Abstract
This study investigates the mechanisms behind direct aqueous carbonation in concrete manufacturing, focusing on its effects on workability and strength. Through an analysis of cement paste under varying water-to-cement ratios (0.5, 0.55, and 0.6) and CO2 additive amount (0%, 0.1%, and 1%), the results demonstrate that early carbonation accelerates the setting process. Higher CO2 additive amounts and lower w/c ratios further reduce setting time and fluidity, also increase free water consumption, which negatively affects the pore structure and compressive strength. However, as the transformation of amorphous calcium carbonate to calcite, enhancing hydration during curing and ultimately improving the final compressive strength. This study elucidates the various effects of CO2 addition to cement paste on fresh and hardened paste during the direct aqueous carbonation process, shedding light on how it influences hydration, workability, and strength, contributing to sustainable concrete production.
Similar content being viewed by others
Introduction
Since September 2020, when China committed to achieving carbon neutrality by 2060, Carbon Capture, Utilization, and Storage (CCUS) has attracted significant attention as a key technology for large-scale emission reduction. Although China has certain cost advantages over other countries, the current demonstration costs of CCUS technology remain relatively high, limiting its competitiveness compared to alternative technologies and hindering its broader adoption1. Specifically, in the coal and oil chemical industries, integrated enhanced oil recovery demonstration projects exhibit the lowest capture costs, reaching as low as 120 RMB per ton of CO2. In contrast, the cement industry is among the sectors with the highest capture costs in China, ranging from 180 to 730 RMB per ton of CO22,3.
In response to the high costs associated with CCUS technology in the cement industry, researchers have developed innovative carbonation techniques aimed at enhancing the efficiency and effectiveness of CO2 sequestration, with the goal of rapidly forming strength at an early stage3,4,5. The carbonation reaction, as shown in Eqs. 1–8, is considered to occur spontaneously at ambient temperature. CO2 first dissolves in water and subsequently reacts with hydration products such as calcium hydroxide and C-S-H in later stages, forming stable carbonates6,7 .
Traditional carbonation methods, conducted under natural environmental conditions, typically require a carbonation process of 28 days or more. However, this approach is time-consuming, inefficient, and the carbonation effect is difficult to control8,9,10. In response to this challenge, researchers have investigated the use of CO2 as an admixture in concrete during truck mixing. The reaction between CO2 and the hydrating cement produces finely distributed CaCO3, which subsequently influences hydration11. Durability tests indicated that this CO2 treatment did not compromise the expected durability of the treated concrete, confirming that CO2 is a viable admixture for improving concrete performance11.
Existing research indicates that the introduction of carbon dioxide can cause rapid hardening of cement-based materials, and the flowability decreases sharply with increasing carbon dioxide content. This is mainly related to the coagulation structure of hydration and carbonization products formed on the surface of cement clinker. Monkman et al.11 found that introducing carbon dioxide (0.15 -0.30%) into fresh concrete shortened the initial and final setting times by 27.6–22.2% and 25.3–20.9%, respectively. The change in setting time is attributed to the accelerated hydration and nucleation of calcium carbonate, which results in a large amount of carbonization and hydration products covering the surface of cement particles, reducing the content of free water. Wang et al.12 found that adding 0.30 -0.90% carbon dioxide can reduce the compressive strength of cement by about 10 -20%. This is because the compaction degree of carbon mixed cement is poor, and the pore structure deteriorates and the pore size distribution is poor after hardening. Similarly, He et al.13 reported that injecting carbon dioxide into concrete not only reduces the formation of C-S-H, but also prevents the dissolution of cement clinker due to the attachment of carbide products to the surface of cement particles, resulting in a decrease in compressive strength. However, Hosseinpanahi14 found that after adding 0.50% carbon dioxide to concrete, the compressive strength increased by about 5% at 7 days and 3% at 28 days, indicating that carbon dioxide has a positive effect on compressive strength.
Although existing studies15,16,17 and the Canadian company CarbonCure18 have explored the reaction mechanisms of CO2 in C-S-H and Ca(OH)2 of concrete, the detailed processes of microstructural changes and chemical reactions remain poorly understood. The mechanism behind the reduced workability observed when CO2 is introduced during concrete mixing is not yet fully clarified. This also leads to a lower absorption of CO2 when injected into the paste, accompanied by a sharp decrease in fluidity, which results in the paste’s absorption of CO2 being much less than 1% of the cement mass. Additionally, during the injection of CO2 into fresh concrete, small bubbles may form within its internal structure, but few studies have focused on high-resolution analyses of pore structures in hardened concrete using direct aqueous carbonation (DAC).
This study aims to address the challenge of limited CO2 penetration during carbonation curing, where carbonation remains superficial and does not reach deeper into the material17. To tackle this issue, CO2 is directly introduced during the cement paste mixing process, a technique referred to as DAC in this study. By selecting three different water-to-cement (w/c) ratios and three different CO2 additive amount for the preparation of cement paste, the study analyzes changes in workability during mixing, as well as the macro- and microscopic performance of the samples after molding. The microscopic features also explain the strength variations observed in cement paste with DAC. This study evaluates the impact of CO2 mixing on cement paste, particularly the effects on hardened samples and high-resolution pore structures. It elucidates the DAC mechanism from various perspectives, including the variation in hydration and workability when CO2 is intermixed with fresh cement paste, paving the way for the practical application of DAC in concrete preparation.
Materials and methods
Material
The cement used in this study is P.O. 42.5 Portland cement, with an initial setting time of 172 min and a final setting time of 236 min. The measured specific surface area is 358 m2/kg. The main components of the cement, as determined by XRF analysis, are listed in Table 1.
The CO2 used in this study was supplied by Hangzhou Jingong Special Gas Co., Ltd., with a purity of ≥ 99.5%. The gas is stored under pressure in steel cylinders and requires depressurization through a regulator before use, followed by preprocessing. The detailed apparatus for this process is described in Sect. 2.2.1.
DAC device and sample preparation of DAC cement paste
Device
For the preparation of cement paste with DAC, a custom-made mixing apparatus and gas supply system, as shown in Fig. 1(a) and (c), were used. And Fig. 1(d) is the complete process equipment diagram. The mixing apparatus has a volume capacity of 5–20 L and operates under a working pressure of 0–6 bar. In the free state, the free rotational speed of the blade is 700r/min. Internal pressure is monitored in real-time via a pressure gauge. The CO2 cylinder is connected externally to a pressure-reducing valve and linked with a copper tube, part of which is submerged in a 20 °C constant temperature water bath. This setup ensures that the CO2 gas temperature is close to 20 °C before entering the mixing apparatus. Flow meters, as depicted in Fig. 1(b), are installed at both the gas inlet and outlet of the mixing apparatus to measure the CO2 additive amount. These flow meters measure in cubic meters and can only detect unidirectional gas flow.
Additionally, to complete the preparation and curing process, a constant temperature and humidity curing chamber, cement paste molds, and a demolding gun were used. The curing chamber has a working volume of 50 L, with a temperature range of 0–85 °C and a humidity range of 20-99.9%.
Sample preparation and curing regime
This study utilized different water-to-cement (w/c) ratios and CO2 fluxes to prepare the cement paste. The setting of w/c ratio is based on the conclusions of our group’s previous studies. A ratio higher than 0.6 or lower than 0.5 will result in poor paste performance and prevent normal molding. The setting of CO 2 additive amount is also based on the previous research of our group, as detailed in the analysis in 3.2.1. The pressure inside the tank can be adjusted according to the tank volume and the amount of paste used. In this study, the maximum pressure used is 0.1 MPa, which is based on actual production to avoid safety risks caused by excessive pressure. Table 2 presents the detailed mix proportions for each group.
To prepare cement paste under DAC regimes, the required quantities of materials were measured according to the pre-set mix formulations, and the raw materials were mixed in the mixing device. Initially, a 1-minute preliminary mixing was conducted to ensure thorough blending. Subsequently, CO2 was rapidly introduced into the sealed container within 10 s, after which the gas inlet was closed. The mixture was then continuously stirred for 4 min in a high-pressure CO2 atmosphere. After mixing, the gas outlet was opened, and the outflow rate was recorded. The reacted paste was poured into pre-prepared molds, vibrated on a vibrating table for 30 s to smooth the surface, then covered and cured for 24 h before demolding.
The DAC-treated cement paste samples were demolded and placed in a constant temperature and humidity curing chamber, set at 25 °C and 65% humidity, for curing. Finally, the samples were removed at the target age for relevant testing.
Methodologies
Properties of fresh paste
The carbonation process alters the composition of the hydration products in OPC, transforming C-S-H and Ca(OH)2 into CaCO3 and silica gel, which affects the overall workability of the paste. Therefore, slump and setting time of fresh paste with or without DAC were measured accordingly.
(a) Fluidity.
The slump test for cement paste was conducted according to GB/T 8077 − 1987. First, the glass plate and truncated cone mold were moistened with a damp cloth. The glass plate dimensions were 400 × 400 × 5 mm3, and the truncated cone mold had an upper diameter of 36 mm, a lower diameter of 60 mm, and a height of 60 mm. The mixed cement paste was swiftly poured into the truncated cone mold, leveled with a straight-edged trowel, and then the mold was vertically lifted. After allowing the cement paste to flow on the glass plate for 30 s, the maximum diameters in two perpendicular directions were measured with a ruler, and the average value was calculated to represent the fluidity for that group.
(b) Setting time.
The setting time test for DAC cement paste was conducted according to GB/T 1346–2011, using a Vicat apparatus to determine the initial and final setting times. After mixing, the DAC cement paste was placed in a cone mold on a glass plate and then transferred to a constant temperature and humidity curing chamber set at 20 °C and 90% humidity. During the test, the sample was removed from the curing chamber as needed. The initial setting was determined when the initial setting needle of the Vicat apparatus sank 4 mm ± 1 mm above the bottom plate. The final setting time was determined when the Vicat needle left no trace on the sample. The initial and final setting times for each group were recorded in minutes.
(c) Semi-adiabatic calorimetry.
Semi-adiabatic calorimetry was used to describe the hydration and carbonation process of the DAC cement paste. During the semi-adiabatic calorimetry, temperature variations associated with the mixture hydration and carbonation were recorded from 20 °C by a temperature measurement instrument (HIOKI LR8400-21, SHANGHAI, CHINA). The sample preparation process can be seen in 2.2.2. Afterward, 125 g of the DAC cement paste was taken into a container, then it was placed into the instrument to record temperature variations for up to 120 h.
Properties of hardened paste
For CO2-cured concrete, the density difference before and after curing is often used as an indicator of the degree of carbonation. Therefore, the absolute dry density of all samples was measured. Additionally, DAC can affect compressive strength to varying degrees. To explain the variations in strength and carbonation levels, multiscale pore structural analyses, including the pycnometer method and X-ray computed tomography (X-CT), were conducted.
(a) Absolute dry density.
The density of DAC cement paste reflects changes in porosity and the formation of carbonation products. Because the water content in each group could not be controlled uniformly, the cement paste samples were dried in an oven at 105 °C for 3 h until a constant weight was achieved, defined as a mass difference of less than 0.5% every 30 min. The volume of each sample was measured using the displacement method: the sample was submerged in a graduated cylinder filled with water, and the volume difference before and after submersion indicated the sample’s volume. The absolute dry density of the DAC cement paste was then calculated by dividing the mass by the volume.
(b) Compressive strength.
The compressive strength of DAC cement paste samples was tested using an AI-7000 M servo-controlled tensile testing machine with a loading rate of 0.5 mm/min. Each group consisted of three parallel samples, and the average value and standard deviation were calculated.
(c) Pore structural analysis.
The testing of pore structure is uniformly carried out using 28 day old specimens to avoid inter group differences caused by incomplete carbonation and hydration reactions in the early stage. The pycnometer method was employed to measure the finer pores of the cement paste. This method relies on the volume displacement principle. A specific mass of ground powder, which passed through a 0.075 mm sieve, was placed in a pycnometer filled with anhydrous kerosene. As shown in Eq. 9, The true volume of the powder was determined by comparing volume readings before and after adding the powder. The true density of the sample was calculated from its volume and mass. Additionally, using the absolute dry density of a cubic sample, the true porosity was determined.
The total porosity of the sample, denoted as K, is calculated using the pycnometer method. Here, Vtotal represents the total volume of the sample, Vpore refers to the pore volume, and Vsolid to the volume of the solid material. These volumes are related by the equation Vtotal=Vpore+Vsolid, which similarly applies to their respective densities. The pycnometer method requires that the ground powder pass through a 0.075 mm fine sieve and be soaked in anhydrous kerosene. Since the sample is finely powdered, its true porosity is ultimately determined through calculation. To account for kerosene’s volume changes due to temperature fluctuations, the pycnometer should be immersed in a water bath throughout the experiment, and readings should be taken swiftly and precisely to minimize experimental errors.
Additionally, the pore structure of selected concrete samples was analyzed using X-ray computed tomography (X-CT). The X-CT system employed in this experiment was the XTH 320 LC from X-TEK SYSTEMS LTD, equipped with a high-resolution detector (2000 × 2000 pixels). The applied acceleration voltage was 180 kV, and the current was 220 µA. During the X-CT scan, the sample was rotated uniformly by 360° to acquire 2000 projections from different angles, with an exposure time of 0.5 s for each projection. The pixel resolution of the X-CT images was 12.5 μm/pixel. CTPro software, which uses a back-projection algorithm, was utilized to convert the dataset of each sample into grayscale images, with manual controls for noise, beam hardening, and ring artifacts. After reconstruction, VGStudio software was employed to analyze the pore structure of the samples.
Microstructural analysis of hardened paste
The characterization tests, including X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetric analysis (TG-DTG), and Fourier-transform infrared spectroscopy (FTIR), aim to investigate the hydration and carbonation degrees of cement paste with or without DAC.
To identify the mineral phases formed, XRD and FTIR analyses are performed. XRD is conducted using a Bruker D8 Advance diffractometer (Bruker Corporation, Germany) with Cu-Kα radiation (40 kV, 40 mA). The scan is executed at a rate of 0.02° 2θ/step within a range of 5° to 90° 2θ. FTIR testing is carried out using a NICOLET iS50 FT-IR spectrometer from Thermo Fisher Scientific (USA), covering a spectral range of 4000 –400 cm⁻¹ with a resolution of 4 cm⁻¹ and 32 scans.
Additionally, the Scherrer formula (Eq. 10) is used to calculate the crystal size of the CaCO3 produced by the DAC reaction.
where, Gxrd represents the grain size (nm); K is the Scherrer constant, with a value of 0.89; λ is the wavelength of the X-ray, which is 0.1541 nm for Cu-Kα; β is the full width at half maximum (FWHM) of the most intense diffraction peak (in radians); θ is the angle of diffraction.
The TG-DTG test was utilized to calculate the hydration and carbonation degrees of cement paste with and without DAC. The analysis was conducted using a Mettler Toledo STARe System TGA2 from the Swiss company Mettler Toledo. The samples were heated from 30 °C to 1000 °C in a nitrogen atmosphere at a heating rate of 10 °C/min.
The microstructural morphologies of the mineral phases in cement paste with DAC were examined using a scanning electron microscope (SEM). The samples were coated with gold to enhance their conductivity for observation. The SEM analysis was performed using an SU 8010 model from Hitachi, Ltd. (Japan), with an operating voltage of 3 kV and a working distance of 9.5 mm.
Results and discussion
Properties of fresh paste
Fluidity
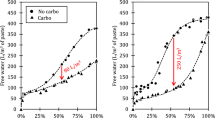
Figure 2 illustrates the fluidities of cement paste with and without DAC. As expected, the fluidity of the cement paste increases with higher water-to-cement (w/c) ratios due to the greater availability of free water in samples with larger w/c ratios. However, the incorporation of DAC significantly impairs the workability of the cement paste, as indicated by reduced fluidity. For example, introducing 0.1% CO2 results in a 16.5%, 13.2%, and 12.6% decrease in fluidity for samples with w/c ratios of 0.5, 0.55, and 0.6, respectively, compared to the control group (~ 0% CO2 additive amount). Thus, DAC markedly deteriorates the workability of the paste, even at a low CO2 additive amount of 0.1%, leading to decreased fluidity. This observation is further supported by Fig. 3, which presents on-site photographs of the flow test for selected cement pastes. The picture (a) shows good fluidity in cement paste without DAC, while the pictures (b)-(d) depict a significant reduction in fluidity and loss of plasticity following the introduction of 1% CO2 during mixing. From the graph, it can be seen that when the w/c ratio is 0.6, the expansion degree of the cement paste decreases significantly with the amount of CO2. The most severe workability damage is 0.5-1, and the paste has completely lost its fluidity. Moreover, the reduced workability of DAC cement paste could negatively impact its CO2 sequestration capacity, as discussed in Sect. 3.2.1.
Setting time
Based on the fluidity data, the initial and final setting times of cement paste with DAC are recorded in Table 3. The table shows that for samples with w/c ratios of 0.5, 0.55, and 0.6 under a 0.1% CO2 additive amount, the final setting time is accelerated by 5.1%, 6.6%, and 9.0%, respectively, compared to the control group (~ 0% CO2 additive amount). As expected, increasing the CO2 additive amount from 0.1 to 1% further shortens the final setting time. Interestingly, while a 0.1% CO2 additive amount decreases the fluidity of cement paste regardless of the w/c ratio, it has only a minimal effect on the final setting time. This is because the brief period of mixing with 0.1% CO2 primarily affects localized areas of the paste by forming nano-scale CaCO319, which increases viscosity locally without significantly altering the overall setting time20. However, when the CO2 additive amount is increased to 1%, both fluidity and setting time decrease. This effect may be attributed to accelerated hydration and carbonation processes, which increase the content of hydration and carbonation products, leading to enhanced stiffness and viscosity21.
Semi-adiabatic calorimetry
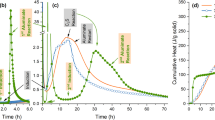
Figure 4 illustrates the hydration heat test analysis of cement paste with and without DAC process from 0 to 120 h. It can be observed from the figure that the entire DAC process can be roughly divided into three stages: (i) 0 to 40 h, (ii) 40 to 102 h, and (iii) 102 to 110 h.
Firstly, between 0 and 40 h, it can be seen that the temperature change trends of cement paste with and without DAC treatment generally remain consistent over time. This can be observed in the magnified figure, where both show a simultaneous rise and fall. However, it is evident that the early hydration temperature of the cement paste treated with 1% CO2 DAC is lower than that of the basic group. This implies that the introduction of CO2 leads to a decrease in hydration temperature during the 0 to 40-hour period. Since the CO2 is preheated before being introduced into the paste, and the carbonation reaction is an exothermic reaction, it should not result in a decrease in the temperature of the cement paste. Therefore, this early temperature reduction can only be attributed to the slowing down of the hydration reaction22. Studies have shown that the hydration process of cement slows down as the pH decreases, as the hydration reaction requires a suitable alkaline environment to proceed smoothly23. However, the introduction of CO2 leads to an increase in carbonate ions within the paste, which causes a decrease in pH, thereby weakening the early hydration reaction rate to some extent.
During the 40–102 h stage, the rate of hydration reaction gradually slows down, and there is no significant difference between the two during this period. Until 102 h, the cement paste treated with DAC suddenly experiences a small temperature peak, rising by about 1.5 °C, followed by an overall large temperature increase in the sample, which rapidly rises by 31 °C within 8 h, and then the temperature drops sharply. Since the cement paste treated with DAC only has an additional 1% CO2 compared to the basic group, only carbonation or hydration reactions are likely to occur within the paste. After consulting the literature, it is found that the temperature rise at 102 h is likely related to the crystalline transformation of calcium carbonate, and the \(\:\varDelta\:\text{H}\) between the ACC and calcite is about 53.33 ± 1.38 kJ/mol24. Many studies believe that the CO2 introduced into the paste early on forms calcium carbonate crystals mostly in the form of Amorphous Calcium Carbonate (ACC)13 and this ACC is also one of the main reasons for the decrease in fluidity. Around 102 h, the paste has hardened, and with the progress of the hydration reaction, the water content is also rapidly decreasing. At this time, ACC quickly transforms into calcite or vaterite, and the related transformation sequence is as follows24:
Due to the enthalpy difference between ACC and calcite, heat is released during the transformation process. Combining the heat release with temperature changes, the calculation formula is as follows:
where \(\:\varDelta\:\text{T}\) represents the change in temperature, m is the mass of the substance, C is the specific heat capacity of the substance.
Given the mass of the cement paste is 150 g, the W/C ratio is 0.6, and the CO2 flux is 1%, we can roughly calculate that the temperature rise is about 1 °C. However, since it is assumed that all forms of calcium carbonate have transformed from ACC to calcite, the actual transformation amount will be slightly less, and the specific heat capacity of cement will also be lower. Therefore, there will be a slight discrepancy between the actual and calculated values, but the overall change is basically consistent, which can confirm that this part of the temperature difference is due to the crystalline transformation of calcium carbonate. Subsequently, between 102 h and 110 h, the temperature climbed significantly by 31 °C. The reason for this temperature change is the concentrated and rapid hydration of cement, leading to a sharp increase in the temperature of the paste. Thus, although the decrease in pH early on led to a slower hydration rate of the DAC cement paste, around 100 h, the paste will undergo rapid hydration again. Further quantitative analysis is needed to determine the final degree of hydration and its impact on strength. In summary, after CO2 is added to the cement paste, the early hydration heat decreases and the hydration reaction weakens due to the decrease in pH value. But under the action of calcium carbonate, the hydration heat is increased in the later stage, and similar situations have also appeared in other studies25,26.
Properties of hardened paste
CO2 absorption
By recording and calculating the difference between the CO2 additive amount entering and exiting during the preparation process, the amount of CO2 encapsulated in the paste can be determined, as shown in Table 4. This table excludes data from groups without DAC. The results indicate that at a 0.1% CO2 additive amount, the outflow for all three w/c ratios of DAC cement paste is recorded as 0 m3. However, at a 1% CO2 additive amount, only the cement paste with a w/c ratio of 0.6 encapsulated all the CO2, while 33% and 15% of the CO2 were not absorbed in the other two groups; this unabsorbed CO2 was expelled from the stirring vessel. Initially, CO2 dissolves in the pore solution of the cement paste, forming CO32− ions. These ions then react with Ca²⁺ leached from the cement paste to form CaCO3 precipitates. As the reaction progresses, the availability of free Ca2+ ions and unoccupied reaction sites within the pore structure diminishes, leading to a lower reaction rate and reduced efficiency in capturing additional CO227. Thus, at a 1% CO2 additive amount, although the hydration process is accelerated, the finite nature of Ca²⁺ leaching from the cement paste limits the amount of Ca2+ available for reaction with CO32−, reducing CaCO3 formation and CO2 capture efficiency. Additionally, as CO2 reacts to form CaCO3, the concentration of CO32− ions in the pore solution increases, which decreases the solubility of additional CO2, thereby slowing the rate of CO2 dissolution and participation in the carbonation reaction. However, a higher w/c ratio increases the amount of free water, enhancing CO2 solubility and capture efficiency of DAC cement paste, as reflected in Table 4. Therefore, the w/c ratio is a crucial factor in the DAC process, with the amount of free water determining the CO2 capture capacity of DAC cement paste27.
Compressive strength
Figure 5 clearly illustrates the changes in compressive strength of DAC cement paste with different water-to-cement (w/c) ratios over curing periods of 3, 7, and 28 days. It can be observed that as the w/c ratio increases, the compressive strength of the samples without CO2 addition tends to decrease. This is due to the higher w/c ratio leading to increased porosity, which in turn reduces the material’s density and strength.
However, during the early curing stage (around 3 days), samples with DAC addition exhibit higher compressive strength compared to the control group without DAC. This indicates that DAC treatment can effectively enhance the strength of cement paste at an early stage. With the increase of CO2 additive, the compressive strength at the early stage is significantly improved, which is attributed to the transformation of Ca(OH)2 into nano-sized CaCO3, reducing porosity and increasing material density28.
In the later curing stage (around 28 days), the changes in compressive strength of the samples are more complex. For a fixed w/c ratio, the strength of the samples initially decreases and then increases with the increase of CO2 additive. At w/c ratios of 0.5 and 0.55, the strength of samples with 1% CO2 additive is lower than that of the control group, possibly due to the increased CO2 content affecting the workability of the paste, thereby affecting the strength. However, at a w/c ratio of 0.6, a 1% CO2 additive significantly enhances the paste strength by 10.8% compared to the control group, indicating that under appropriate w/c ratios, DAC treatment can significantly improve the long-term strength of the material.
Overall, the impact of CO2 additives on the strength of cement paste is multifaceted and closely related to the w/c ratio and curing time. Under suitable conditions, DAC treatment can significantly enhance the strength of cement paste, but the specific mechanisms of enhancement require further research to clarify.
Pore structural analysis
X-ray computed tomography (X-CT) is employed to analyze the pore structure of selected concrete, complemented by the pycnometer method for testing micro-pores below the resolution of X-CT (< 3 μm). Figure 6 illustrates the three-dimensional pore distribution for a w/c ratio of 0.55 under CO2 additive amount of 0%, 0.1%, and 1%. Data analysis reveals the average and maximum pore volumes for each group, as shown in Table 5. When the CO2 additive amount is 0%, the sample exhibits a more compact structure with a uniform pore distribution. Upon introducing 0.1% CO2, the pore distribution visibly increases, with the maximum pore volume reaching 0.886 mm3. At a 1% CO2 additive amount, the paste’s loss of plasticity leads to larger pores, with the maximum pore volume reaching 1.435 mm3.
Table 5 also compares the porosity obtained from X-CT with that from the pycnometer method, indicating the proportion of micro-pores (< 3 μm). The data show that after CO2 introduction, the proportion of micro-pores gradually decreases, suggesting that carbonate precipitates are filling voids within the sample. This filling primarily occurs on the surface of cement particles and between hydration products, where amorphous CaCO3 adheres and reduces the proportion of micro-pores. However, research indicates that interlayer pores of C-S-H and pores between hydration products do not significantly affect strength29,30. Consequently, early DAC treatment has a notably detrimental effect on the pore structure of hardened samples, leading to a significant reduction in sample strength.
Microstructural analysis of hardened paste
XRD
Figure 7 presents the X-ray diffraction (XRD) patterns of cement paste with a w/c ratio of 0.55 under CO2 additive amounts of 0%, 0.1%, and 1% at ages of 3, 7, and 28 days. Given that the mineral phase changes primarily depend on the CO2 additive amount, the XRD patterns for the other w/c ratios (0.5 and 0.6) are similar and thus omitted for brevity. The XRD patterns reveal diffraction peaks for, Portlandite (CH), Quartz (Q), Calcite (C), and the phases of C2S and C3S. As the CO2 additive amount increases, the intensity of the calcite peaks gradually strengthens. Additionally, the peak intensity of Ca(OH)2 at 28 days is highest with no CO2, followed by 1%, and lowest at 0.1%. This trend is attributed to the formation of CaCO3 precipitates by CO2, which serve as nuclei for subsequent hydration and accelerate the hydration rate. However, because CO2 initially consumes a significant amount of Ca(OH)2, there is a notable difference in Ca(OH)2 content among the groups at earlier ages. Although C3S and C2S also react with CO2, their reaction is slower, and the amount consumed by carbonation is relatively low.
Therefore, in this study, the difference in C3S content between 3 and 28 days is used to assess the hydration rate. The XRD patterns were quantitatively analyzed using Jade 9.0, and the results are shown in Fig. 8. It is important to note that the relative content represents only the proportion of a specific phase within the current group. Due to differences in mix ratios among the groups and the presence of products such as C-S-H and other amorphous substances that cannot be quantitatively analyzed by XRD, accurate comparisons between groups are not possible. Thus, only a semi-quantitative analysis can be performed.
Figure 8 shows that at 3 days, the C3S content among the groups is nearly identical, indicating they start from a similar baseline. By 7 days, noticeable differences emerge among the groups. By 28 days, the C3S consumption in the group with 1% CO2 has increased by 51.5% compared to the control group. This suggests that the introduction of CO2 not only produces a substantial amount of carbonate precipitates at an early age, enhancing early strength but also that these precipitates act as nuclei, promoting the growth of hydration products. This, in turn, benefits the strength gain of the samples at later stages.
Figure 9 presents the XRD patterns for three w/c ratios at CO2 additive amounts of 0.1% and 1%, highlighting the early precipitation of calcite. The samples were analyzed on the third day to minimize interference from ongoing curing effects on calcite formation.
According to Eq. (10), the crystal size of CaCO3 was calculated, as shown in Fig. 10. As the carbonation rate increases, the diffraction peaks become sharper, indicating a more complete calcite lattice. Additionally, under the same CO2 additive amount, sharper peaks are observed with increased water usage, highlighting the crucial role of free water in the early carbonation process of cement paste. When CO2 dissolves in water, more free water facilitates the formation of CO32− ions. This leads to a lower concentration of CO32−, thereby extending the carbonation duration. Research indicates that calcite crystallinity improves with carbonation time; longer carbonation periods result in better-crystallized calcite31. As seen in Fig. 10, with a w/c ratio of 0.6 and a 1% CO2 additive amount, the calcite crystals exhibit the best crystallization. Well crystallized calcite can better improve the strength of the sample32, which is consistent with the results of previous strength analysis.
FTIR
Figure 11 presents the FTIR spectra of DAC cement paste with a w/c ratio of 0.55 at curing ages of 3 and 28 days. The characteristic peak at 3644 cm− 1 is associated with the stretching vibrations of the hydroxyl group (O-H). Peaks in the 2900 cm− 1 to 3700 cm− 1 range and at 1640 cm− 1 correspond to the stretching vibrations of H-O-H. The CO32− absorption bands at 1105 cm− 1 and 876 cm− 1 are attributed to symmetric stretching (ν1 mode) and bending vibrations (ν2 mode), respectively, while the broad band at 1463 cm− 1 corresponds to the asymmetric stretching vibration (ν3 mode). The peaks at 815 cm− 1, 940 cm− 1, and 1010 cm− 1 are associated with the C-S-H spectral bands. Research indicates that under carbon curing, the C-S-H characteristic peaks would shift from the Q1 to the Q2 peak, leading to the formation of amorphous calcium silicate carbonate33,34 .
In this study, the C-S-H characteristic peaks did not exhibit any shift, indicating that CO2 does not significantly react with C-S-H in DAC. This is primarily because, during the early stages of DAC, C-S-H has not yet formed in large quantities, so CO2 mainly reacts with Ca(OH)2. Furthermore, at 3 days of curing, the calcite peak for the group with 1% CO2 is already significantly higher than in the other two groups. This observation, combined with previous sections, suggests that within 3 days, CO32− ions are rapidly consumed and almost entirely transformed into CaCO3 precipitates.
TG-DTG
Figure 12a, b display the TG-DTG curves for each group. Based on the thermogravimetric curves in this study, alongside previous research, the temperature range from 30 °C to 950 °C has been divided into five regions: (1) 30–300 °C, where dehydration of hydrated calcium silicate and ettringite primarily occurs; (2) 300–500 °C, where Ca(OH)2 begins to decompose, with the associated weight loss due to the release of hydroxyl groups; (3) 500–600 °C, where CaCO3 begins to decompose and release CO2, particularly involving the decomposition of vaterite and poorly crystalline CaCO3; (4) 600–720 °C, where calcite decomposition occurs; and (5) 720–950 °C, where additional decomposition and weight loss of CaCO3 take place.
In Fig. 12 (a), it can be observed that when the w/c ratio is 0.6, the peak at 675 °C becomes increasingly pronounced with the introduction of 0.1% and 1% CO2, confirming the continuous production of well-crystallized calcite as the carbonation rate increases. Figure 12 (b) presents the thermogravimetric curves for a 1% CO2 additive amount at different w/c ratios. It is evident from the figure that the groups with w/c ratios of 0.5 and 0.55 not only exhibit a shift in the thermal decomposition temperature to 630 °C but also show changes between 720 °C and 950 °C. These changes could be related to the poor crystallinity of calcite and the formation of vaterite. When the w/c ratio is 0.55 and 0.5, the decrease in the content of free water leads to an increase in the concentration of dissolved CO32−, shortening the carbonation time. This leads to a change in the crystal form of CaCO3, resulting in the formation of more vaterite and smaller calcite crystals, with an earlier thermal decomposition temperature.
To investigate the relationship between the CO2 encapsulated during the mixing stage and the weight loss observed in thermogravimetric testing, weight loss calculations were performed across different temperature intervals, as shown in Fig. 13. Given that the CO2 additive amount in the mix ratio is based on the mass of the cementitious material, and the TG data indicates the mass loss of CO2 as a percentage of the total sample, it is essential to account for the w/c ratio in converting the thermogravimetric test results. Therefore, the CO2 mass derived from the TG data for the three groups was adjusted by dividing by the corresponding w/c ratio. This adjustment converts the TG data to reflect the encapsulated CO2 amount during the preparation stage, resulting in values of 0.555%, 0.992%, and 1.344% of the cement mass. The difference in carbon sequestration between different groups is mainly due to the incomplete sequestration of CO2 during the mixing stage. The specific sealing situation can be found in Sect. 3.2.1.
Microscopic morphology
When CO2 is introduced into cement paste, it first dissolves in water, forming CO32−. These ions then combine with cations in the paste, leading to the formation of carbonate precipitates that settle within the matrix. Research indicates that CO2 preferentially reacts with calcium hydroxide Ca(OH)2 in the paste to produce CaCO3 precipitates35. Figure 14 displays the micromorphology of Ca(OH)2 crystals under different CO2 additive amounts. It can be clearly observed that when the CO2 additive amount is 0.1%, the CO32− dissolved into the paste react with Ca(OH)2 to form a small amount of CaCO3 precipitates that adhere to the surface of the Ca(OH)2, as shown in Fig. 14 (a). Due to the low concentration of CO32−, the amount of carbonate precipitates is also limited and does not significantly affect the paste. However, when the CO2 additive amount reaches 1%, Ca(OH)2 is noticeably disrupted, the thickness of the crystals is significantly reduced, and a large amount of carbonate precipitates appear near the fragmented Ca(OH)2 crystals, as seen in Fig. 14 (b).
Additionally, small amounts of vaterite were detected in the DAC cement paste. Although distinct diffraction peaks for vaterite were not visible in the XRD analysis, the presence of vaterite was confirmed, as shown in Fig. 15. Research has shown that vaterite can effectively improve the workability of pastes36. Therefore, the formation of vaterite observed in this study is likely related to the free water content of the paste. This observation indicates that the DAC process indeed leads to the formation of vaterite, which remains present in the samples at both 3 days and 28 days of curing.
The persistence of vaterite under typical curing conditions suggests that it does not readily transform into the more stable and stronger calcite phase during subsequent curing. Despite the relatively low content of vaterite, its presence could potentially influence the overall material properties. Therefore, if the curing conditions can be optimized to facilitate the transformation of vaterite into calcite, it is likely that the strength of the DAC cement paste could be further improved.
This insight opens opportunities for further research aimed at exploring and adjusting the curing conditions to promote this phase transformation, which could enhance the structural integrity and durability of DAC-treated cementitious materials.
Discussion
The most important factors affecting the strength of the sample are the composition of hydration and carbonization products. Cement hydration primarily involves the reactions of C3S, C2S, C3A, and C4AF with water, typically occurring over five stages and lasting 24 to 48 h37,38. Since the cement paste is mixed for only one minute before the introduction of CO2, the production of calcium hydroxide (CH) is minimal. While previous studies indicate that CO2 enhances cement hydration, even in water-rich environments, the CH formed within this short time frame is insufficient to fully react with 1% of the introduced CO239. As a result, during the paste mixing process, the introduced CO2 likely exists in three forms: (i) as CaCO3 precipitates, (ii) as dissolved CO32− ions, and (iii) as unreacted CO2 gas, which may lead to pore formation in the hardened sample. But it can be certain that DAC treatment resulted in a slower hydration rate due to a decrease in the pH value of the paste. It was not until around 108 h that the promoting effect of calcium carbonate on hydration was demonstrated. This result is consistent with XRD results. Meanwhile, this promoting effect also led to an increase in intensity.
Another important factor affecting strength is pore structure. In addition to the pore structure caused by the presence of unreacted CO2, the changes in pore structure observed by X-CT results are more due to changes in fluidity. As the fluidity decreases, the hardened paste structure is more prone to larger pores, as the paste cannot flow freely and completely fill the mold. Therefore, when the w/c ratio is low and too much CO2 is added, the overall pore structure will be poor.
Meanwhile, the combination of carbonation products and hydration products was also observed in the SEM images. When the hydration product used calcium carbonate as the crystal nucleus, it not only promoted the hydration process, but also further enhanced the strength of the specimen through their van der Waals forces. In addition, in the SEM images, it can be seen that calcium carbonate not only precipitates in the form of calcite, but also some vaterite aggregates. Previous studies have shown that calcite increases the consumption of free water, while vaterite increases the fluidity of the paste. Moreover, because the vaterite in this study was only observed in pastes with poor workability, the crystal structure control of calcium carbonate in DAC cement pastes is closely related to the free water content. According to the crystal size of calcite, it can be observed that the size of calcium carbonate also changed according to changes in the w/c ratio and the amount of CO2 added. Undoubtedly, different crystal forms contribute significantly to strength, but there is currently no definitive conclusion on how to control the generation of crystal forms in DAC. But there is no doubt that the addition of CO2 has a significant promoting effect on the strength of the specimens.
In summary, when CO2 is added as an additive to cement paste, it has both positive and negative effects. Through compressive strength testing from 3 to 28 days, it can be seen that the positive impact is greater than the negative impact. Therefore, if the negative impact of pore structure caused by DAC is further eliminated and the flowability of the slurry is improved, the strength of DAC specimens will be further enhanced.
Conclusion
This study investigates the macroscopic properties and microstructure of cement paste under varying w/c ratios and carbonation gradients, emphasizing the substantial impact of early CO2 introduction on paste performance, hydration products, and carbonated structures. The underlying mechanism behind the observed changes in workability and strength when CO2 is introduced during mixing is fully elucidated. Several key findings include:
-
1.
DAC treatment leads to a decrease in pH due to CO2, resulting in a slower early hydration rate of cement. Until around 108 h, the cement underwent rapid hydration for about 8 h, ultimately improving its compressive strength.
-
2.
Due to the introduction of CO2, calcium carbonate is generated, resulting in the filling of micropores and improve the overall compactness of the specimen. However, due to the deterioration of work performance, the paste is more prone to large pores after hardening.
-
3.
Microstructural analysis confirms that all introduced CO2 is eventually absorbed as CaCO3 precipitates, with CO32− ions and unreacted CO2 gas integrated into subsequent reactions. However, a mix proportion with high free water content is more likely to produce well crystallized calcite, which is beneficial for the development of strength. The resulting interwoven structure of CaCO3 and hydration products contributes to the cement’s final hardness.
These findings provide valuable insights into DAC technology’s potential to enhance cement performance, with a DAC reaction model is proposed, offering a promising pathway for more sustainable concrete production.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Wei, N. et al. A strategic framework for commercialization of carbon capture, geological utilization, and storage technology in China. Int. J. Greenhouse Gas Control. 110, 103420 (2021).
Zheng, B., Niu, J. & Zhang, K. Current Status and Outlook of CCUS Industry in China. In Annual Report on China’s Petroleum, Gas and New Energy Industry (2022–2023), 289–306 (2024).
Zhang, D. & Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 113, 134–143 (2016).
Li, L. & Wu, M. An overview of utilizing CO2 for accelerated carbonation treatment in the concrete industry. J. CO2 Util. 60, 102000 (2022).
Zhang, D., Li, V. C. & Ellis, B. R. Optimal pre-hydration age for CO2 sequestration through Portland cement carbonation. ACS Sustain. Chem. Eng. 6 (12), 15976–15981 (2018).
Ashraf, W. Carbonation of cement-based materials: challenges and opportunities. Constr. Build. Mater. 120, 558–570 (2016).
Zhang, D., Ghouleh, Z. & Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Utilization. 21, 119–131 (2017).
Wang, X., Guo, M. & Ling, T. Review on CO2 curing of non-hydraulic calcium silicates cements: mechanism, carbonation and performance. Cem. Concr. Compos. 133, 104641 (2022).
Song, Q. et al. CO2 curing of SCMs blended cement blocks subject to elevated temperatures. Constr. Build. Mater. 374, 130907 (2023).
Liang, C. et al. Effects of early-age carbonation curing on the properties of cement-based materials: a review . J. Build. Eng. 108495 (2024).
Monkman, S. et al. Properties and durability of concrete produced using CO2 as an accelerating admixture. Cem. Concr. Compos. 74, 218–224 (2016).
Wang, M. et al. Effect of CO2-mixing dose and prolonged mixing time on fresh and hardened properties of cement pastes. J. Zhejiang University-Science A. 24 (10), 886–897 (2023).
He, Z., Li, Z. & Shao, Y. Effect of carbonation mixing on CO2 uptake and strength gain in concrete. J. Mater. Civ. Eng. 29 (10), 4017176 (2017).
Hosseinpanahi, A. et al. Properties and microstructure of concrete and cementitious paste with liquid carbon dioxide additives. J. Clean. Prod. 414, 137293 (2023).
Ho, H. et al. CO2 utilization via direct aqueous carbonation of synthesized concrete fines under atmospheric pressure . ACS Omega. 5 (26), 15877–15890 (2020).
Kim, N. et al. Hydration kinetics of ordinary Portland cement mixed under a direct CO2 inject condition. J. Building Eng. 77, 107531 (2023).
Ma, M. et al. Effect of direct carbonation routes of basic oxygen furnace slag (BOFS) on strength and hydration of blended cement paste. Constr. Build. Mater. 304, 124628 (2021).
Wagner, J. & Monkman, S. Making an Impact: Sustainable Success Stories: CarbonCure. How to Commercialize Chemical Technologies for a Sustainable Future, 255–262 (2021).
Tu, Z. et al. Effects of limestone powder on CaCO3 precipitation in CO2 cured cement pastes. Cem. Concr. Compos. 72, 9–16 (2016).
Duran-Herrera, A. et al. Accelerated and natural carbonation of concretes with internal curing and shrinkage/viscosity modifiers. Mater. Struct. 48, 1207–1214 (2015).
Zhang, S. et al. CO2 utilization and sequestration in ready-mix concrete—a review. Sci. Total Environ. 907, 168025 (2024).
Scrivener, K. L., Juilland, P. & Monteiro, P. J. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 78, 38–56 (2015).
Chen, H. et al. The coupling effect of calcium concentration and pH on early hydration of cement. Constr. Build. Mater. 185, 391–401 (2018).
Radha, A. V. et al. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. 107(38), 16438–16443 (2010).
Xie, Y. et al. Hydration heat and microstructure of SCM-blended cement under semi-adiabatic conditions: Effect of initial temperatures. Constr. Build. Mater. 356, 129329 (2022).
Li, X. et al. Properties and hydration mechanism of cement pastes in presence of nano-ZnO. Constr. Build. Mater. 289, 123080 (2021).
Vance, K. et al. Direct carbonation of Ca(OH)2 using liquid and supercritical CO2: implications for carbon-neutral cementation. Ind. Eng. Chem. Res. 54 (36), 8908–8918 (2015).
Li, L., Cao, M. & Yin, H. Comparative roles between aragonite and calcite calcium carbonate whiskers in the hydration and strength of cement paste. Cem. Concr. Compos. 104, 103350 (2019).
Zhang, Z. et al. Endowing strength to calcium silicate hydrate (CSH) powder by high pressure mechanical compaction. Cem. Concr. Res. 159, 106858 (2022).
Ma, Y. et al. Effect of drying method on calcium silicate hydrate (CSH): experiments and molecular dynamics simulations study. Constr. Build. Mater. 411, 134367 (2024).
Jiang, J. et al. Calcite crystallization in the cement system: morphological diversity, growth mechanism and shape evolution. Phys. Chem. Chem. Phys. 20 (20), 14174–14181 (2018).
Rodríguez-Sánchez, J. et al. Mechanisms of phase transformation and creating mechanical strength in a sustainable calcium carbonate cement. Materials 13 (16), 3582 (2020).
Hyvert, N. et al. Dependency of C–S–H carbonation rate on CO2 pressure to explain transition from accelerated tests to natural carbonation. Cem. Concr. Res. 40 (11), 1582–1589 (2010).
Rostami, V. et al. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 42 (1), 186–193 (2012).
Hu, L. et al. An insight of carbonation-hydration kinetics and microstructure characterization of cement paste under accelerated carbonation at early age. Cem. Concr. Compos. 134, 104763 (2022).
Zhou, Y. et al. Production of vaterite via wet carbonation of carbide residue: enhancing cement properties and CO2 sequestration. Cem. Concr. Compos. 150, 105549 (2024).
Bullard, J. W. et al. Mechanisms of cement hydration. Cem. Concr. Res. 41 (12), 1208–1223 (2011).
Beaudoin, J. & Odler, I. Hydration, setting and hardening of Portland cement. Lea’s Chem. Cem. Concrete. 5, 157–250 (2019).
Han, S. et al. Carbon dioxide capture using calcium hydroxide aqueous solution as the absorbent. Energy Fuels. 25 (8), 3825–3834 (2011).
Acknowledgements
This work was financially supported by the “Pioneer” R&D Program of Zhejiang (2022C03003), Horizon 2021(CSTO2NE) (101086302) and Ningbo Public Welfare Science and Technology Plan Project (Grant No.2023056).
Author information
Authors and Affiliations
Contributions
Zhiguang Wang: Methodology, Writing – review & editing. Hangjie Zhou: Formal analysis, Writing – review & editing. Songsong Lian: Formal analysis. Tao Meng: Formal analysis. Haiying Yu: Writing – original draft. Xiangming Zhou: Writing – original draft. Su Wang: Conceptualization. Shaoqin Ruan: Conceptualization, Funding acquisition, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Zhou, H., Lian, S. et al. Hydration behaviors, workability, and strength variations in direct aqueous carbonation (DAC) of Portland cement paste. Sci Rep 15, 5517 (2025). https://doi.org/10.1038/s41598-025-89875-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89875-4