Abstract
Vector-borne infections impose a significant burden on global health systems and economies due to their widespread impact and the substantial resources required for prevention, control, and treatment efforts. In this work, we formulate a mathematical model for the transmission dynamics of a vector-borne infection with the effect of vaccination through the Atangana-Baleanu derivative. The solutions of the model are positive and bounded for positive initial values of the state variable. We presented the basic concept and theory of fractional calculus for the analysis of the model. We determine the threshold parameter, denoted by \(\mathcal {R}_0\), using the next-generation matrix method. The local asymptotic stability of the system at the disease-free equilibrium is analyzed. To establish the existence of solutions for the proposed model, we employ fixed-point theory. A numerical scheme is developed to visualize the system’s dynamical behavior under varying input parameters. Numerical simulations are conducted to illustrate how these parameters influence the dynamics of the system. The results highlight key factors affecting the transmission and control of vector-borne diseases, offering insights into strategies for prevention and mitigation.
Similar content being viewed by others
Introduction
Vector-borne diseases (VBDs) include malaria, dengue fever, Zika virus, and Chikungunya, all of which are major health issues in the world especially in the tropics and subtropics. Malaria alone, for example, caused 247 million cases and 619,000 deaths in 2021-mainly in Africa. Changing climate patterns, urbanization as well as global mobility have made these diseases persist despite various preventive strategies which are put in place to fight them. Infections that are spread by vectors are illnesses passed on to people and animals via insects like mosquitoes, ticks, flies, and fleas1. These insects carry germs like viruses, bacteria, and parasites which they introduce into new hosts during meals taken from their blood2. Vector-borne diseases have been a significant issue in public health since ancient times. Malignant fevers like malaria and typhus left Greece and Rome devastated, as they noted down back then. It was not until the late 19th century and early 20th century that scientists learned of the bacteria responsible for such diseases. A turning point in vector-borne disease research occurred leading to substantial advancements in preventive strategies; however, vectors are still major health challenges globally due to factors like climate change, urbanization, and human migration, which hastened their spread3.
Vector-borne diseases are very big health problems globally. If we take a look at the World Health Organization (WHO), they estimate that there are more than 17% of all infections that are posed by vector-borne diseases leading to over 700,000 deaths every year. Among the most significant concern is malaria, with approximately 219 million cases occurring and over 400,000 deaths yearly mostly in children below 5 years old living in sub-Saharan Africa4. As social and economic factors are now included as important influencing variables on malaria transmission, the new model has been developed. In fact, malaria really digs deep into the health of countries around the world, most severely infecting millions of people, especially younger children in tropical areas. Unlike earlier models, this one incorporates family conditions and household conditions, such that these models are better equipped to describe the influence of such conditions on both disease transmission and disease severity5. The Marburg virus is an infectious disease; due to this infection more deaths are reported. It even causes more cases in places where healthcare is really much more difficult to contain. The cause of this surge is entirely delaying action on speedy interventions to fight against the virus6. Dengue fever threatens an estimated 3.9 billion people across 129 nations around the globe with around 96 million symptomatic cases and about 40,000 related deaths each year7. Climate change has a direct impact on these ailments due to its effect on the ecosystem of vectors such as their distribution patterns, physiology or behavior which implies that it will lead to higher chances of transmission and more severe epidemics8.
Diseases borne by significant vectors include malaria, dengue fever, Zika virus, Lyme disease, and chikungunya. The weight of these infections is particularly high in tropical and subtropical areas where environmental conditions favor the proliferation of vectors. Climate change, urbanization, and increased global travel worsen the situation9. To effectively control and prevent them, an integrated approach that includes vector control measures, public health education, vaccination programs as well as strong healthcare systems, is essential10. The comprehension that diseases spread via vectors and the influence of different factors on that spread is of utmost importance for the creation of specific measures and mitigation of their global health effects11. One of the major components of understanding infectious diseases, predicting epidemics, assessing control efforts, guiding treatment policies, stimulating research as well as improving education, and raising public awareness is through mathematical models12. They are integral to the global efforts in controlling and preventing these significant public health threats13. In addition, deforestation has a significant effect on transmission and spread through breeding areas, healthcare services access, movement and migration over time as well as population density in specific regions. Understanding how these variables work together is important for devising effective prevention and control strategies for such illnesses. In this perspective, vaccination is modeled as a compartmental type model assuming that the reduced probability of infection is with vaccinated individuals. The effects of vaccination on transmission dynamics are shown through the use of the Atangana-Baleanu derivative to allow a more realistic representation of the effects of vaccination on the spread of disease14.
Fractional differential operators are increasingly being applied in different scientific and engineering fields, particularly in the area of mathematical modeling, which has emerged recently17. Within this perspective, it is crucial that we focus on Atangana-Baleanu fractional differential operators since they factor in the intricacy of binding illnesses such as Dengue and Zika18. Furthermore, fractional differential operators have found applications in addressing complex real-life issues like drug trafficking, corruption, and new chaotic systems apart from their use in epidemiology19. These operators can be distinguished from traditional integer order operators through the employment of non-locality and long-term memory effects by the use of the Mittage-Leffler function as their kernel20. They have been employed in several epidemiological models, such as Covid-19 model, HPV model, and syphilis model, which demonstrate how closely they capture disease behaviors21. Measles is a leading cause of child deaths, killing about 142,300 people yearly, even though there’s a safe vaccine. Cases have gone up recently, especially in young children and adults with weak immune systems. The main reason for this rise is fewer people getting vaccinated22. It is possible to qualitatively and quantitatively analyze vector-borne infections through a fractional framework that makes use of non-integer derivatives to model their dynamics accurately23. This approach takes into account complex behaviors and memory processes that usually go unrecognized by traditional integer order models during the transmission of diseases24. When there are two or more epidemics at once, then such a situation can be very difficult for public health systems. Modeling mathematics shows that the diseases affect each other. The current study employs a special sort of mathematical modeling to evaluate interventions in monkeypox and COVID-19 among high-risk populations25. For instance, it helps control diseases such as yellow fever, chikungunya, and HBV where vaccination and treatment are key factors26. In addition, these aspects may be incorporated into the epidemiological models through fractional frameworks so as to make them more realistic27. Unlike the other mpox outbreaks in recent years, this outbreak has largely spread through sexual contact. The target group had already a very high risk of acquiring HIV, it was primarily affected. Because it affected basically the same people, both diseases raised special health concerns because of this characteristic. Reductions in health focus were required so that sexual and social restrictions, such as those in that community, facilitate the spread of disease. Later this would help curb the outbreak and protect people from mpox and HIV28.
Consequently, the Caputo-Fabrizio fractional derivative was proposed after briefly looking at some basic properties of the exponential kernel used to model various engineering and physical processes29. Despite its exciting methods, there were some issues associated with the locality of this kernel that can serve to reduce its accuracy and applicability in certain areas30. To solve these problems, Atangana and Baleanu came up with the Atangana-Baleanu-Caputo (ABC) fractional derivative, which has the Mittag-Leffler function as a non-local, non-singular kernel31. The Caputo-Fabrizio fractional derivative has been associated with a new fractional integral which is defined using some exponential decay kernel such that it smoothens out singularities that are normally present in classical fractional calculus. In other words, this integral gives a more flexible and steady basis for system modeling in which there are memory and hereditary properties devoid of complications originating from singular kernels32. Analyzing the behavior of a model with memory effects requires an operator that extends differentiation for fractional orders. The model is based on a kernel, which is free from singularities and smooth. Thus, the solution is well-defined and stable unlike ones produced by classical fractional calculus with their singularities33.
The Atangana-Baleanu fractional derivative is a useful instrument for modeling complex systems that exhibit memory characteristics due to its non-singular and non-local kernel. Its unique features make it a prime candidate for stability and adaptability necessitating areas including higher level methods in image processing and so on34.The ABC fractional derivative has been employed in a variety of fields, such as epidemiology, where it more accurately depicts the spread of infectious diseases35. It also provides an insight into COVID-19 models through diffusions and dynamics control on infectious diseases for instance36. Moreover, the ABC derivative describes anomalous diffusion and viscoelastic materials better than ordinary methods used in physics and engineering for example37. To comprehend the systems through which various diseases spread and develop, advanced mathematical techniques facilitate the inclusion of historical information and the impacts of long-term memory38,39. In turn, researchers may use these fractional models in devising methods to stop or manage sicknesses, thus improving overall health of people who are impacted40. This study is thus driven by the desire for greater accuracy in predictive models that can guide public health initiatives and policy decisions in combating vector-borne diseases41.
Our studies focus on the dynamics of non-integer order and nonlocal, nonsingular kernels in modeling vector-borne diseases. The approach we have taken is a better representation of real-life dynamics because it incorporates fractional order calculus, which takes into account memory effects and long-range interactions more efficiently. Therefore, predictions about disease propagation and control are made more precisely using this methodology than is done with ordinary integer models. Furthermore, nonlocal and nonsingular kernel formation results in smoother and realistic output thus increasing the stability of the model when compared to others based on classical methods. The Atangana-Baleanu derivative is introduced, whose nonlocal and nonsingular kernels define the kernel for capturing spatial and temporal correlations in the transmission dynamics. The effects of past infections on current transmission dynamics are modelled by the kernels. This paper presents a fractional framework for vector-borne diseases, where a nonlocal and nonsingular kernel has been used to describe the complicated and non-integer patterns of disease transmission. The Atangana-Baleanu Caputo derivative is included in our model that gives a better and comprehensive representation of different relationships among vectors, their hosts and their environments that would lead to optimal prediction and understanding of epidemic trends.
The layout of this article: In section "Fractional-calculus preliminaries", we explain the fundamental results and ideas related to the AB fractional operator. Section "Evaluation of fractional dynamics" formulates a mathematical model of vector-borne disease infections incorporating vaccination. Section "Analysis of the model" gives an in-depth account of stability analysis, while section "Solution of the model" emphasizes on existence and uniqueness of solutions. A numerical scheme is constructed for the proposed model in section "Numerical scheme for the dynamics", while In section "Results and discussion" describes the results and discussion. Section "Conclusion" provides an extensive conclusion of the whole work.
Fractional-calculus preliminaries
In this paper, we demonstrate the important theory related to the A-B operator comprising the Caputo derivative mentioned in literature15. This is also shown for the AB operator in reference15. All these outcomes and analyses were utilized for assessing the model.
Definition 2.1
15. Suppose we define a function \(l\) where, \(l:[p,q]\rightarrow \mathbb {R}\) then according to this definition, the Caputo fractional derivative of order \(\varepsilon\) with respect to \(l\) can be written in terms of \(p\):
Let s belong to \(\textbf{Z}\) and \(\varepsilon\) belong to the interval \((s-1,s)\).
Definition 2.2
15. Let’s assume there’s a function l such that for \(q > p\), l belongs to the space \(H^1(p, q)\) and \(\varepsilon \in [0,1]\). The ABC operator fractional in Caputo representation is defined by:
Definition 2.3
15. let \(^{ABC}_{p}I^{\varepsilon }_{t}l(u)\) where AB derivative integral can be described as:
It implies that the initial function is reachable with fractional-order \(\varepsilon \rightarrow 0\).
Theorem 2.4
15. For a function l defined on the interval \(l \in C[m,n]\), then the following is true
In addition, the Lipschitz condition is satisfied by the ABC derivative as follows:
Theorem 2.5
15. A unique solution of this fractional differential equation system
could potentially be expressed in the following manner:
Evaluation of fractional dynamics
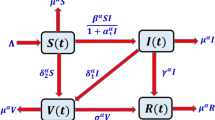
In this section, we will define a mathematical model regarding how the transmission takes place in relation with vector as a means of conveying infection including vaccination and spraying of insecticides. The term \(N_h\) will indicate humans’ population whilst \(N_v\) refers to population of people who have structured diseases that lead to their inability for reproduction. We clearly delineate four classes of humans which are the susceptible \(S_h\), those who were vaccinated \(V_h\), those who contracted diseases \(I_h\) and those who have recovered from certain infections \(R_h\); on the other hand, vector is classified into two groups that include either susceptible or infected forms they are supposed to be represented as \(S_v\) and \(I_v\) respectively. In this expression, the recruitment rate for humans is represented by \(\Lambda _h\), while the recruitment of vectors is denoted by \(\Lambda _v\). The death rate for humans is indicated by \(\mu _h\), and the death rate for vectors is represented by \(\mu _v\). We assume that some amount p of the population vulnerable to infection get vaccinated against it and take their place among those who have received a shot of medication. The recovery rate from this infection will be represented as \(\gamma\), disease-caused mortality will be denoted \(\delta\), and vaccine efficacy will be referred to as \(\nu\). A fraction p of the susceptible population is vaccinated and moves to the susceptible class. We assumed that the vaccination was not fully effective and moved to the susceptible class after losing the efficacy of the vaccine. Hence, our model with these aforementioned proposals can be described mathematically as follows:
where r is the rate of using treated bed nets and \(\epsilon\) is the efficacy of bed nets which reduce the contact between susceptible and infected individuals of both the populations. In this formulation, \(\theta\) is the rate at which insecticide spray reduces the population vectors. Here, we have the initial condition
In accordance with Model (1), all initial conditions for a system should be non-negative; thus, their representation is presented below:
The model mentioned above (1) in fractional form can be expressed as:
where \(0<\upsilon \le 1.\) In order to make epidemic modeling more efficacious in forecasting and controlling the diffusion of contagions. It is given that the Atangana-Baleanu derivative has a great capacity to represent its non-local nature as well as its non-singular behaviors, which can be important in describing some physical phenomena accurately. The atangana-Baleanu derivative is a mathematical structure that can easily morph into a model of memory systems with long-term relationships. It has a wide application area owing to its versatility.
Theorem 3.1
The solutions of the proposed model (3) of vector-borne infection are non-negative and bounded.
Analysis of the model
This part will emphasize the vector-born disease model with regard to DFE, \(R_0\) and LAS. Let \(\mathcal {E}_0\) represents the DFE obtained by taking the steady state of system (3) devoid of any infections. Fo this.
We presume that the basic reproduction number is denoted by \(\mathcal {R}_0\) and various methods could be used to compute it. Our model’s \(\mathcal {R}_0\) can be determined by following these steps:
and
From the above, we have \(R_0\)=\(\mathcal {F} \mathcal {V}^{-1}\) as
Putting DFE points, Then \(\mathcal {R}_0\) is:
The threshold parameter \(\mathcal {R}_0\) is determined using the next-generation matrix approach. An explicit expression for \(\mathcal {R}_0\) is derived within the framework of the Atangana-Baleanu derivative, and the stability of the disease-free equilibrium is analyzed in this context.
Theorem 4.1
If \(\mathcal {R}_0\) is less than 1, then the steady-state \(\mathcal {E}_0\) locally asymptotically stable, otherwise unstable.
Proof
In order to attain the stability outcome that is desired, we consider the Jacobian matrix at \(\mathcal {E}_0\) which is given by
It is clear that the eigenvalues of \(\mathcal {J}(E_0)\) are negative hence giving us the desired results. Thus we define the characteristic equation as follows:
Based on the information provided, As like the \(5^{th}\) and \(6^{th}\) eigenvalues are negative, we reduce the \(\mathcal {J}(E_0)\) to the sub-matrix formed:
\(3^{rd}\) and \(4^{th}\) eigenvalues are also negative, therefore
as \(\text {Tr}(\mathcal {J}(E_1))< 0\) and \(\text {det}(\mathcal {J}(E_1))> 0\)), For this \(R_0 < 1\), so the system 3 is LAS for DFE. \(\square\)
Here, we determined the local stability of DFE of our system, which shows that the diseases dies out for \(\mathcal {R}_0<1\).
Solution of the model
In this paper we use the fixed-point theory to prove the existence and uniqueness of solutions in our model. It presents a proper theory to analyze the behavior of vector borne disease model. The above system describes a vector carried sickness with A-B derivative as follows:
this can be further expressed as follows:
\(w(t)=(S_h,V_h,I_h,R_h,S_v,R_v)\) is our state variable in this context. In addition, \(\mathcal {J}\) is continuous function. To elaborate more;
when appropriate initial conditions like \(w_0(t)=(S_h(0),V_h(0),I_h(0),R_h(0),S_v(0),I_v(0))\) were defined. More so, it possesses the Lipschitz property as follows:
The existence and uniqueness of system (4) will be analysed in the next result.
Theorem 5.1
If this condition holds, there exists a unique solution for the proposed system (4) involving vector-borne illness.
Proof
To get the solution, we employ the A-B fractional integral in equation (2.3) on the system in equation (5) resulting in:
The same interval \(I\) needs to be taken as \((0,\mathcal {T})\), and the operator \(T\) is represented as follows: \(\Lambda :\mathcal {P}(I,\textrm{R}^6)\rightarrow \mathcal {P}(I,\textrm{R}^6)\)
subsequently, we have equation (8) such as:
For an arbitrary set I, we denote its supreme norm by \(\Vert .\Vert _I\), which is denoted mathematically as follows.
According to the fact that \(\mathcal {P}(I,\textrm{R}^6)\) can be classified as a Banach space given the existence of norms \(\Vert .\Vert _I\). In addition, it is evident that
\(\mathcal {P}(I,\textrm{R}^6)\) and \(\mathcal {P}(I^2,\textrm{R})\) respectively are places where both w(u) and \(\mathcal {K}(u,\varpi )\) belong. The situation is such that
Employing the definition of \(\Lambda\) as given in (10), we get the below outcomes:
Additionally, as a result of employing the Lipschitz restriction (6), together with the result from equation (1), we get that
As a result, we get the following
where
It is evident that in the event when the previously stated condition (7) is satisfied, \(\Lambda\) becomes a contraction. As a result, it follows that the vector-borne infection model, described by system (4), has a unique solution. \(\square\)
The solution of our model is investigated with the help of fixed-point theory in the context of the fractional ABC derivative.
Numerical scheme for the dynamics
Here, we are to solve our system (4) numerically. To this end, we first discuss the Atangana-Baleanu derivative, which is stated as follows.
the equation that precedes it should be converted into another one as per the instructions given in16:
The aforementioned towards \(u_{s+1}=(s+1)\Delta u\) might be expressed as
this can be converted into:
In this next step, the Newton polynomial method is utilized for approximating g(u, f(u)) as follows.
If we involve the polynomial that has been mentioned earlier in (20), then we can derive:
moreover, we get
Through simplification, the following result is achieved:
The method described below can be used to compute the integrals mentioned above.
So, \(V_1=2(s-\imath )^2+(3\varepsilon +10) (r-\imath )+2\varepsilon ^2+9 \varepsilon +12,\) and \(V_2=2(s-\imath )^2+(5\varepsilon +10) (s-\imath )+6\varepsilon ^2+18 \varepsilon +12\). We obtain after simplification:
The Atangana-Baleanu-Caputo (ABC) fractional derivative is a robust mathematical tool for capturing memory and nonlocal effects in epidemic models. However, it has several limitations that warrant consideration. The nonlocal nature of the ABC derivative increases computational complexity, making simulations more resource-intensive, while the derivation of analytical solutions is often nontrivial. The accuracy of models incorporating the ABC derivative is highly sensitive to precise parameter calibration, with misestimations potentially affecting stability and reliability. Furthermore, the physical interpretation of the ABC derivative in the context of epidemiology remains less well-established, and its applicability is constrained in systems exhibiting abrupt changes, such as those caused by sudden interventions or shifts in transmission dynamics. Despite these challenges, the ABC derivative remains a valuable framework when paired with high-quality data and a clear understanding of its underlying assumptions and limitations.
Results and discussion
The proposed infectious model time series will be depicted using the above approach. Monitoring, controlling, and understanding infectious diseases primarily involves time series analysis. It gives an insight into how they spread with time, warns before they could actually spread and measures up well the effectiveness of measures taken. This type of information can better inform specific public health measures. We showed the impact of input factors on the output of the proposed system. The subsequent illustrations indicate that certain parameters influence the process of infection.
-
The fractional parameter \(\upsilon\) is analysed in the first simulation presented in Figs. 1 and 2 with regards to its impact on vector-borne disease transmission over time.
-
The values of \(\upsilon\) were set 1.00, 0.95, 0.90, and 0.85 in Fig. 1.
-
As depicted in Fig. 2, values of \(\upsilon\) are ; 0.45, 0.55, 0.65 and 0.75.
-
A thorough understanding of a system response in different situations can be obtained through systematic investigation of those fractional values.
-
Infection dynamics are significantly affected by the fractional parameter \(\upsilon\).
-
Among the methods for controlling epidemics, \(\upsilon\) seems to be the most effective.
-
Public authorities should prioritize research on \(\upsilon\) to better understand and potentially reduce disease impact.
-
The input parameter p has an effect on the dynamics of vector-borne infections as shown in Fig. 3.
-
While infection levels can be reduced by p, it is advisable to increase vaccination efficacy for improved control.
-
The simulation used \(\rho\) values of 0.26, 0.36, 0.46, and 0.56.
-
Biologically impact are seen Changing input parameters as seen in the Figs. 4 and 5.
-
For different values of \(\beta _h\) being 0.43, 0.53, 0.63, and 0.73 shown in Fig. 4 while \(\theta\) set at the same time was 0.037, 0.067, 0.097, and 0.127 in Fig. 5.
-
A detailed study of that which factors affect asymptomatic and infected hosts is performed.
-
Insight from these results is important so that public health and intervention strategies can be effectively designed.
-
In order to develop successful control measures for the transmission of infections via vectors it is important to comprehend how these variables interact among themselves.
The input parameters were chosen based on reasonable values found in existing research on vector-borne diseases. These parameters are used to show that the model works and to study that changes in important factors, like transmission rates and vaccination coverage, affect the results. As such, the results indicative the significance and sensitivity of the model to certain key parameters, as well as their effects on the observed dynamic features of disease transmission. The results show that \(R_0\) is most sensitive to changes in vaccination rates.
Conclusion
Vector-borne infections posed a significant challenge to global health systems and economies due to their widespread impact and the considerable resources required for prevention, control, and treatment. In this study, a mathematical model was formulated to describe the transmission dynamics of a vector-borne infection, incorporating the effect of vaccination through the Atangana-Baleanu derivative. The model’s solutions were shown to be positive and bounded for positive initial values of the state variable. The fundamental concepts and theoretical framework of fractional calculus were introduced to facilitate the model’s analysis. The threshold parameter, denoted by \(\mathcal {R}_0\), was derived using the next-generation matrix method, and the local asymptotic stability of the system at the disease-free equilibrium was investigated. Fixed-point theory was employed to establish the existence of solutions for the proposed model. Additionally, a numerical scheme was developed to examine the system’s dynamical behavior under varying input parameters. Numerical simulations were performed to demonstrate the influence of these parameters on the model’s dynamics. The findings underscored critical factors affecting the transmission and control of vector-borne infections, providing valuable insights into strategies for their prevention and mitigation.
Future studies will investigate the impact of pulse immunization on the dynamics of vector-borne infections. Additionally, the individual effects of these immunization strategies will be examined, and the transmission dynamics of vector-borne infections will be incorporated within a stochastic framework.
Data availability
The data that support the findings of the study are available from the corresponding author upon reasonable request.
References
Müller, R., Reuss, F., Kendrovski, V. & Montag, D. Vector-borne diseases. Biodivers. Health Face Clim. Change 67-90 (2019).
Ramalho-Ortigao, M. & Gubler, D. J. Human diseases associated with vectors (arthropods in disease transmission). In Hunter’s tropical medicine and emerging infectious diseases (pp. 1063-1069). Elsevier. (2020).
Athni, T. S. et al. The influence of vector-borne disease on human history: socio-ecological mechanisms. Ecol. Lett. 24(4), 829–846 (2021).
World Health Organization. “Vector-borne diseases.” Accessed August 3. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. (2024).
Ullah, M. A., Raza, N., Alshahrani, M. Y. & Omame, A. Analysis and interpretation of a novel malaria transmission mathematical model with socioeconomic structure. Nonlinear Dyn. 1-20 (2024).
Mustafa, N., Rahman, J. U. & Omame, A. Modelling of Marburg virus transmission dynamics: a deep learning-driven approach with the effect of quarantine and health awareness interventions. Model. Earth Syst. Environ. 1-21 (2024).
Centers for Disease Control and Prevention. “Climate Impact on Vector-Borne Diseases.” Accessed August 3. https://www.cdc.gov/climateandhealth/effects/vectorborne.htm. (2024).
U.S. Department of Health and Human Services. “West Nile Virus and Other Mosquito-Borne Diseases.” Accessed August 3. https://www.hhs.gov/west-nile-virus/index.html. (2024).
Wilson, A. L. et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14(1), e0007831 (2020).
Jan, R., Ahmad, I., Ahmad, H., Vrinceanu, N. & Hasegan, A. G. Insights into dengue transmission modeling: Index of memory, carriers, and vaccination dynamics explored via non-integer derivative. AIMS Bioeng. 11(1) (2024).
Jan, R. et al. Fractional perspective evaluation of chikungunya infection with saturated incidence functions. Alex. Eng. J. 83, 35–42 (2023).
Jan, R., Qureshi, S., Boulaaras, S., Pham, V. T., Hincal, E. & Guefaifia, R. Optimization of the fractional-order parameter with the error analysis for human immunodeficiency virus under Caputo operator. Discrete Contin. Dyn. Syst.-S 0-0 (2023).
Shah, Z., Bonyah, E., Alzahrani, E., Jan, R. & Aedh Alreshidi, N. Chaotic phenomena and oscillations in dynamical behaviour of financial system via fractional calculus. Complexity (2022).
Jan, R. et al. Fractional view analysis of the impact of vaccination on the dynamics of a viral infection. Alex. Eng. J. 102, 36–48 (2024).
Atangana, A. & Baleanu, D. New fractional derivatives with nonlocal and non-singular kernel: Theory and application to heat transfer model. Therm. Sci. 20, 763769 (2016).
Toufik, M. & Atangana, A. New numerical approximation of fractional derivative with non-local and non-singular kernel: Application to chaotic models. Eur. Phys. J. Plus 132, 444 (2017).
Deressa, C.T., Etemad, S., & Rezapour, S. On a new four-dimensional model of memristor-based chaotic circuit in the context of nonsingular Atangana-Baleanu-Caputo operators. Adv. Diff. Equ. 1-24 (2021).
Deebani, W., Jan, R., Shah, Z., Vrinceanu, N. & Racheriu, M. Modeling the transmission phenomena of water-borne disease with non-singular and non-local kernel. Comput. Methods Biomech. Biomed. Engin. 26(11), 1294–1307 (2023).
Ogunrinde, R. B., Nwajeri, U. K., Fadugba, S. E., Ogunrinde, R. R. & Oshinubi, K. I. Dynamic model of COVID-19 and citizens reaction using fractional derivative. Alex. Eng. J. 60(2), 2001–2012 (2021).
Khan, M. A., Ullah, S. & Farooq, M. A new fractional model for tuberculosis with relapse via Atangana-Baleanu derivative. Chaos, Solitons & Fractals 116, 227–238 (2018).
Baskonus, H. M. New trends on the mathematical models and solitons arising in real-world problems. Symmetry 16(1), 1 (2023).
Peter, O. J., Cattani, C. & Omame, A. Modelling transmission dynamics of measles: The effect of treatment failure in complicated cases. Model. Earth Syst. Environ. 10(5), 5871–5889 (2024).
Jan, R., Degaichia, H., Boulaaras, S., Ur Rehman, Z., & Bahramand, S. Qualitative and quantitative analysis of vector-borne infection through fractional framework. Discrete Contin. Dyn. Syst.-S 0-0 (2024).
Alyobi, S. & Jan, R. Qualitative and quantitative analysis of fractional dynamics of infectious diseases with control measures. Fractal Fract. 7(5), 400 (2023).
Omame, A., Raezah, A. A., Okeke, G. A., Akram, T. & Iqbal, A. Assessing the impact of intervention measures in a mathematical model for monkeypox and COVID-19 co-dynamics in a high-risk population. Model. Earth Syst. Environ. 10(5), 6341–6355 (2024).
Tang, T.-Q. et al. Analysis of the dynamics of a vector-borne infection with the effect of imperfect vaccination from a fractional perspective. Sci. Rep. 13(1), 14398 (2023).
Redding, D.W., Gibb, R., & Jones, K.E. Ecological impacts of climate change will transform public health priorities for zoonotic and vector-borne disease. medRxiv, 2024-02 (2024).
Omame, A., Han, Q., Iyaniwura, S. A., Ebenezer, A., Bragazzi, N. L., Wang, X., Kong, J. D. & Woldegerima, W. A. Understanding the impact of HIV on mpox transmission in an MSM population: A mathematical modeling study. Infect. Dis. Model. (2024).
Amouch, M., & Karim, N. Fractional-order mathematical modeling of COVID-19 dynamics with different types of transmission. Numer. Algebra, Control Optim. 0-0 (2023).
Khan, F.S., Majeed, A.H., & Khalid, M. Integrating freelance models with fractional derivatives, and artificial neural networks: A comprehensive approach to advanced computation. Heliyon10(9) (2024).
Higazy, M. & Alyami, M. A. New Caputo-Fabrizio fractional order SEIASqEqHR model for COVID-19 epidemic transmission with genetic algorithm based control strategy. Alex. Eng. J. 59(6), 4719–4736 (2020).
Moumen, B. M., Guebbai, H., Kurulay, M. & Benmahmoud, S. A new fractional integral associated with the Caputo-Fabrizio fractional derivative. Rendiconti del Circolo Matematico di Palermo Series 2 70(3), 1277–1288 (2021).
Caputo, M. & Fabrizio, M. A new definition of fractional derivative without singular kernel. Progr. Fract. Diff. Appl. 1(2), 73–85 (2015).
Ghanbari, B. & Atangana, A. A new application of fractional Atangana-Baleanu derivatives: Designing ABC-fractional masks in image processing. Physica A 542, 123516 (2020).
Bekiros, S. & Kouloumpou, D. SBDiEM: A new mathematical model of infectious disease dynamics. Chaos, Solitons & Fractals 136, 109828 (2020).
Yao, S., Fan, N. & Jie, H. Modeling the spread of infectious diseases through influence maximization. Optim. Lett. 16(5), 1563–1586 (2022).
Gul, R., Shah, K., Khan, Z. A. & Jarad, F. On a class of boundary value problems under ABC fractional derivative. Adv. Diff. Equ. 2021, 1–12 (2021).
Iheonu, N. O., Nwajeri, U. K. & Omame, A. A non-integer order model for Zika and Dengue co-dynamics with cross-enhancement. Healthcare Anal. 4, 100276 (2023).
Aliyu, A.I., Alshomrani, A.S., Li, Y., Inc, M. & Baleanu, D. Existence theory and numerical simulation of HIV-I cure model with new fractional derivative possessing a non-singular kernel. Adv. Diff. Equ. 1-17 (2019).
Bonyah, E., Gómez-Aguilar, J. F. & Adu, A. Stability analysis and optimal control of a fractional human African trypanosomiasis model. Chaos, Solitons & Fractals 117, 150–160 (2018).
Abro, K.A., & Gómez-Aguilar, J.F. Role of Fourier sine transform on the dynamical model of tensioned carbon nanotubes with fractional operator. Math. Methods Appl. Sci. (2020).
Adom-Konadu, A., Sackitey, A.L. & Anokye, M. Local Stability Analysis of epidemic models using a Corollary of Gershgorin’s Circle Theorem. (2022).
Hussain, S. et al. Fractional dynamics of vector-borne infection with sexual transmission rate and vaccination. Mathematics 9(23), 3118 (2021).
Aljahdaly, N. H., Agarwal, R. P., Shah, R. & Botmart, T. Analysis of the time fractional-order coupled burgers equations with non-singular Kernel operators. Mathematics 9(18), 2326. https://doi.org/10.3390/math9182326 (2021).
Funding
Project financed by Lucian Blaga University of Sibiu (Knowledge Transfer Center) & Hasso Plattner Foundation research grants LBUS-HPI-ERG-2023-05.
Author information
Authors and Affiliations
Contributions
All authors participated equally to the conceptualization, investigation, analysis, original draught Writing, review, and editing of the work.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no conflicts of interest.
Ethical approval
There is no ethical issue in this work. We all the authors of this work revised and approved the work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ullah, N., Shah, Z., Jan, R. et al. Modeling the non-integer dynamics of a vector-borne infection with nonlocal and nonsingular kernel. Sci Rep 15, 6262 (2025). https://doi.org/10.1038/s41598-025-90182-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90182-1