Abstract
Quantitative polymerase chain reaction (qPCR) technology offers advantages such as easy operation and high specificity, but also faces issues like lengthy detection time and low sensitivity. This paper proposes a method by using novel SERS-active silver flower-like material to enhance the fluorescence signal generated by qPCR instrument, so as to shorten the detection time and improve the detection sensitivity. In this method, silver flower-like material is first prepared on substrates using a lyotropic liquid crystal soft template combined with electrodeposition growth. Then, an ultrasonic device is used to peel the active material into aqueous solution, and the peeling efficiency reaches 90%. Using rhodamine 6G (R6G) as the qPCR amplification detection molecule, the fluorescence signal generated from the flower-like material-containing sample is increased by 20%. Finally, the qPCR amplification effect is detected with the commercially available new coronavirus nucleic acid detection kit. The results showed that the CT value decreased from 35 to 31 after adding the silver flower-like material, and the detection efficiency increased by 20%. Meanwhile, no non-specific amplification is observed. For COVID-19 samples at S7 concentration, the positive detection rate rises from 1/200 to 2/3 by our method.
Similar content being viewed by others
Introduction
Quantitative real-time Polymerase Chain Reaction (qPCR) technology, with the advantages of simple operation, good reproducibility, and low sample contamination, has been widely used in gene expression analysis, pathogen detection, genetic disease screening, and many other fields1,2,3,4,5. Nevertheless, nucleic acid fragments to detect sample, and the detection time usually costs more than 1 h. Further, for very low concentration samples, the fluorescence signal produced by DNA is very weak, which may result in false-negative detection. To improve the detection sensitivity efficiency of qPCR, the PCR-enhancing additives (PCR enhancers) are widely researched6,7,8,9. Up to now, the PCR enhancers include nanomaterial, non-ionic detergents, organic molecules, low molecular weight amides, and co-solvents, etc10,11,12,13,14. In terms of the mechanism of action, it consists the facilitation of GC-rich sequence amplification, and the mitigation of the effects of PCR inhibitors. Organic solvents, compatible solutes, and nucleotide analogues are used to facilitate the GC-rich sequence amplification. For example, betaine (N, N, N-trimethylglycine) can reduce the melting temperature by eliminating the GC-dependency of DNA melting; Dimethylsulfoxide (DMSO) may prevent the secondary structure formation of GC-rich templates15,16. In the other hand, proteins and non-ionic detergents are utilized to reduce the effect of PCR inhibitor. For example, Bovine serum albumin (BSA) is able to protect Taq DNA polymerase from inactivation by tannic acids, proteinase, and melanin; Tween 20 (polysorbate-20) may counteract the inhibitory effects from low levels of sodium dodecyl sulfate (SDS) on Taq DNA polymerase17,18.
In recent years, nano-PCR has been developed to enhance the PCR signals by adding nanomaterials. With the advantages of large surface-to-volume ratios, good thermal conductivity and miniature structure, the nanomaterials may increase thermal conductivity and interaction between PCR components19. In 2005, Li et al. added the AuNPs to the reaction solution and the PCR sensitivity is greatly increased20. Subsequently, many kinds of nanomaterials, such as metal-based nanoparticles, carbon nanotubes, and quantum dots, were investigated to improve the sensitivity, efficiency, and specificity of PCR21,22. To increase the sensitivity of PCR furtherly, in 2021, Hyo Geun Lee et al. proposed a method with the combination of surface-enhanced Raman spectroscopy (SERS) and PCR. The DNA sample is firstly amplified with PCR, then transferred to SERS substrate and measured with Raman spectroscopy lastly. With this combined method, the detection sensitivity of DNA is significantly increased23. However, although the sensitivity is improved greatly, this method is very complicated as both the PCR and Raman spectroscopy are used and the operation process is involute.
Overall, improving the sensitivity, efficiency, and specificity of qPCR detection through PCR enhancers requires binding to DNA, or altering the properties of DNases, which makes the selection of enhancers complex and the applicability of enhancers limited. Meanwhile, all the PCR enhancers improve the performance by the manipulation of the testing sample. These methods only increase the number of nucleic acid fragments, but not consider to enhance the intensity of fluorescence signal directly. Hyo Geun Lee et al. intend to improve the sensitivity of PCR with the SERS technique, but the method is complex. On the other hand, the current nano-enhancers, such as gold nanoparticles were blamed for their high cost, carbon nanotubes requiring complex modifications before use, and quantum dots were only specific for some genes, these drawbacks hindered their widespread application in their application24,25,26. In this study, we propose a simple and effective approach by adding a silver flower-like LSPR-active material into the reaction reagents to directly enhance the fluorescence signal generated during PCR to improve the sensitivity. The proposed method has the advantages of low cost, no additional modifications, and universal enhancement effects.
Principle of fluorescence signal enhancement by silver flower-like materials
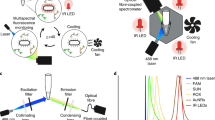
SYBR Green dye I and TaqMan probe methods are among the most commonly used detection techniques in qPCR instruments27,28,29. The SYBR Green dye I method relies on the binding of a fluorescent dye to double-stranded DNA, with the fluorescence intensity directly correlating to the quantity of qPCR products. In contrast, the TaqMan probe method utilizes a probe molecule containing both a fluorescent reporter group and a quencher group. In its intact state, the quencher absorbs the fluorescence emitted by the reporter, rendering the signal undetectable. During qPCR amplification, DNA polymerase cleaves the probe, separating the reporter from the quencher. This process generates a detectable fluorescence signal, which is then measured by the qPCR instrument30,31,32.
Due to the highly branched and porous micro-nanoscale structure of the silver flower-like material33, when light irradiates on the surface, the free electrons within the structure interact with the light, generating a phenomenon known as Localized Surface Plasmon Resonance (LSPR)34. While free electrons on the surface of metallic nanostructures are excited, the LSPR effect will be produced. Such effects lead to collective oscillations that enhance the local electromagnetic field. This enhancement significantly increases the excitation efficiency and emission intensity of nearby fluorescent molecules, resulting in a strong fluorescence signal35,36,37,38. The effect is particularly pronounced in the “hotspot” regions at the tips of the nanostructures, where it provides even stronger signal enhancement. Additionally, as the silver flower-like material possess outstanding physicochemical properties such as large surface area-to-volume ratio, high surface charge density, and good thermal conductivity, they can accelerate the qPCR reaction process39,40, further enhancing detection efficiency and sensitivity. Based on the multiple advantages mentioned above, the LSPR-active silver flower-like material is chosen to add into the qPCR reagents, so as to enhance the testing efficiency and sensitivity directly. Figure 1 illustrates the principle of fluorescence signal generation and enhancement in the probe method.
Preparation and detachment of silver flower-like materials
Firstly, the silver flower-like materials are prepared by the method of lyotropic liquid crystal soft template combined with electrochemical deposition38. By precisely controlling the ratio of the aqueous phase AgNO3 (99 wt%; Sigma-Aldrich, St. Louis, MO, USA), oil-phase p-xylene (99 wt%) and anionic surfactant sodium bis (2-ethylhexyl) sulfosuccinate (AOT; 98 wt%), a stable reverse hexagonal lyotropic liquid crystal soft template is achieved. Subsequently, by controlling parameters such as applied voltage and temperature, the uniform silver flower-like structure may be prepared with good reproducibility. The detailed preparation method is referred41. Figure 2a shows the prepared substrate and it can enhance the Raman signal of R6G greatly which confirms the LSPR effects of the silver flower-like materials. Additionally, the substrate may be stored with the external oxygen barrier method developed by our group with the temporal stability of half a year or so42.
To ensure the integrity of the silver flower-like material, it is detached from the substrate using ultrasonic vibration. Considering that the materials would be mixed with qPCR reagents, they are detached into an aqueous solution using the following method. The prepared substrate, after alcohol cleaning and drying, is placed in a 50 mL beaker, and 10 mL of distilled water is added. Then it is ultrasonic vibrated by ultrasonic cleaner (FUYANG Company, F-030ST) at 600 W and 30 ºC for half an hour. Figure 2b shows the substrate after ultrasonication. Most of the silver flower-like material on the substrate has been stripped away. Figure 2c displays the flower-like material dispersed solution after ultrasonication, which is clear, transparent, and free of precipitation, indicating uniform dispersion of the particles in the aqueous solution.
To confirm the effectiveness of the stripping and transferring process, the silver flower-like material in the solution are characterized using a thermal field emission scanning electron microscope (SEM) with the scanning voltage of 2 kV and working distance of 4.1 mm. Figure 3a shows the SEM of the silver nanoflower structure grown on an ITO glass substrate. At a scale of 1 μm, the flower-like structure of the material is observed to be relatively intact, with a large size and a diameter of approximately 4 μm, which helps avoid unnecessary interactions with the PCR reagents. The particle size distribution in the aqueous solution is measured by potential and nano particle size analyzer (Malvern Panalytical Company, Zetasizer Nano ZS). The result presented in Fig. 3b indicates that the particle sizes in the solution are mainly concentrated in the range of 40–50 nm and 3–4 μm, attributing to the length of the nanometals and the diameter of the flower structure. Additionally, the approximately 40 nm particles observed in the dynamic light scattering (DLS) measurement. From TEM image we can see, there are lots of nano-scale silver structure at the center of the silver flower. Furthermore, as an aqueous ultrasound device is used to detach the silver flower-like materials from the ITO glass surface, the small silver material is possibly fractured during the process. Hence, the DLS measurement results shows the nano-scale silver structures in the solution.
Experiments
Fluorescence enhancement verification
To validate the enhancement of the silver flower-like material on the fluorescence signal, the Rhodamine 6G (R6G) is chosen as the test sample for qPCR detection. First, an aqueous solution of R6G with a concentration of 1 × 10−6 mol/L is prepared. Subsequently, the R6G solution is added to 2 groups of the 8-tube strip and each group with the amount of 10 µL. For the experimental group, 10 µL of the silver flower-like material dispersion solution is added, while for the control group, 10 µL of nuclease-free water is added. Finally, the capped 8-tube strip is placed into the qPCR instrument (Thermo Fisher, QuantStudio 1), and an appropriate cycle number is set for fluorescence signal detection. Figure S1 shows the qPCR instrument and the 8-tube strip used in the experiment. The fluorescence signal detection results of Rhodamine 6G are shown in Fig. 4.
Novel coronavirus test
Amplification of COVID-19
To further explore the fluorescence enhancement effect of the silver flower-like material on biological samples, novel coronavirus 2019-nCov nucleic acid detection kit (Fluorescent PCR method) from BioPerfectus Biotechnology company is chosen for detecting. The detailed compositions and quantities in the kit are listed in Table 1. Nucleic acid amplification reaction solution is a critical component for conducting PCR, comprising buffer solution, primers, and DNA polymerase. Enzyme mix includes reverse transcriptase and RNase inhibitor, facilitating the transcription of RNA viruses (such as the RNA genome of the virus) into the corresponding cDNA for subsequent qPCR amplification. 2019-nCoV reaction solution contains specific primers and fluorescent probes. RNase-free water is used to prevent the interference from RNA degradation. According to the prescribed ratios, a total of 20 µL of the above mixed reaction solution is added to each 8-tube strip with 6 tubes. Then, the positive samples should be added in the mixed reaction solution.
According to the kit illustration, positive samples with S6 concentration should be tested as positive and the amount of 5 µL should be added in the reaction solution. As the initial concentration of the positive sample is S1, it needs to be diluted at a ratio of 1:2187 to obtain the S6 concentration sample. Then, 5 µL of the S6 concentration positive sample is added to each tube and centrifuged, followed by thorough mixing. Now, the total volume of the mixed reaction system is 25 µL. To improve the repeatability and reliability of the experiment, 3 tubes are set up for both the experimental and control group, respectively. In the experimental group, 10 µL of the silver flower-like material dispersion solution is added to each tube, while in the control group, 10 µL of nuclease-free water is added to each tube instead. The qPCR instrument parameters are set as shown in Table SI. After amplification, the fluorescence intensity results are presented in Fig. 5.
Specificity validation
To verify that the enhanced fluorescence signal originates from positive samples rather than negative ones, we conduct two experimental validations. The qPCR amplification experiment of negative samples is performed to validate whether the silver flower-like material enhance fluorescence signal. So, the negative sample is prepared with S6 concentration and then, added to 3 tubes: each has 20 µL reaction solution and 5 µL negative sample. Next, 10 µL of the silver nanoflower material dispersion solution is added to both the positive and negative samples. The amplification reaction is then performed under the same conditions as described above and the test results are shown in Fig. 6a. Simultaneously, the melting curves are obtained after the amplification process as shown in Fig. 6b. The specificity of the qPCR reaction with silver flower-like material may be checked by the melting curve.
Detection sensitivity
To investigate the effect of the silver flower-like material dispersion solution concentration on the enhancement of fluorescence signals, the initial silver flower-like material dispersion solution is concentrated to obtain solutions with 2-fold, 4-fold, and 8-fold concentrations. When concentrated to 8-fold, precipitation of the silver flower-like material occurred in the solution, indicating that the 8-fold concentration is approaching saturation, as shown in Fig. S2.
These concentrated solutions are named 2×, 4×, and 8×, while the original solution is referred to as 1×. 10 µL of silver flower-like material dispersion solutions at concentrations of 1×, 2×, 4×, and 8× were mixed with positive samples at an S6 concentration, following the same experimental procedure as described earlier. Figure 7a shows the fluorescence intensity curves obtained after qPCR amplification of the silver flower-like material dispersion solutions and nuclease-free water, along with the corresponding Ct values. The Ct values obtained at each concentration were averaged, and a fitting curve of Ct value versus concentration was plotted, as shown in Fig. 7b.
As instructed by the 2019-nCoV nucleic acid detection kit, the samples have only single-batch (200 samples) positivity at S7 concentration. That is, 200 samples at S7 concentration could obtain only one positive result. This suggests that the detecting sensitivity decreases dramatically with reducing sample concentration. Based on the results from above experiments, silver flower-like material dispersed solution with 4-fold concentration is chosen for the further investigation of its effect on sensitivity enhancement at very low sample concentration.
Sample with S6 concentration is diluted 2-fold, 3-fold (S7 concentration), and 4-fold for the following detections. As stated above, in the experimental group, 10 µL of 4X dispersed solution is added, while the control group uses the same volume of nuclease-free water. The PCR amplification curves of samples with different concentrations are shown in Fig. 8.
Results and discussions
Figure 4 shows the fluorescence signal detection results of Rhodamine 6G. After adding the flower-like material dispersed solution, the signal intensities are enhanced by approximately 20%. These preliminary results indicate that the added material is effective in improving fluorescence signal in qPCR analysis.
Figure 5 shows the PCR amplification curves after adding 10 µL of silver flower-like material dispersed solution (experimental group) and nuclease-free water (control group) to positive samples at S6 concentration. In the control group with nuclease-free water, the number of amplification cycles required for fluorescence intensity to reach the detection threshold (Cycle threshold value, Ct value) is 35. While in the experimental group with the flower-like material, the Ct value is 31. This demonstrates that the proposed LSPR-active flower-like material can enhance the fluorescence signal and thus reduce the Ct value in qPCR analysis.
The specificity validation experiment results are shown in Fig. 6. It indicates that the negative samples with the dispersed solution did not exhibit enhanced fluorescence signal after qPCR amplification as shown in Fig. 6a. This confirms that the fluorescence signal enhanced by the silver flower-like material originates from positive samples. Moreover, Fig. 6b shows the melting curves for positive and negative samples after amplification. The positive samples exhibit single-peak characteristic, while the negative samples have no peak. It indicates that the introduction of the silver flower-like material will not bring any nonspecific products, which further validates the excellent specificity of the material in enhancing the fluorescence signal for the novel coronavirus.
According to Fig. 7a, we can see that for 1X, 2X and 4X solutions, the reduction of Ct value upon increasing concentration exhibits an approximately linear trend. However, when the concentration reaches 8-fold, the decrease in Ct value becomes relatively small. The horizontal blue dash line in Fig. 7b shows the averaged Ct value of the 8× solution, which intersects with the fitting curve at approximately 4.4-fold concentration. It indicates that, while the solution is concentrated with 4.4×, the concentration of silver flower approaches saturation. Therefore, further concentrating the solution has no meaning and then, we chose 4× solution to perform the sensitivity experiment which is very close to 4.4×. Additionally, for 8× and 4× solutions, which needs only 27 cycles to reach fluorescence threshold. As the detection efficiency is inversely proportional to the Ct value, the addition of silver flower-like material can enhance the efficiency of COVID-19 detection by approximately 20%.
Impact of concentrations of silver flower-like material on qPCR amplification efficiency for positive samples with S6 concentration: (a) PCR amplification curves under 1×, 2×, 4×, and 8× concentrations of silver flower-like material dispersed solution, the experimental groups consist of 3 for 1×, 5 for 2×, 4 for 4×, 5 for 8×, and the control groups are 3; (b) Averaged Ct value as a function of solution concentration, the saturation concentration is 4.4× and the corresponding CT is about 27.
Figure 8 show the PCR amplification curves of positive samples at different concentrations. According to the kit instructions, the result is considered negative when the Ct value exceeds 40 or there is no detectable signal. When the Ct value is between 37 and 40, the sample is flagged as suspicious and requires multiple detections. When the Ct value is less than or equal to 37, the sample is confirmed to be positive. As can be seen from the results, all COVID-19 samples are detected positive at the S6 concentration. Meanwhile, the Ct value of the experimental group is lower than that of the control group. After a 2-fold dilution of the S6 sample, all the samples in the experiment group are detected positive. While in the control group, two samples are positive, while one sample is marked as suspicious. At the S7 concentration, the experimental group shows two positive and one suspicious result. However, in the control group, one sample is suspicious, and no fluorescence signal is detected in the other two. Upon a 4-fold dilution of S6, seven samples are detected in the experimental group. One positive, four suspicious and two no-signal samples are obtained. In the control group, no fluorescence signal is detected.
PCR amplification curves for different concentration positive samples with 4× silver flower solution: (a) S6 concentration, both the experimental and control groups are 3; (b) 2-fold dilution of S6 concentration, the experimental groups are 2 and the control groups are 3; (c) S7 concentration, both the experimental and control groups are 3; (d) 4-fold dilution of S6 concentration, the experimental groups are 6 and the control groups are 2.
These results powerfully illustrated that under the condition of low sample concentrations, detection sensitivity can be improved by introducing the silver flower-like material. Especially, fluorescence signals are detected in all the tested samples at S7 concentration, and 2 out of 3 samples can meet the positive criteria. Compared to the normal 1/200 detection standard at S7 concentration, the addition of the silver flower-like material can significantly lower the detection limit and enhance the detection sensitivity of the qPCR technology.
Meanwhile, to further discuss the enhancement effect of silver flower-like material dispersed solutions at different concentrations on other qPCR dye, the triplex DNA is selected to perform the experiments. The triplex DNA mixed solution is prepared according to Table SII and then, 10 µL of nuclease-free water, and 10 µL of 4×, 2×, and 1× silver flower solutions are mixed with the triplex DNA solution in the PCR 8-tube strip with two groups. Then, the test experiment is done with the amplification cycles of 45 and the results are shown in Fig. 9 It is shown that, while adding 4× silver flower solutions, the Ct value is decreased from 33 to 27. Moreover, the Ct value has a linear relationship with solution concentrations which is similar to that of Fig. 7(b).
To demonstrate the enhancement performance of silver flower-like materials in qPCR, it is compared with other commonly used PCR enhancers and the results are shown in Table 2. It indicates that, the AuNPs enhancer has the specificity for different genes, and others have no enhancement effects. However, the silver flower-like material can significantly reduce the Ct values for both the triplex DNA and COVID-19. Consequently, the silver flower-like material is very suitable for the qPCR as an enhancer.
Moreover, traditional silver nanoparticles usually appear yellow in a dispersed state due to their absorption of light around 400 nm. But, our silver flower-like material dispersed solution appears nearly colorless. To confirm this difference, the ultraviolet-visible(UV-vis) absorption spectroscopy is measured for the 4X concentration using a UV-2600 ultraviolet-visible spectrophotometer (Shimadzu Corporation) and the result is shown in Fig. 10. As can be seen that the silver flower-like material exhibits the absorbance between 250 and 300 nm and then, the dispersed solution appears colorless. We consider this phenomenon is caused by the complex structure of the silver flower-like material.
Conclusion
This work focuses on a high-sensitivity qPCR detection method, which achieves efficient enhancement of fluorescence signals by introducing LSPR-active silver flower-like material to the reaction reagents. The active material is prepared on ITO glass substrates using lysotropic liquid crystal soft templates and electrodeposition growth, and is effectively transferred to aqueous solution by ultrasonic vibration. Rhodamine 6G and COVID-19 samples with the flower-like material are tested using a qPCR instrument, and compared with the control group containing nuclease-free water. The results successfully validate the effectiveness of the flower-like material in enhancing fluorescence signals, while maintaining high specificity of the samples. Furthermore, the impact of the concentration of the active material dispersed solution is investigated, and the optimal concentration of 4-fold was determined. With the 4-fold concentration solution, the Ct value for COVID-19 decreased from 34 to 27, indicating a nearly 20% improvement in detection efficiency. Additionally, for samples at S7 concentration, the positive detection rate rises from 1/200 to 2/3, significantly increasing the detection sensitivity. The method of enhancing qPCR detection efficiency and sensitivity based on the silver flower-like material has great potential advantages in practical applications, and holds broad prospects in molecular diagnostics and biological research.
Data availability
Data is provided within the manuscript, and the datasets used and analysed during the current study available from the corresponding author, Zhaoliang Cao, on reasonable request.
References
Heid, C. A., Stevens, J., Livak, K. J. & Williams, P. M. Real-time quantitative PCR. Genome Res. 6, 986–994 (1996).
Kim, D. W. Real-time quantitative PCR. Exp. Mol. Med. 33, 101–109 (2001).
Rocha, D. J. P. G., Castro, T. L. P., Aguiar, E. R. G. R. & Pacheco, L. G. C. Gene expression analysis in bacteria by RT-qPCR. Methods Mol. Biol. 2065, 119–137 (2020).
Huggins, L., Massetti, L., Schunack, B., Colella, V. & Traub, R. Novel high-throughput multiplex qPCRs for the detection of canine vector-borne pathogens in the Asia-Pacific. Microorganisms 9, 1092 (2021).
Czibere, L. et al. High-throughput genetic newborn screening for spinal muscular atrophy by rapid nucleic acid extraction from dried blood spots and 384-well qPCR. Eur. J. Hum. Genet. 28, 23–30 (2020).
Shaik, G. M., Dráberová, L., Dráber, P., Boubelík, M. & Dráber, P. Tetraalkylammonium derivatives as real-time PCR enhancers and stabilizers of the qPCR mixtures containing SYBR Green I. Nucleic Acids Res. 36, e93 (2008).
Tan, M. et al. Super-enhancer-associated SNHG15 cooperating with FOSL1 contributes to bladder cancer progression through the WNT pathway. Pharmacol. Res. 197, 106940 (2023).
Horáková, H. et al. 1,2-Propanediol-trehalose mixture as a potent quantitative real-time PCR enhancer. BMC Biotechnol. 18, 11–41 (2011).
Karunanathie, H., Kee, P. S., Ng, S. F., Kennedy, M. A. & Chua, E. W. PCR enhancers: types, mechanisms, and applications in long-range PCR. Biochimie 197, 130–143 (2022).
Yuce, M., Kurt, H., Mokkapati, V. R. S. S. & Budak, H. Employment of nanomaterials in polymerase chain reaction: insight into the impacts and putative operating mechanisms of nano-additives in PCR. RSC Adv. 4, 36800–36814 (2014).
Bachmann, B., Lüke, W. & Hunsmann, G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 18, 1309 (1990).
Seifi, T. et al. Amplification of GC-rich putative mouse PeP promoter using betaine and DMSO in ammonium sulfate polymerase chain reaction buffer. Avicenna J. Med. Biotechnol. 4, 206 (2012).
Chakrabarti, R. & Schutt, C. E. The enhancement of PCR amplification by low molecular weight amides. Nucleic Acids Res. 29, 2377–2381 (2001).
Pratyush, D. D., Tiwari, S., Kumar, A. & Singh, S. K. A new approach to touchdown method using betaine as co-solvent for increased specificity and intensity of GC-rich gene amplification. Gene 497, 269–272 (2012).
Rees, W. A., Yager, T. D., Korte, J. & Von Hippel, P. H. Betaine can. Eliminate base pair composition depend. DNA Melt. Biochem. 32, 137–144 (1993).
Simonović, A. et al. Dimethyl sulfoxide improves sensitivity and specificity of RT-PCR and QRT-PCR amplification of low-expressed transgenes. Arch. Biol. Sci. 64, 865–876 (2012).
Kreader, C. A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl. Environ. Microbiol. 62, 1102–1106 (1996).
Samarakoon, T., Wang, S. Y. & Alford, M. H. Enhancing PCR amplification of DNA from recalcitrant plant specimens using a trehalose-based additive. Appl. Plant. Sci. 1, 1200236 (2013).
Devika, P. P. et al. Nano-PCR for the early detection of tomato leaf curl virus. 3 Biotech 14, 5 (2024).
Li, M., Lin, Y. C., Wu, C. C. & Liu, H. S. Enhancing the efficiency of a PCR using gold nanoparticles. Nucleic Acids Res. 33, e184 (2005).
Wang, L. et al. Effects of quantum dots in polymerase chain reaction. Phys. Chem. B. 113, 7637–7641 (2009).
Du, X., An, H., Jin, B., Meng, L. & Liu, Q. J. Carbon nanotubes altering specificity of repeated PCR and DNA integrity properties. Nanosci. Nanotechnol. 14, 5547–5551 (2014).
Lee, H. G. et al. PCR-coupled paper-based surface-enhanced Raman scattering (SERS) sensor for rapid and sensitive detection of respiratory bacterial DNA. Sens. Actuators B Chem. 326, 128802 (2021).
Lin, Y. et al. Mechanism of gold nanoparticle induced simultaneously increased PCR efficiency and specificity. Chin. Sci. Bull. 58, 4593–4601 (2013).
Jung, S., Kim, J., Kim, J., Yang, S. H. & Kim, S. K. Extensible multiplex real-time PCR for rapid bacterial identification with carbon nanotube composite microparticles. Biosens. Bioelectron. 94, 256–262 (2017).
Yang, Z. et al. Application of nanomaterials to enhance polymerase chain reaction. Molecules 27, 8854 (2022).
Navarro, E., Serrano-Heras, G., Castaño, M. J. & Solera, J. Real-time PCR detection chemistry. Clin. Chim. Acta. 439, 231–250 (2015).
Tao, Y. et al. Comparison of analytical sensitivity and efficiency for SARS-CoV-2 primer sets by TaqMan-based and SYBR Green-based RT-qPCR. Appl. Microbiol. Biotechnol. 106, 2207–2218 (2022).
Shahrajabian, M. H. & Sun, W. The significance and importance of dPCR, qPCR, and SYBR Green PCR kit in the detection of numerous diseases. Curr. Pharm. Des. 30, 169–179 (2024).
Green, M. R. & Sambrook, J. Constructing a standard curve for real-time polymerase chain reaction (PCR) experiments. Cold Spring Harb. Protoc. 2018 (10). (2018).
Cawthon, R. M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 37, e21 (2009).
Zhang, H. et al. Determination of advantages and limitations of qPCR duplexing in a single fluorescent channel. ACS Omega. 6, 22292–22300 (2021).
Song, C. Y. et al. Gold nanoflowers with tunable sheet-like petals: facile synthesis, SERS performances, and cell imaging. J. Mater. Chem. B. 4, 7112–7118 (2016).
Wei, L. et al. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov. Today. 20, 595–601 (2015).
He, X., Wang, H., Li, Z., Chen, D. & Zhang, Q. ZnO-Ag hybrids for ultrasensitive detection of trinitrotoluene by surface-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 16, 14706–14712 (2014).
Liu, P., Yang, S., Fang, M., Luo, X. & Cai, W. Complex nanostructures synthesized from nanoparticle colloids under an external electric field. Nanoscale 3, 3933–3940 (2011).
Chang, Y. C., Huang, B. H. & Lin, T. H. Surface-enhanced Raman scattering and fluorescence on gold nanogratings. Nanomaterials (Basel). 10, 776 (2020).
Yaraki, M. T. & Tan, Y. N. Metal nanoparticles-enhanced biosensors: synthesis, design, and applications in fluorescence enhancement and surface-enhanced Raman scattering. Chem. Asian J. 15, 3180–3208 (2020).
Yin, I. X. et al. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 15, 2555–2562 (2020).
Jaiswal, K., Mahanta, M. & De, M. Nanomaterials in photocatalysed organic transformations: development, prospects, and challenges. Chem. Commun. 59, 5987–6003 (2023).
Zhang, S. et al. Effect of the duty cycle of flower-like silver nanostructures fabricated with a lyotropic liquid crystal on the SERS spectrum. Molecules 26, 6522 (2021).
Song, C. et al. Surface-enhanced Raman spectroscopy substrate time stability improvement using an external oxygen barrier method. Appl. Spectrosc. 78, 289–295 (2024).
Zeng, R. et al. The 60 nm gold nanoparticles improve qPCR amplification efficiency through specific palindromic sequences (GGATCC or ACCGGT) in primers. Biochim. Biophys. Acta Gen. Subj. 1868, 130560 (2024).
Lenka, G. & Weng, W. H. Nanosized particles of titanium dioxide specifically increase the efficiency of conventional polymerase chain reaction. Digest. J. Nanomater. Biostruct.. 8, 1655–1662 (2013).
Humes, S. T. et al. Overcoming qRT-PCR interference by select carbon nanotubes in assessments of gene expression. Biotechniques 63, 81–84 (2017).
Funding
This work was supported by Jiangsu Key Disciplines of the Fourteenth Five-Year Plan (Grant No. 2021135), the National Natural Science Foundation of China (Grants No. 22205155), the Natural Science Foundation of Jiangsu Province (Grant No. BK20220640), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (Grant No. 22KJB150011), the Young Elite Scientists Sponsorship Program by Jiangsu Association for Science and Technology (Grants No. JSTJ-2023-XH042), Basic Research Pilot Project of Suzhou (No. SJC2021018), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (Grant No. KYCX23_3311).
Author information
Authors and Affiliations
Contributions
C.S. (Congxi Song) contributed to writing the original draft and data analysis. K.L. (Keliang Li) processed the spectrum data. Z.J. (Zhihui Jiang) was responsible for validation. Y.T. (Yibo Tang) conducted the investigation and data curation. W.H. (Wusheng Hu) contributed to data curation and formal analysis. H.M. (Hongming Mao) developed the methodology. X.Z. (Xin Zhao) provided supervision. J.Y. (Jian Yin) contributed to conceptualization, methodology, validation, and supervision. H.L. (Huanjun Lu) was responsible for funding acquisition and writing—review and editing. Z.C. (Zhaoliang Cao) contributed to conceptualization and writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, C., Li, K., Jiang, Z. et al. High-sensitivity qPCR detection method based on silver flower-like LSPR-active material. Sci Rep 15, 5964 (2025). https://doi.org/10.1038/s41598-025-90369-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90369-6