Freeman, E. A., Ellis, D. A., Bagi, J., Tytheridge, S., & Andrés, M. Perspectives on the manipulation of mosquito hearing. Curr. Opin. Insect Sci., 66, 101271.https://doi.org/10.1016/j.cois.2024.101271 (2024).
Acknowledgements
We would like to thank the Research Scholars and staff Ms. Jhuma Samanta, Ms. Krishveni Vijayakumar, Ms. Bidisha Das, H. Worchan. Mr. Himalaya, Sukram Boro, and Bubai Guwala of Entomology and Bio-threat Management Division of Defence Research Laboratory for their support throughout this research. We are also wish to thank all technical and non-technical staff of Defence Research Laboratory, Tezpur.
Funding
There was no funding received for this research.
Author information
Authors and Affiliations
Contributions
P. R. Conceived the research, edited the manuscript, P. R. performed the experiments, carried out the statistical analyses and drafted the manuscript, D. G & V.M. conceived the research, coordinated the project and edited the manuscript, S. D. reviewed and edited the manuscript. V. R. & D.V.K. reviewed the research. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rajan, P., Goswami, D., Vanlalhmuaka et al. Acoustic behaviour and flight tone frequency changes in adult Aedes albopictus and Culex quinquefasciatus mosquitoes. Sci Rep 15, 15499 (2025). https://doi.org/10.1038/s41598-025-89608-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89608-7