Abstract
Nomograms for predicting survival are well-established for colorectal adenocarcinoma but are less common for rectal mucinous adenocarcinoma (MAC). This study leverages Surveillance, Epidemiology, and End Results (SEER) database to develop a prognostic nomogram for rectal MAC, enhancing survival prediction and clinical decision-making. Data from the SEER (2004–2017) were analyzed, focusing on surgically treated rectal MAC patients. The study excluded cases with inadequate follow-up or incomplete data. Demographic and pathological variables, along with treatment modalities (radiotherapy and chemotherapy), were examined. A nomogram was developed to predict 1-, 3-, and 5-year cancer-specific survival (CSS), and its accuracy was validated using receiver operating characteristic (ROC) curves, calibration, and decision curve analysis (DCA). The study included 690 patients, segmented into training (483) and validation (207) cohorts. Significant factors affecting survival included age and cancer stage. The resulting nomogram, incorporating age and TNM stages, demonstrated a concordance index of 0.722 and was further validated through ROC analysis, calibration, and DCA, showing robust predictive performance and clinical utility. Age and TNM staging are critical predictors of CSS in rectal MAC patients post-surgery. The developed nomogram, utilizing these factors, offers improved prognostic accuracy and is a valuable tool for clinical practice, facilitating more informed treatment decisions.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) ranks third in incidence and second in mortality among all cancer types. In 2022, it was estimated that there were more than 1.9 million new cases and 904,000 deaths attributable to this disease, accounting for nearly one-tenth of both global cancer diagnoses and fatalities1. The majority of CRC cases are classified as adenocarcinomas (AC). Mucinous carcinoma, a distinct subtype, constitutes 10–15% of CRC cases. Studies have shown that mucinous carcinoma is associated with a poorer response to treatment compared to AC2.
Nitsche et al.3 conducted an analysis of 28,056 patients diagnosed with CRC, specifically examining subtypes including AC, mucinous adenocarcinoma (MAC), and signet-ring cell carcinomas (SC). Their study demonstrated that AC were the most prevalent subtype, occurring in 89.7% of patients, followed by MAC at 9.7%, and SC at 0.6%. The findings indicated that MAC and SC were associated with higher tumor (T), node (N), and metastasis (M) categories, more frequent lymphatic invasion, and poorer grading compared to AC. Furthermore, significant disparities were noted in the 10-year cumulative incidences of locoregional and distant recurrences among the subtypes. Survival rates also varied, with the 5-year overall survival (OS) being 59% for AC, 52% for MAC, and notably lower at 40% for SC. Zhang et al.4 analyzed 620 patients with locally advanced rectal cancer who underwent neoadjuvant treatment. Within this cohort, 8.5% of the patients were diagnosed with MAC. They observed a pathological complete response rate of 7.5% in MAC patients versus 22.0% in AC patients. Similarly, tumor downstaging rates were 20.8% for MAC compared to 48.7% for AC. Furthermore, the study reported 3-year disease-free survival (DFS) rates of 58.4% for the MAC group versus 77.6% for the AC group. Additionally, the 3-year locoregional recurrence rates were significantly higher in the MAC group at 26.0%, compared to 5.7% in the AC group.
Although the literature includes nomograms for MAC of CRC5, such tools are scarce for rectal cancers, especially regarding post-surgical prognosis. In this retrospective analysis, we examined the demographic and pathological characteristics of rectal cancer patients with MAC types using data from the Surveillance, Epidemiology, and End Results (SEER) database. Our objective was to identify predictive factors associated with survival and to develop a nomogram based on these factors for predicting 1-, 3-, and 5-year cancer-specific survival (CSS) after surgery. The creation of this nomogram aims to enhance the prediction of patient survival and to serve as a reference for clinical decision-making.
Materials and methods
Data source and patient selection
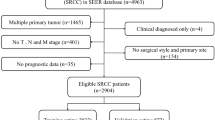
Data for this study were obtained from the SEER database (version 8.4.3), covering a cohort from 2004 to 2017. Inclusion criteria were limited to patients diagnosed with MAC of the rectum who underwent surgical treatment, as surgical intervention provides comprehensive pathologic reports essential for detailed analyses. Exclusion criteria encompassed patients with less than one month of follow-up, unknown survival status, age under 20, unclear histological grade, and indeterminate TNM staging, including those classified as stage Tis, thus ensuring clarity and completeness of the data for robust analysis.
Variables
The study focused on a set of demographics and pathological characteristics including gender; race categorized as white and others; age groups (< 65, 65–74, ≥ 75). Pathologic details captured post-surgery included the grade of the tumor (well differentiated, moderately differentiated, poorly differentiated, and undifferentiated) and the American Joint Committee on Cancer (AJCC) stage, determined using the TNM classification in accordance with the 7th edition of the AJCC Cancer Staging Manual. Treatment modalities, specifically radiotherapy and chemotherapy, were also documented for their roles in patient outcomes.
Statistical analysis
Statistical analyses were performed using R software, version 4.3.2. The Kaplan–Meier method was employed to estimate CSS rates at 1, 3, and 5 years after surgical treatment. Patients were randomly split into a training cohort and a validation cohort at a 7:3 ratio. A univariate Cox proportional hazards model was applied to identify significant prognostic factors (p < 0.05) in the training cohort, which were then assessed through a multivariate Cox analysis to elucidate independent predictors of survival. The performance of prognostic factors was quantified using the concordance index (C-index); a C-index exceeding 0.70 indicated the feasibility of constructing a nomogram based on these factors. The nomogram was developed to predict CSS at 1, 3, and 5 years.
The reliability of the nomogram was initially validated within the training cohort and subsequently tested in the validation cohort. Validation techniques included the use of receiver operating characteristics (ROC) to assess the discriminative ability of the nomogram. The rationale behind employing ROC analysis lies in its effectiveness in evaluating the sensitivity and specificity of the predicted probabilities against actual outcomes. Calibration, which measures the agreement between observed outcomes and predictions, was used to ensure the accuracy of the nomogram. Lastly, decision curve analysis (DCA) was applied to determine the clinical usefulness of the nomogram by evaluating the net benefits at various threshold probabilities, providing insights into the practical impact of adopting the nomogram in clinical settings.
Ethics approval and consent to participate
The patient data in the SEER database are de-identified and anonymized; therefore, ethical consent was not required for the use of this information. The procedures were meticulously conducted in full compliance with relevant guidelines and regulations. Research involving human participants or human data was performed in accordance with the ethical principles established in the “Declaration of Helsinki”.
Results
The flowchart of the patients’ selection was listed in the Fig. 1. A total of 690 patients were enrolled. Overall, the 1-, 3-, and 5-year CSS rates were 92.5%, 73.4%, and 62.2%, respectively. Further stratified analysis revealed 5-year CSS rates of 82.5% for stage I, 74.9% for stage II, 63.0% for stage III, and 22.8% for stage IV. Table 1 provides baseline characteristics of patients distributed into two cohorts, a training cohort with 483 participants and a validation cohort with 207 participants, from a study on rectal MAC. The demographic and clinical data are detailed for both cohorts, facilitating comparisons and evaluations of homogeneity across groups. Sex distribution shows a slight majority of males in both cohorts, with 61.5% in the training and 63.8% in the validation groups, indicating a balanced representation. Racial composition is predominantly white in both cohorts, comprising 84.5% in the training and 79.7% in the validation group, with the remainder classified as “other” races. Age is segmented into three categories: below 65, between 65 and 74, and 75 or older. The training cohort and the validation cohort display similar age distributions, ensuring that age-related variables impact both groups equally in subsequent analyses. Tumor grade, a critical clinical variable, shows a major proportion of patients classified as moderately differentiated (64.4% in the training and 61.4% in the validation cohorts), followed by poorly differentiated, well differentiated, and undifferentiated. The cancer stage, categorized according to the AJCC system from I to IV, illustrates that most patients are in stage III, followed by stages II, I, and IV, respectively, across both cohorts. The detailed TNM classification corroborates the staging with proportions maintaining consistency between cohorts. Treatments administered include radiotherapy and chemotherapy, with the majority of patients receiving both therapies across the groups. Statistical significance tests (p-values) across all variables show no significant differences between the cohorts, indicating that the two groups are statistically comparable, which is vital for validating study findings reliably.
Table 2 presents the results of both univariate and multivariate analyses of CSS among the training cohort, detailing the impact of various demographic and pathological factors on survival outcomes. The univariate analysis served as the initial screening to identify potential predictors. Age (specifically patients aged ≥ 75), stage III, stage IV, and TNM classifications T4 and N2, as well as M1 (metastatic disease), showed significant associations with poorer survival outcomes, indicating a higher risk compared to their reference groups. In the multivariate Cox regression analysis, factors adjusted for each other to discern the independent effect of each variable on survival. As the stage is derived directly from the TNM classification, we acknowledge the potential issue of collinearity if both variables are included simultaneously in the analyses. To address this, we exclude stage in the multivariable analyses. Including the TNM classification allows us to assess the specific prognostic impact of each component, which is not possible when only the stage is analyzed. Patients aged 75 or older had a hazard ratio (HR) of 2.55, indicating a significantly higher risk of mortality compared to younger age groups. Stage IV cancer was identified with a HR of 7.59, highlighting a considerably elevated risk of cancer-specific mortality. In the TNM classifications, T4, N2, and M1 were significant predictors of survival, with T4 exhibiting a HR of 5.98, which underlines its crucial impact on survival. Similarly, N2 and M1 were strongly linked to poorer outcomes, with HRs of 2.58 and 3.36, respectively. The selection of these factors such as age, T, N, and M stages for the nomogram was based on their statistically significant and clinically relevant impacts on survival, as demonstrated through rigorous multivariate analysis.
We developed a nomogram using age, T, N, and M stages (Fig. 2), which achieved a C-index of 0.722, indicating a good fit and suitability for development. The ROC curves for this nomogram’s prognosis prediction are presented in Fig. 3. The areas under the curve for the 1-, 3-, and 5-year CSS rates were 0.822, 0.765, and 0.770 in the training cohort, and 0.702, 0.683, and 0.763 in the validation cohort, respectively. In Fig. 4, the calibration plots compare the actual CSS outcomes with the probabilities predicted by the nomogram, providing a visual representation of the good agreement between the nomogram’s predictions and the observed outcomes in both the training and validation cohorts. In Fig. 5, we have depicted DCA curves to evaluate the clinical utility of the nomogram by quantifying its net benefits. The DCA curves demonstrate that using the nomogram to predict 1-, 3-, and 5-year CSS probabilities offers greater clinical net benefits compared to the treat-all or treat-none strategies. These results illustrate that our prognostic model possesses substantial clinical application value, affirming its potential utility in informed clinical decision-making.
Discussion
Presently, there is a scarcity of nomograms designed for predicting outcomes after surgery for rectal MAC. To bridge this gap, we identified risk factors and constructed a nomogram to predict CSS. This nomogram has demonstrated substantial reliability in its prognostic predictions.
Al-Abed et al.6 conducted a retrospective study involving 374 patients who underwent radical rectal surgery over a ten-year follow-up. They reported significantly lower OS in elderly patients, with 1-year survival rates of 91.3% for patients under 50 and 75.5% for those over 80. The 5-year survival rates showed a similar trend, with 87.0% for patients under 50 compared to 57.1% for those over 80. The hazard ratio for patients aged over 80 compared to those under 50 was 4.79, indicating a significantly higher risk of mortality. Gefen et al.7 also found significant age-related difference in outcomes of CRC post-surgery. Their study categorized patients into three age groups and highlighted that the very old (≥ 80 years) experienced higher 30-day (5.9% vs 1.3% vs 0.3%) and 90-day (10.3% vs 2.6% vs 0.7%) mortality rates post-rectal cancer surgery compared to the middle-aged (50–79 years) and young (< 50 years) groups. Our own research corroborates these findings, showing that patients older than 75 with MAC have notably poorer CSS rates.
However, Pilleron et al.8 examined the influence of histology and disease stage on age-specific one-year relative survival ratios (RS) for CRC. Their findings indicated that differences in RS among various histologies—specifically AC, MAC, and SC—ranged from 7.4 to 10.6 percentage points. Notably, these differences escalated dramatically to 42 percentage points for patients categorized under unknown or other histologies, which represented less than 6% of cases. The study posited that age-related survival discrepancies were consistent across all histologies, suggesting that such differences in RS are not predominantly due to age-based variations in these factors. Regarding disease stage, Pilleron et al. observed that RS differences between individuals aged 50–64 and those aged 75–84 widened with advancing disease stage—from 6 percentage points in localized disease to 27 percentage points in distant disease. The greatest disparities were noted in cancers of unknown stage, exceeding 28 percentage points. The researchers inferred that age disparities in stage distribution likely contribute to these observed differences in survival. They further hypothesized that within these stage groups, variances in survival rates could be attributed to factors such as frailty and therapeutic approaches. The authors advocate for the inclusion of frailty and comorbidity assessments in future research to elucidate the age-related disparities in CRC survival, thereby enhancing the precision of prognostic evaluations and potentially guiding more tailored treatment strategies.
Yoshioka et al.9 observed that patients with high-grade colorectal MAC experienced significantly shorter DFS and OS compared to those with low-grade MAC. Notably, high-grade cases were associated with a higher incidence of progressive disease, particularly marked by an elevated prevalence of positive lymph nodes (92.3% versus 55.5%). The authors advocated for a clinical distinction between low- and high-grade MAC. However, their study was limited by a small sample size, encompassing only 40 patients with MAC, of which just 13 were diagnosed with the high-grade variant, thereby diminishing the statistical robustness of their findings. In contrast, Williams et al.10 reported that among patients with stage II or III CRC, the status of tumor-infiltrating lymphocytes was a robust independent predictor of DFS for mucinous tumors and proved to be a more effective prognostic indicator than histological grade. These findings suggest a limited influence of histological grade on the prognosis of MAC. Our larger-scale study corroborates the findings of Williams et al., demonstrating that the impact of histological grade on CSS in patients with MAC of the rectum is negligible.
Xie et al.11 examined a cohort of 1380 patients diagnosed with CRC, of which 165 were identified as having the mucinous subtype. Their findings indicated that stage III mucinous carcinoma patients exhibited significantly poorer survival rates compared to those with non-mucinous carcinoma. Moreover, they identified stage as an independent and significant prognostic factor for mucinous CRC in a multivariate analysis. In a subsequent study, Sun et al.12 investigated 145 cases of rectal MAC and determined that a positive N stage significantly predicted early recurrence in these patients. Furthermore, Huang et al.13 analyzed data from 162 patients and found that N2 stage was a significant predictor of local recurrence, while T4 stage substantially predicted distant metastasis. Consistent with these findings, our own research involving patients with N2 stage (HR = 2.58, p < 0.001) or T4 stage (HR = 5.98, p < 0.001) demonstrated significantly worse survival outcomes. We propose that stage is a critical factor to consider for CSS in patients with rectal MAC.
Liao et al.14 assessed the necessity of adjuvant radiotherapy for stage III rectal MAC by analyzing data from the SEER database spanning 2004 to 2015, which included 665 patients. Their findings indicated no significant differences in OS or CSS between the group treated with chemotherapy alone and the group treated with both chemotherapy and radiotherapy, even after adjusting for potential confounders through propensity score matching. The authors concluded that adjuvant radiotherapy did not confer any survival benefit in patients with rectal MAC. Due to our dataset not distinguishing between pre-operative and post-operative radiotherapy, we are unable to verify these findings conclusively. Hugen et al.15 examined the efficacy of radiotherapy in 103 patients with rectal mucinous carcinoma. Their study found that patients who received preoperative radiotherapy did not demonstrate an improvement in 10-year OS compared to those who did not receive radiotherapy (44.7% vs. 49.4%, p = 0.475). Our study concentrated on CSS and similarly found no discernible benefit in CSS attributable to radiotherapy.
Powers et al.16 asserted that patients with resected stage III colon cancer should receive adjuvant chemotherapy irrespective of histological subtype. In a study encompassing 3,052 cases of colorectal MAC, Hugen et al.17 demonstrated no significant difference in the effectiveness of chemotherapy between MAC and non-MAC, suggesting that histological classification should not influence adjuvant treatment recommendations. Vernmark et al.18 recently investigated the benefits of adjuvant chemotherapy for patients with stage II-IV rectal MAC. Their findings indicated enhanced OS (HR = 0.42) and CSS (HR = 0.41) in patients who received adjuvant chemotherapy compared to those who did not, though their study was limited by a small sample size of 56 patients with MAC. Liang et al.19 advocate for adjuvant chemotherapy in moderate and high-risk stage II rectal MAC patients following neoadjuvant chemoradiotherapy, based on observed benefits over stage II rectal AC patients. However, they recommend against adjuvant chemotherapy for low-risk patients. In our analysis, approximately three-fourths of the patients received chemotherapy, but the data did not distinguish between preoperative and postoperative administration. Thus, the specific benefits of neoadjuvant versus adjuvant chemotherapy warrant further investigation.
This retrospective study utilizes the SEER database, which presents several limitations inherent in its design. First, the SEER database lacks information on patients’ underlying diseases, which can significantly influence prognostic outcomes. Second, there is no available data on patients’ performance status in the database, another crucial factor affecting patient prognosis. Third, while SEER records whether patients received chemotherapy or radiotherapy, it does not differentiate between specific protocols, such as the use of neoadjuvant versus adjuvant treatments, nor does it include information on drug types or radiotherapy techniques (e.g., short-course vs. long-course radiotherapy). Fourth, metastatic lesions can significantly influence survival outcomes in MAC. Unfortunately, the SEER database does not provide detailed information on metastatic lesions (e.g. liver or lung). Fifth, certain common predictors, including lymphatic invasion, venous invasion, perineural invasion, and resection margin status, which are known to be important prognostic factors in CRC. Unfortunately, the SEER database does not include detailed information on these pathological characteristics, which limits our ability to analyze their impact on CSS in this study. Finally, the database does not include lifestyle factors such as smoking, which has been proven to be associated with rectal cancer risk20. Despite these limitations, what sets this study apart is the development of a clinically validated nomogram, specifically tailored for predicting 1-, 3-, and 5-year CSS in rectal MAC patients. Unlike prior research, which often focused broadly on colorectal MAC or did not emphasize post-surgical prognosis, our study provides a specialized tool with robust predictive accuracy. This nomogram not only offers practical utility for individualized patient counseling and clinical decision-making but also fills a critical gap in the literature by addressing the paucity of prognostic models for this rare and aggressive cancer subtype. These results contribute to the growing understanding of rectal MAC by integrating population-level data from the SEER database and providing a novel, validated tool that enhances clinical prognostic assessments. Future research should aim to incorporate additional variables such as comorbidities, lifestyle factors, and detailed treatment protocols to further refine prognostic models and guide tailored therapeutic strategies for this high-risk patient population.
Conclusion
This study identifies age and TNM stage as significant independent predictors of CSS in patients with MAC of the rectum undergoing surgical treatment. These findings highlight the critical prognostic impact of advanced tumor, nodal, and metastatic stages, along with advanced age, in predicting poorer survival outcomes. Notably, our analysis highlights the unique challenges encountered by patients with rectal MAC, characterized by significantly poorer survival outcomes.
Data availability
The data analyzed in this study are accessible through the Surveillance, Epidemiology, and End Results (SEER) program database, available at https://seer.cancer.gov/.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263 (2024).
Hugen, N., Brown, G., Glynne-Jones, R., de Wilt, J. H. & Nagtegaal, I. D. Advances in the care of patients with mucinous colorectal cancer. Nat. Rev. Clin. Oncol. 13(6), 361–369 (2016).
Nitsche, U. et al. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J Cancer Res. Clin. Oncol. 142(11), 2357–2366 (2016).
Zhang, J. et al. Mucinous adenocarcinoma predicts poor response and prognosis in patients with locally advanced rectal cancer: a pooled analysis of individual participant data from 3 prospective studies. Clin. Colorectal Cancer 20(4), e240–e248 (2021).
Xu, J. et al. Construction of novel prognostic nomogram for mucinous and signet ring cell colorectal cancer patients with a survival longer than 5 years. Int. J. Gen. Med. 15, 2549–2573 (2022).
Al-Abed, Y., Parker, M., Arulampalam, T. & Tutton, M. Survival following rectal cancer surgery: Does the age matter?. Acta Chir Belg. 119(5), 282–288 (2019).
Gefen, R., Emile, S. H., Horesh, N., Garoufalia, Z. & Wexner, S. D. Age-related variations in colon and rectal cancer: An analysis of the national cancer database. Surgery 174(6), 1315–1322 (2023).
Pilleron, S., Withrow, D. R., Nicholson, B. D. & Morris, E. J. A. Age-related differences in colon and rectal cancer survival by stage, histology, and tumour site: An analysis of United States SEER-18 data. Cancer Epidemiol. 84, 102363 (2023).
Yoshioka, Y. et al. Clinicopathological and genetic differences between low-grade and high-grade colorectal mucinous adenocarcinomas. Cancer 121(24), 4359–4368 (2015).
Williams, D. S. et al. Tumour infiltrating lymphocyte status is superior to histological grade, DNA mismatch repair and BRAF mutation for prognosis of colorectal adenocarcinomas with mucinous differentiation. Mod. Pathol. 33(7), 1420–1432 (2020).
Xie, L., Villeneuve, P. J. & Shaw, A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int. J. Oncol. 34(4), 1109–1115 (2009).
Sun, Y. et al. A refined prediction of early recurrence combining tumor deposits in patients with resected rectal mucinous adenocarcinoma. Surg. Today 53(7), 762–772 (2023).
Huang, Q. et al. Risk factors for recurrence of radically resected mucinous colorectal adenocarcinoma. Cancer Manag. Res. 13, 4777–4790 (2021).
Liao, H. et al. Adjuvant radiotherapy is not necessary for stage III mucinous rectal cancer: Evidence based on long survival analysis from SEER data. J. Gastrointest. Surg. 27(12), 2857–2866 (2023).
Hugen, N. et al. Modern treatment of rectal cancer closes the gap between common adenocarcinoma and mucinous carcinoma. Ann. Surg. Oncol. 22(8), 2669–2676 (2015).
Powers, B. D., Felder, S. I., Imanirad, I., Dessureault, S. & Dineen, S. P. The impact of histologic subtype on receipt of adjuvant chemotherapy and overall survival in stage III colon cancer: a retrospective cohort analysis. J. Gastrointest. Cancer 52(2), 719–727 (2021).
Hugen, N. et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann. Oncol. 24(11), 2819–2824 (2013).
Vernmark, K., Knutsen, A., Loftås, P. & Sun, X. F. The impact of adjuvant chemotherapy on survival in mucinous and non-mucinous rectal adenocarcinoma patients after TME surgery. PLoS ONE 18(2), e0282211 (2023).
Liang, Y. et al. Risk stratification of stage II rectal mucinous adenocarcinoma to predict the benefit of adjuvant chemotherapy following neoadjuvant chemoradiation and surgery. Front Oncol. 14, 1352660 (2024).
Sharp, L., McDevitt, J., Brown, C., Carsin, A. E. & Comber, H. Association between smoking at diagnosis and cause-specific survival in patients with rectal cancer: Results from a population-based analysis of 10,794 cases. Cancer 123(13), 2543–2550 (2017).
Acknowledgements
The authors thank the Surveillance, Epidemiology, and End Results (SEER) database for supplying the clinical information necessary for this study.
Funding
This study did not receive any external funding.
Author information
Authors and Affiliations
Contributions
Y.C.: Conceptualization, Methodology, Writing—original draft. C.D.: Conceptualization, Methodology, Writing—original draft. X.Z.: Conceptualization, Methodology, Writing—original draft. K.L.: Conceptualization, Methodology, Project administration, Writing—review and editing. Y.X.: Conceptualization, Methodology, Writing—original draft. C.-Y.H.: Conceptualization, Methodology, Formal analysis, Project administration, Writing—review and editing, Supervision. J.H.: Conceptualization, Methodology, Formal analysis, Project administration, Writing—review and editing, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Duan, C., Zhang, X. et al. A nomogram for predicting cancer-specific survival in patients with rectal mucinous adenocarcinoma following surgery. Sci Rep 15, 6809 (2025). https://doi.org/10.1038/s41598-025-90428-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90428-y