Abstract
Thioredoxin Related Transmembrane Protein 1 (TMX1) encodes a thiol-disulfide oxidoreductase and regulates cleavage, formation, or isomerization of disulfide bonds among cysteine residues in proteins. Using bioinformatic analysis, we firstly prove TMX1 is significantly upregulated in lower-grade gliomas (LGGs) and associated with poor prognosis of LGG patients. Besides, it’s also highly expressed in higher WHO grade, 1p/19q non-co-deleted and isocitrate dehydrogenase (IDH) wildtype LGGs, thus we established a TMX1-based nomogram model, which exhibits a strong and stable predictive ability in the prognosis of LGG patients. Then, we also noticed that TMX1 was significantly associated with the immune cell infiltrations in LGGs, especially in B cell, CD8+ T cell, CD4+T cell, dendritic cell, macrophage and neutrophil. Meanwhile, it was also highly correlated to the expressions of MKI67, PCNA, PROM1 and SOX2 in LGGs, and that higher TMX1 LGGs showed a stronger resistance to temozolomide (TMZ). Finally, in our in vitro and in vivo experiments, we verified that TMX1 is highly expressed in LGG patients clinically, and it not only regulates the proliferative ability of SW1088 and SW1783 cells both in vitro and in vivo, it can also be inhibited to increase the TMZ therapy sensitivity in vivo. These results revealed that TMX1 acts as a strong prognostic biomarker in LGGs, and targeting TMX1 can be an efficient way to increase the TMZ therapy in LGG patients.
Similar content being viewed by others
Introduction
Gliomas, deriving from the neuroepithelium, constitute approximately 80% of brain tumors and represent the most common cancer type within the central nervous system (CNS)1,2. The World Health Organization (WHO) classifies gliomas into grades I–IV based on malignancy, with grades II, III, and IV having comparatively poorer prognoses because of infiltrative characteristic, challenges in surgical resection, and chemoradiotherapy resistance3,4,5. Despite several tumor therapeutics like immunotherapy and target therapy showing promise as treatment strategies, its association with an unsatisfied 5-year survival rate in glioma patients poses a challenge. Following standard treatment, some lower-grade gliomas (LGGs, including WHO II and WHO III grade gliomas) recur and progress into high-grade gliomas6, and acquired drug-resistance always occurs7,8. Consequently, there is an urgent need for effective biomarkers and targets to enhance the treatment and overcome the chemotherapy resistance of glioma patients.
Thiol-disulfide oxidoreductases play crucial roles in catalyzing reduction/oxidation (redox) reactions, facilitating the cleavage, formation, or isomerization of disulfide bonds among cysteine residues in substrate proteins9,10,11. The establishment or rearrangement of these disulfide bridges holds significant importance for the precise folding and assembly of protein12. The Thioredoxin-related transmembrane protein (TMX) subfamily comprises five thiol-disulfide oxidoreductases anchored to the membrane (TMX1, TMX2, TMX3, TMX4, and TMX5). These proteins share an N-terminal signal sequence for endoplasmic reticulum (ER) targeting and a catalytically active thioredoxin (TRX)-like domain, known as the type-a TRX-like domain, which contains the active sites13. TMX1, the most extensively studied member of the TMX family, demonstrates a preference for interacting with both membrane-bound folding-competent and folding-defective clients14.
The roles of thiol-disulfide oxidoreductases in cancer chemotherapy have been widely reported, many oxidoreductases confer cancer cells resistance to various kinds of chemotherapy. For example, protein disulfide-isomerase a4 promotes glioblastoma angiogenesis which leads to GBM resistant to anti-angiogenic therapy11; inhibition PDIA1 increase the cisplatin sensitivity of ovarian cancer cells15; TXNDC17 enhances paclitaxel resistance via inducing autophagy in ovarian cancer16.However, it is still unknow if TMX1 is involved in cancer drug resistance. Despite TMX1 being the most extensively studied member of the TMX family, its roles in cancer initiation and progression are relatively less explored. In this study, we found that TMX1 expression is significantly elevated in various cancer samples, particularly in LGG, and higher TMX1 expression correlates with a poorer prognosis for LGG patients. Additionally, we developed a TMX1 expression-based nomogram model to predict the clinical outcomes of LGG patients. Furthermore, we conducted an analysis of TMX1-related cancer hallmarks and pathways, revealing potential interactions between TMX1 and immune cell infiltrations in cancer. Finally, through a series of in vitro and in vivo experiments, we present the novel finding that TMX1 not only promotes LGG cell proliferation but also imparts resistance to TMZ (temozolomide) in LGGs.
Methods and materials
Acquisition and processing of data
Transcriptomic data from human normal tissues and tumor samples were obtained from the Genotype-Tissue Expression (GTEx) dataset17 and The Cancer Genome Atlas (TCGA) dataset, respectively. All retrievable data were downloaded from the UCSC Xena database (https://xenabrowser.net/datapages/) and CGGA dataset (http://www.cgga.org.cn/). The data format was converted to transcripts per kilobase million (TPM) prior to analysis. Cancer abbreviations are detailed in Supplemental Table 1.
Receiver operator characteristic (ROC) curve analysis
Prognostic predictive capacities for 1/3/5-year overall survival of TMX1 expression and other predictors (including WHO grade, gender, age, IDH status, and 1p/19q status) were assessed through receiver operating characteristic (ROC) curves using the R package “timeROC” (version 0.4)18. Area under the curve (AUC) values were calculated to indicate the prognostic predictive capacities of these predictors.
Nomogram model construction and validation
Nomogram construction and validation analyses were performed on the R programming platform using the “rms” package. The Cox regression method was employed to establish the TMX1-based nomogram using the TCGA-LGG cohort as the training cohort, and the prognostic ability of this nomogram model was validated in the CGGA-LGG cohort. Calibration plots of the nomogram model indicated the prognosis-predictive accuracy, and C-indexes were computed in both training and validation nomogram cohorts.
Gene set enrichment analysis (GSEA)
Before GSEA analysis, differential expression analysis was conducted between high-TMX1 (top 30% samples) and low-TMX1 (bottom 30% samples) subgroups in each cancer type by R package “limma”19. Differential expression genes (DEGs) meeting criteria (adjusted p value < 0.05, log2FC > 2) were obtained. Using the DEGs, a “gmt” formatted Hallmark file (including 50 gene-sets) was acquired from the Molecular Signatures Database (MSigDB, https://www.gsea-msigdb.org/gsea/msigdb). GSEA was performed using the R package “clusterProfiler” (version 4.10.0)20. False discovery rate (FDR) and normalized enrichment score (NES) values were calculated for each hallmark in each cancer type and visualized in a bubble plot using the “ggplot2” R package, and it was visualized by referring to a previous publication21.
Gene ontology analysis
DEGs in LGG samples were utilized for Gene Ontology (GO) analysis with the R package “clusterProfiler” (version 4.10.0). Three modules, including biological process (BP), molecular function (MF), and cellular component (CC), were analyzed for upregulated or downregulated genes. Q values were computed for each term and visualized in bar plots.
Cell line and cell culture
SW1088 and SW1783 cells were obtained from the American Type Culture Collection (ATCC, https://www.atcc.org/) and cultured in Minimum Essential Medium (MEM, Gibco, Cat.No.# 11095080, USA) with 10% fetal bovine serum (FBS, Gibco, Cat.No.# 10437028, USA) and 100U/ml Penicillin-Streptomycin Solution (Gibco, Cat.No.#15140122, USA). Both cell lines were maintained in a 37 °C cell incubator with 100% humidity.
Clinical sample collection
In total nineteen glioma samples and seven adjacent brain samples were collected from the Neurosurgery Department of the First Affiliated Hospital of Xinxiang Medical University from 2022 to 2023. The excision and storage of these samples adhered to hospital rules and strictly followed Helsinki guidelines. All patients enrolled in have signed informed consent forms. This study has been approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University (No. EC-024-081).
Antibodies and reagents
TMX1 Polyclonal antibody (Cat No. 27489-1-AP), Beta-Tubulin Polyclonal antibody (Cat No. 10068-1-AP), KI67 Polyclonal antibody (Cat No. 27309-1-AP) and horseradish peroxidase (HRP)-conjugated affinipure goat anti-rabbit IgG (Cat No. SA00001-3) were purchased from the Proteintech company. Temozolomide (Cat. No. HY-17364) was bought from MedChemExpress (MCE) company.
shRNA plasmids and Lentivirus transfection
Plasmids designed to express shRNA target (shTMX1-1 sequence:5′-CGTGCCAAGCAATAAGATTTA-3′, shTMX1-2 sequence:5′-AGGACTGAGTGGACGGTTTAT-3′, shTMX1-3 sequence:5′-GCTGTGTGAATCCATTAGATT-3′) were constructed by Genechem Company (Shanghai, China). Subsequently, the lentivirus packaging for these plasmids was conducted in accordance with the procedure on the addgene website (https://www.addgene.org/protocols/lentivirus-production/) strictly.
Western blot
Glioma cells were processed for protein extraction using RIPA lysis buffer (Beyotime, Cat. No. P0013B), and the protein concentration was determined via bicinchoninic acid (BCA) method (Beyotime, Cat. # P0010). Equal amounts of lysates from each sample were separately loaded onto SDS-PAGE. Subsequent to electrophoresis, the proteins in the gel were transferred to a polyvinylidene difluoride (PVDF) membrane with a 0.2 μm pore size (Thermo Scientific, Cat. No. # 88520). Following the transfer, the PVDF membrane underwent blocking in a 5% Bovine Serum Albumin (BSA) solution for 60 min. Subsequently, in accordance with the primary antibodies’ protocol, the primary antibodies were diluted in 5% BSA solution, and the PVDF membranes were fully incubated at 4 °C overnight. On the following day, the membranes were rinsed three times with TBST solution before undergoing the staining process with the secondary antibody. After a two-hour incubation with the secondary antibody at suitable concentration, protein bands were visualized using an enhanced chemiluminescence kit (ECL, ThermoFisher, Cat. No. #32106), and the quantification of protein expression was carried out using Image J software.
RNA extraction and quantitative real‑time PCR
Total RNA of glioma cells was extracted using the RNAsimple Total RNA Kit (TIANGEN, Cat. No#DP419) following the manufacturer’s instructions strictly. Subsequently, 1 µg of total RNA from each sample was utilized to synthesize complementary DNA (cDNA). Quantitative real-time PCR (RT-qPCR) was conducted on a qTower3 PCR system (Analytikjena, Germany) to measure RNA expression levels. The primer sequences used in this study are as follows:
-
TMX1 forward primer: 5′-TTGCGAAAGTAGATGTCACAGAG-3′;
-
TMX1 reverse primer: 5′- CTGATAGCGCCTAAATTCACCAT-3′;
-
ACTB forward primer: 5′-CATGTACGTTGCTATCCAGGC-3′;
-
ACTB reverse primer: 5′-CTCCTTAATGTCACGCACGAT-3′.
Cell viability assessment (CCK-8 method)
Cell viability was assessed using CCK-8 solution (Beyotime, China). Logarithmic cells (2000 cells) were seeded into each well of a 96-well plate with 100 µl of culture medium. At each designated time point, 10 µl of CCK-8 solution was added to each well and incubated at 37 °C for 60 min. The optical density (OD) values were measured at a wavelength of 450 nm.
Colony formation assay
For the colony formation assay, 200 cells from each cell group were seeded in a well of a six-well plate, and the medium was changed every three days. On the 15th day after seeding, the medium was discarded, and cells were washed twice with PBS. Subsequently, 4% paraformaldehyde was added to fix the cells for 30 min. After fixation, 1% crystal violet was applied to stain the cells for 1 h. Following thorough PBS washing, pictures were taken, and the cell colonies were quantified.
Temozolomide (TMZ) sensitivity bioinformatic analysis
Referring to previous research22, we conducted the TMZ sensitivity analysis to predict the correlation between TMX1 expression and TMZ sensitivity in LGG patients. TMZ sensitivity data for 835 cancer cell lines were obtained from the Cancer Therapeutics Response Portal (CTRP version 2.0, https://portals.broadinstitute.org/ctrp). This portal provided the dose–response score of TMZ in each cancer cell line, where this score is indicative of drug sensitivity (with a higher value indicating greater TMZ resistance). To address missing values for each cell line, K-nearest neighbor (k-NN) imputation was performed using the R package “impute” (version 3.18). Additionally, Ridge regression was employed to predict TMZ sensitivity for LGG samples in the TCGA and CGGA cohorts, utilizing the “pRRophetic” R package23.
Animal experiments
In vivo experiments were carried out in accordance with the guidelines set by the Medical Ethics Committee of the First Affiliated Hospital of Xinxiang Medical College, following the UK Animals Act (1986). The file number of the animal experiments of this study is EC-024-444. To establish the in vivo astrocytoma model, we subcutaneously injected 10 × 106 TMX1 knock-down or control SW1088 or SW1783 cells (in 100 µl PBS) per nude mouse (6-week-old BALB/c strain female, GemPharmatech, Nanjing). The tumor size was measured and recorded weekly by a researcher who was blinded to the experiments. The tumor volume was calculated using the formula V =A* B2/2, where A and B represent the major and minor axes of each tumor, respectively. The PBS or TMZ was administrated by intraperitoneal (i.p.) injection on days 5, 10, 15, 20 and 25 after tumor cells were inoculated (30 mg/kg per dose).
Statistical analysis
The Wilcoxon sum test was employed to determine the statistical significance of variations in TMX1 expression levels between cancer and para-cancerous normal tissues. To assess the prognostic impact of TMX1 expression in each cancer, univariate Cox regression and Kaplan–Meier analysis were utilized. In the univariate Cox regression analysis, p-values and Hazard Ratio (HR) were examined, and log-rank p-values with a 95% Credibility Interval (CI) were calculated using the Kaplan–Meier method, and the survival analysis data visualization heatmap was referring to a previous publication24. For assessing the significance of the correlation between TMX1 and immune cell infiltrations, cell proliferation, stemness markers, and TMZ sensitivity scores, Pearson’s correlation method was employed. The correlation significance was evaluated through R values and p-values.
Results
TMX1 is aberrantly upregulated in cancers
Through comparative analysis of TMX1 expression levels in normal human tissues sourced from the GTEx dataset and cancer samples from the TCGA dataset, we observed heightened TMX1 mRNA expression in numerous human cancer samples (Fig. 1A). Specifically, TMX1 exhibits high expression in 27 out of 33 cancer types, with downregulation noted in only 4 types of cancer, including KIRP, UCS, ACC, and KICH. This observation underscores the potential significance of TMX1 in the context of cancer biology.
(A) TMX1 expression was compared between TCGA tumor samples and GTEx normal tissue samples across different cancer types. (B) Summary of the correlations between TMX1 expression and overall survival (OS), disease-free interval (DFI), disease-specific survival (DSS) and progression-free interval (PFI) of distinct cancer patients according to univariate Cox regression and Kaplan-Meier model. Red indicates that TMX1 is the risk factor affecting prognosis of cancer, and green indicates a protective factor. Only p value < 0.05 are colored. (C) Through univariate Cox regression method, forest map shows the prognostic role of TMX1 in pan-cancer. The red label indicates that TMX1 is a statistically significant risk factor of LGG patients. (D, E) Kaplan-Meier survival curves of TMX1 of TCGA-LGG patients in overall survival (D) and disease-free survival (E).
Higher TMX1 expression correlates poor prognosis of LGG patients
Furthermore, we investigated the potential impact of TMX1 on cancer prognosis through log-rank test and univariate Cox regression analysis for each cancer type. The results are depicted in the heatmap (Fig. 1B) and forest plot (Fig. 1C), indicating that elevated TMX1 expression is associated with poorer clinical prognosis for LGG patients (HR = 1.75, p-value = 3.8e-4). Subsequent Kaplan-Meier survival curve analysis further validated that higher TMX1 expression in LGG patients correlates with worse overall survival (Fig. 1D) and disease-free survival (Fig. 1E) in the TCGA-LGG cohort. These findings were also consistent in the CGGA-LGG cohort (Fig. S1).
Clinical associations of TMX1 in LGG patients
To elucidate the relationships between TMX1 expression and clinicopathological characteristics of LGGs, we compared TMX1 expressions across different LGG subtypes, including WHO grade II vs. grade III, 1p/19q deletion vs. non-co-deletion, and IDH mutation vs. wildtype. As anticipated, TMX1 expressions were significantly upregulated in WHO grade III LGGs compared to grade II LGGs (Fig. 2A) in the TCGA cohort. Similarly, TMX1 expression was elevated in 1p/19q non-co-deletion and IDH wildtype LGGs compared to 1p/19q co-deletion and IDH mutant LGGs, respectively (Fig. 2B, C). These trends were consistent in the CGGA LGG cohort (Fig. 2D–F), confirming the strong association of TMX1 with LGG malignancy. Furthermore, we compared TMX1 expression with gender, age, WHO grade, IDH status, and 1p/19q co-deletion status through univariate Cox regression analysis in both TCGA (Fig. 2G) and CGGA (Fig. 2H) cohorts. The results indicate TMX1 as a stable and prognostic factor in LGG patients. Additionally, Receiver Operator Characteristic (ROC) curve analysis was performed to assess the prognostic predictive ability of TMX1 in LGG patients. TMX1 exhibited a stable and promising predictive capacity in both TCGA and CGGA LGG cohorts (Fig. 2I–N). In conclusion, TMX1 holds promise as a prognostic biomarker for predicting the clinical outcome of LGG.
(A–C) The significant differential expression of TMX1 stratified by WHO grade (A), 1p/19q codeletion status (B) and IDH mutation status (C) of LGG patients in TCGA dataset. (D–F) The significant differential expression of TMX1 stratified by WHO grade (D), 1p/19q codeletion status (E) and IDH mutation status (F) of LGG patients in CGGA dataset. (G, H) Univariate Cox regression analysis of WHO grade, gender, age, IDH mutation status, 1p/19q codeletion status and TMX1 expression in the TCGA (G) and CGGA (H) dataset. (I–K) The area under the curve (AUC) of ROC curves of WHO grade, gender, age, IDH mutation status, 1p/19q codeletion status and TMX1 expression in predicting 1- (I), 3- (J), and 5-year (K) OS events from the TCGA dataset, respectively. (L–N) The area under the curve (AUC) of ROC curves of WHO grade, gender, age, IDH mutation status, 1p/19q codeletion status and TMX1 expression in predicting 1- (L), 3- (M), and 5-year (N) OS events from the CGGA dataset, respectively.
A TMX1-based nomogram predicts LGG patients’ prognosis efficiently
To assess the potential of TMX1 as a prognostic indicator for LGGs, we constructed a TMX1 expression-based nomogram using the training TCGA cohort (refer to Fig. 3A). This nomogram incorporated significant prognostic factors identified through univariate Cox regression analysis in both the TCGA and CGGA cohorts, including WHO grade, IDH status, 1p/19q co-deletion status, and TMX1 expression. Calibration analysis further demonstrated the stability and accuracy of the nomogram model in predicting 1/3/5-year overall survival (OS) of LGG patients in both the TCGA (Fig. 3B–D) and CGGA (Fig. 3E–G) cohorts. These findings suggest that TMX1 holds promise as a predictive marker for clinical outcomes in LGG patients.
(A) The nomogram constructed using WHO grade, IDH status, 1p/19q co-deletion status and the TMX1 expression in the TCGA cohort. (B–D) Calibration plots indicating how the nomogram effectively predicts the 1-, 3-, and 5-year OS of LGG patients in the TCGA cohort. (E–G) Calibration plots indicating how the nomogram effectively predicts the 1-, 3-, and 5-year OS of glioma patients in the CGGA cohort.
Gene set enrichment analysis (GSEA) of TMX1 in cancers
To explore the potential biological implications of TMX1 in various cancers, we conducted Gene Set Enrichment Analysis (GSEA) within each cancer type. Prior to the GSEA analysis, we performed a differential expression analysis (DEA) by comparing the top 30% high-TMX1 samples against the bottom 30% low-TMX1 samples in each cancer type, respectively. Significant differential expression genes (DEGs) with a p-value < 0.05 were identified. Subsequently, these DEGs were utilized to conduct the GSEA analysis based on the 50 hallmark gene sets, and the outcomes were presented in the bubble plot (Fig. 4). Our analysis revealed significant associations of TMX1 with hallmarks such as TNFα signaling via NF-κB, IFNγ response, IFNα response, and Inflammatory response across several cancers, particularly notable in LGG (highlighted in red). These findings suggest that TMX1 may play a role in modulating cancer immune responses.
Analysis of correlations between TMX1 expression level and enriched gene sets. Correlations between TMX1 expression and enriched gene sets in pan-cancer displayed as a bubble plot of NES and log-rank FDR values. NES, normalized enrichment score; FDR, false discovery rate. Result was considered significant only when nominal P < 0.05 and FDR < 0.25.
Gene ontology (GO) analysis of TMX1 in LGGs
After conducting the GSEA analysis, we proceeded to perform Gene Ontology (GO) analysis using the differentially expressed genes (DEGs) related to TMX1 in LGGs, both upregulated and downregulated. In the GO analysis of upregulated DEGs, we observed a positive association of TMX1 with processes such as cell activation, leukocyte activation, cytokine production, and antigen processing and presentation via MHC class II (Fig. 5A). These findings suggest that TMX1 may be involved in cancer immune regulation within the LGG microenvironment. Conversely, in the GO analysis of downregulated DEGs, terms such as synaptic transmission, membrane potential, and neurotransmitter transport were identified (Fig. 5B), which exhibited a negative correlation with TMX1 expression in LGGs.
(A) Gene ontology analysis including biological process (GO-BP), Cellular component (GO-CC) and molecular function (GO-MF) of TMX1 related up-regulated genes in TCGA-LGG cohort. (B) Gene ontology analysis including biological process (GO-BP), Cellular component (GO-CC) and molecular function (GO-MF) of TMX1 related down-regulated genes in TCGA-LGG cohort.
TMX1 correlates immune cell infiltrations, proliferation, stemness, and TMZ sensitivity in LGGs
The aforementioned findings indicate a potential correlation between TMX1 and the immune microenvironment of LGGs. Consequently, we conducted an analysis of immune cell infiltration of TMX1 within the TCGA-LGG cohort using the TIMER platform. Our analysis revealed that TMX1 was positively correlated with tumor purity (Cor = 0.102, p value = 2.55e-2), as well as with infiltration levels of B cells (Cor = 0.431, p value = 4.54e-23), CD8 + T cells (Cor = 0.241, p value = 1.01e-7), CD4 + T cells (Cor = 0.43, p value = 7.96e-23), Macrophages (Cor = 0.491, p value = 4.88e-30), Neutrophils (Cor = 0.382, p value = 5.81e-18), and Dendritic cells (Cor = 0.458, p value = 4.08e-26) (Fig. 6A). Additionally, to explore the associations between TMX1 expression and tumor proliferation and stemness, Pearson analysis was performed between TMX1 and markers of tumor proliferation and stemness. As depicted in Fig. 6B–E, TMX1 exhibited positive correlations with PCNA, MKI67, PROM1, and SOX2 expressions in LGGs, suggesting its potential involvement in the regulation of LGG cell proliferation and stemness. Moreover, considering the role of various thioredoxin proteins in conferring chemotherapy resistance to tumors, we hypothesized that TMX1 might also be implicated in chemotherapy sensitivity in LGGs. To investigate this, we calculated TMZ sensitivity scores for LGG samples from both the TCGA and CGGA cohorts based on the CTRP database. Pearson correlation analysis further supported a significant association between TMX1 expression and TMZ resistance in LGG patients (Fig. 6F, G).
(A) Correlation analysis between TMX1 expression and different immune cell infiltration levels in TCGA-LGG cohort based on TIMER dataset. (B–E) Correlation analysis between TMX1 expression and PCNA (B), MKI67 (C), PROM1 (D) and SOX2 (E) expressions in TCGA-LGG cohort. (F, G) Correlation analysis between TMX1 expression and TMZ resistance values (Calculated based on Cancer Therapeutics Response Portal dataset, higher AUC value means higher TMZ resistance of sample) in TCGA LGG samples (F) and CGGA LGG samples (G).
Knock-down of TMX1 inhibits LGG cell proliferation and promotes TMZ sensitivity in vitro and in vivo
We obtained three paired LGG samples along with adjacent brain tissues and assessed TMX1 expression in these pairs using immunohistochemical assays. Notably, TMX1 exhibited significantly stronger staining in glioma samples compared to paired normal samples (Fig. 7A, B). And TMX1 immunofluorescence images of glioma cells were acquired from the Human Protein Atlas (HPA, https://www.proteinatlas.org/), and consistent with reported, TMX1 is mostly located in endoplasmic reticulum (Fig. 7C). To further validate the impact of TMX1 on LGG cell proliferation, we generated TMX1 knockdown SW1088 and SW1783 cell lines using lentivirus-packaged shRNAs. The efficacy of knockdown was confirmed via RT-qPCR (Fig. 7D) and Western blot analysis (Fig. 7E), demonstrating that all three shRNAs effectively reduced TMX1 mRNA and protein expression in both cell lines. Subsequently, we evaluated the proliferative capacity of SW1088 and SW1783 cells with or without TMX1 knockdown using CCK8 assays and cell colony formation assays. Our data revealed that TMX1 knockdown significantly decreased cell proliferation (Fig. 8A, B) and the ability to form cell colonies (Fig. 8C, D).
(A, B) IHC staining of clinical samples indicates TMX1 expression is significantly upregulated in glioma samples than normal brain samples. Representative images were shown (A) and statistical analysis was performed (B). (C) Immunofluorescent staining images of TMX1 using glioma cells were obtained from the Human Protein Altas (HPA), showing the intracellular localization of TMX1. (D) RT-qPCR showed the knock-down efficiency of TMX1 mRNA using shRNAs in two LGG cell lines. (E) Western blot showed the knock-down efficiency of TMX1 protein expression using shRNAs in two LGG cell lines.
(A, B) CCK8 assay indicates the cell growing ability after TMX1 knock-down in SW1088 (A) and SW1783 (B) cell lines. (C, D) Colony formation assay showed the cell colony formation ability after TMX1 knock-down in SW1088 and SW1783 cell lines. (E, F) The half maximal inhibitory concentration (IC50) curves of astrocytoma cell lines SW1088 (E) and SW1783 (F) with/without TMX1 knock-down were calculated under gradient TMZ concentration treatment.
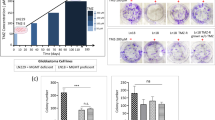
To examine whether knockdown of TMX1 would sensitize SW1088 cells to TMZ treatment in vitro, we compared the half maximal inhibitory concentrations (IC50) of sh-Ctrl and sh-TMX1 LGG cells in vitro using the CCK-8 test. We observed that lower TMX1 expression led to a decrease in the IC50 of both SW1088 and SW1783 cells to TMZ treatment (Fig. 8E, F). Subsequently, to investigate whether knock-down of TMX1 could sensitize LGG cells to TMZ treatment in vivo, we implanted sh-Ctrl or sh-TMX1 SW1088 and SW1783 cells into nude mice subcutaneously, respectively. We then treated each group of tumors bearing nude mice with PBS or TMZ (40 mg/kg p.o). Our results indicated that knockdown of TMX1 significantly reduced the growth of subcutaneous tumors compared with sh-Ctrl SW1088 tumors, and the combination of TMZ and TMX1 knockdown achieved the best therapeutic effect in the SW1088 tumor model (Fig. 9A). Additionally, the cell proliferative biomarker Ki67 of each tumor was also detected by IHC assay (Fig. 9B), suggesting that the proliferative ability was significantly restrained in TMX1 knockdown tumors, and also demonstrating that knockdown of TMX1 sensitizes SW1088 tumors to TMZ therapy in vivo (Fig. 9C). Similarly, these data were also repeated in SW1783 xenograft tumors (Fig. 9D–F), which strongly proved the TMX1 could be a promising target to restrain LGG tumor growth and TMZ resistance.
(A) The SW1088 xenograft tumor volumes were recorded and visualized in the line chart. (B, C) IHC staining of the cell proliferation biomarker Ki67 showing the growing ability of SW1088 xenograft group with different treatment. (D) The SW1783 xenograft tumor volumes were recorded and visualized in the line chart. (E, F) IHC staining of the cell proliferation biomarker Ki67 showing the growing ability of SW1783 xenograft group with different treatment.
Discussion
Currently, novel agents and targets for glioma patients are emerging, but few are suitable for clinical applications, TMZ is still the first-line choice for glioma patients’ postoperative chemotherapy8. Though TMZ has been widely applied in post-operation glioma patients, TMZ resistance still lower their clinical prognosis8. Through a combination of bioinformatic analysis, in vitro, and in vivo experiments, we verified TMX1 could be an ideal and promising target molecule for LGG patients. Targeting TMX1 can be an efficient and promising way to improve the patients’ prognosis.
TMX1 belongs to the thioredoxin superfamily, characterized by its transmembrane domain and redox-active site, which enable it to participate in oxidative protein folding within the endoplasmic reticulum (ER). Initially identified for its role in maintaining cellular homeostasis under oxidative stress, TMX1 has garnered attention for its potential implications in cancer biology12. For example, TMX1 was reported to regulate ER–mitochondria Ca2+ flux while interacting with SERCA2b under oxidizing conditions, which can lead to a decrease of SERCA activity and ER Ca2+ load in cancer cells25. Recently, TMX1 was also identified being a potent controller of TMEM68 driven triacylglycerol biosynthetic pathway, it emphasized and revealed the potential function of TMX1 in cell metabolism26. However, the role of TMX1 in cancers is still less reported.
In this study, we observed upregulation of TMX1 in LGG patients, strongly correlating with LGG prognosis. Further analysis revealed high TMX1 expression in IDH-wild, 1p/19q non-codeletion, and WHO grade III LGG patients, indicating a significant association with clinicopathological biomarkers of LGGs. Univariate Cox regression and ROC curve analysis also suggested TMX1 as an efficient prognostic biomarker for LGG patients, and a TMX1-based nomogram was established to enhance the LGG prognostic predictive ability of TMX1. Further investigation into the molecular basis of TMX1 in LGG involved GSEA and GO analysis, revealing its potential involvement in the immune response of LGG and significant correlation with immune cell infiltrations. Moreover, we found strong positive associaÖtions between TMX1 expression and PCNA, MKI67, PROM1, and SOX2, indicating TMX1’s potential correlations in LGG cell proliferation and stemness regulation. Additionally, strong associations between TMX1 and TMZ sensitivity in LGGs were observed through calculations of TMZ sensitivity for patients in both TCGA and CGGA cohorts. Finally, to verify the cellular biological functions of TMX1 in LGG, we demonstrated that inhibition of TMX1 can restrain proliferation of the astrocytoma cells and confers the cells resistance to TMZ both in vitro and in vivo. Those data revealed that targeting TMX1 can promote efficacy of TMZ therapy for LGG patients.
Though we found inhibition TMX1 can significantly hinder glioma growth and increase TMZ sensitivity, the underlying mechanism is still unrevealed. TMX1 plays a key role in redox regulation within the endoplasmic reticulum (ER), its primary function involves maintaining protein folding and redox homeostasis in the ER by regulating disulfide bond formation12. As we all know, how to maintain redox homeostasis in tumor microenvironment is critical for cancer cells27, TMX1 is reported to interact with SERCA2b under oxidizing conditions in a thiol-dependent manner, which will lead to mitochondria activity blockade in cancer cells, and slow down tumor growth and druge resistance25. This could be a possible and potential mechanism to explain why targeting TMX1 can reduce glioma growth and TMZ resistance.
However, some limitations also exist in our study. We didn’t test if targeting TMX1 via gene therapy methods (like small molecular inhibitor, siRNA/shRNA or Cas9 delivery) would work in xenograft models. Up to now, there is no specific TMX1 inhibitor developed, targeting TMX1 would be a challenging and meaningful topic for researchers. Besides, our conclusion was established based on cell line experiments, it would be much better if the conclusion can be verified in primary astrocytoma cells derived from patients. Taken together, our findings revealed the prognostic role of TMX1 in lower-grade glioma patients, and TMX1 can promote astrocytoma malignant proliferation and TMZ resistance both in vitro and in vitro.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Ostrom, Q. T. et al. The epidemiology of glioma in adults: A state of the science review. Neuro Oncol. 16(7), 896–913 (2014).
Karschnia, P. et al. The oncological role of resection in newly diagnosed diffuse adult-type glioma defined by the WHO 2021 classification: A review by the RANO resect group. Lancet Oncol. 25(9), e404–e419 (2024).
Wesseling, P. & Capper, D. WHO 2016 classification of gliomas. Neuropathol. Appl. Neurobiol. 44(2), 139–150 (2018).
Jiang, T. et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 499, 60–72 (2021).
Lim, M., Xia, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15(7), 422–442 (2018).
Killock, D. CNS cancer: Molecular classification of glioma. Nat. Rev. Clin. Oncol. 12(9), 502 (2015).
Albert, N. L. et al. Translating the theranostic concept to neuro-oncology: Disrupting barriers. Lancet Oncol. 25(9), e441–e451 (2024).
Smerdi, D., Moutafi, M., Kotsantis, I., Stavrinou, L. C. & Psyrri, A. Overcoming resistance to temozolomide in glioblastoma: A scoping review of preclinical and clinical data. Life 14(6) (2024).
Rahman, N. S. A. et al. Functions and mechanisms of protein disulfide isomerase family in cancer emergence. Cell Biosci. 12(1), 129 (2022).
Kanemura, S., Matsusaki, M., Inaba, K. & Okumura, M. PDI family members as guides for client folding and assembly. Int. J. Mol. Sci. 21(24) (2020).
Tu, Z. et al. Protein disulfide-isomerase A4 confers glioblastoma angiogenesis promotion capacity and resistance to anti-angiogenic therapy. J. Exp. Clin. Cancer Res. 42(1), 77 (2023).
Guerra, C. & Molinari, M. Thioredoxin-related transmembrane proteins: TMX1 and little brothers TMX2, TMX3, TMX4 and TMX5. Cells 9(9) (2020).
Cheng, Z., Zhang, J., Ballou, D. P. & Williams, C. H. Jr. Reactivity of thioredoxin as a protein thiol-disulfide oxidoreductase. Chem. Rev. 111(9), 5768–5783 (2011).
Matsuo, Y. Introducing thioredoxin-related transmembrane proteins: Emerging roles of human TMX and clinical implications. Antioxid. Redox Signal. 36(13–15), 984–1000 (2022).
Kullmann, M. et al. Assessing the contribution of the two protein disulfide isomerases PDIA1 and PDIA3 to cisplatin resistance. J. Inorg. Biochem. 153, 247–252 (2015).
Zhang, S. F. et al. TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy 11(2), 225–238 (2015).
Consortium, G. T. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369(6509), 1318–1330 (2020).
Blanche, P., Dartigues, J. F. & Jacqmin-Gadda, H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat. Med. 32(30), 5381–5397 (2013).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43(7), e47 (2015).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2(3), 100141 (2021).
Tu, Z. et al. Sperm autoantigenic protein 17 predicts the prognosis and the Immunotherapy response of cancers: A pan-cancer analysis. Front. Immunol. 13, 844736 (2022).
Tu, Z. et al. Transcriptional patterns of lower-grade glioma patients with distinct ferroptosis levels, immunotherapy response, and temozolomide sensitivity. Oxid. Med. Cell. Longev. 2022, 9408886 (2022).
Geeleher, P., Cox, N. & Huang, R. S. pRRophetic: An R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS One 9(9), e107468 (2014).
Tu, Z. et al. Pan-cancer analysis: Predictive role of TAP1 in cancer prognosis and response to immunotherapy. BMC Cancer 23(1), 133 (2023).
Raturi, A. et al. TMX1 determines cancer cell metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux. J. Cell Biol. 214(4), 433–444 (2016).
McLelland, G. L. et al. Identification of an alternative triglyceride biosynthesis pathway. Nature 621(7977), 171–178 (2023).
Sies, H., Mailloux, R. J. & Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 25(9), 701–719 (2024).
Acknowledgements
We sincerely acknowledge the contributions from the TCGA and CGGA projects.
Funding
This work was supported by Henan Province Key Subjects of Medicine (Other Disciplines of Pharmacy, Clinical Pharmacy) and Medical Science and Technology Research Project of Henan Province (grant nos. LHGJ20230526).
Author information
Authors and Affiliations
Contributions
X.Z. designed lead this study; H.H. collected the data collection, figures visualization, bioinformatic and statistical analysis, cell experiments and manuscript writing. Z.F., P.S., L.G., Z.W., and W.P. collected the clinical samples and did the animal experiments. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement and consent
This study has been approved by Medical Ethics Committee of the First Affiliated Hospital of Xinxiang Medical College (EC-024-081), and strictly adhered to the declaration of Helsinki. Written informed consents have been obtained from all patients enrolled.
Accordance statement of animal work
Animal work of this study has been approved by Medical Ethics Committee of the First Affiliated Hospital of Xinxiang Medical College (EC-024-444). All the animal experiments were conducted under the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hui, H., Zhou, F., Pei, S. et al. Thioredoxin related transmembrane protein 1acts as a prognostic indictor and promotes proliferation and TMZ resistance of lower-grade glioma. Sci Rep 15, 5246 (2025). https://doi.org/10.1038/s41598-025-89908-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89908-y