Abstract
Life’s Essential 8 (LE8) is an emerging composite metric of cardiovascular health encompassing diet, physical activity, smoking, sleep, weight, cholesterol, blood glucose, and blood pressure. Mounting evidence suggests lifestyle factors may play an important role in overactive bladder (OAB), however the link between LE8 and OAB remains unexplored. We aimed to examine the correlation between the two. We analyzed data on 23,187 individuals from the 2005–2018 National Health and Nutrition Examination Survey (NHANES). Participants were stratified into low, moderate and high LE8 groups. Logistic regression examined the association between LE8 and OAB. Restricted cubic splines (RCS) and weighted quantile sum (WQS) regression further probed this relationship. Higher LE8 scores were associated with lower OAB risk, independent of covariate adjustment. The inverse correlation between LE8 and OAB was validated by RCS and WQS analyses. Of LE8 components, glycemic control conferred the greatest contribution. Higher LE8 scores may be protective against OAB. Optimization of cardiovascular health metrics could represent a novel OAB prevention strategy.
Similar content being viewed by others
Introduction
Overactive bladder (OAB) is a common condition characterized by urinary urgency, usually accompanied by frequency and nocturia, with or without urge urinary incontinence, in the absence of urinary tract infection or other obvious pathology1. The prevalence of OAB is reported to be as high as 16.9% globally2, exerting substantial detrimental impacts on quality of life and imposing a tremendous socioeconomic burden3. Therefore, identifying modifiable risk factors for OAB is of great public health significance.
Emerging evidence suggests that lifestyle factors may play an important role in OAB development. In particular, the American Heart Association (AHA) recently proposed “Life’s Essential 8” (LE8), a set of health behaviors and factors including diet, physical activity, nicotine exposure, sleep, weight, cholesterol, blood glucose, and blood pressure (BP), as critical metrics of overall cardiovascular health4. LE8 represents a shift beyond traditional risk factors to focus on the promotion of ideal health. Interestingly, some components of LE8, such as blood glucose, have been associated with increased OAB risk5,6. However, no studies have yet examined the relationship between overall LE8 score and OAB.
Elucidating the link between LE8 and OAB may uncover novel opportunities for improving bladder health through optimization of cardiovascular health metrics. Here, we analyze data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative study of health and nutritional status in the United States, to investigate the association between LE8 score and OAB prevalence. We apply rigorous statistical modeling, including multivariate logistic regression, restricted cubic splines (RCS), and weighted quantile sum (WQS) regression, to delineate this relationship and dissect the relative contributions of individual LE8 components. Our findings may inform preventive strategies and public health messaging to mitigate the rising burden of OAB worldwide.
Methods
Study design and population
This paper mines the NHANES database for seven cycles of data from 2005–2018. NHANES is a research program designed to assess the health and nutritional status of adults and children in the U.S. NHANES is a major program of the National Center for Health Statistics, part of the Centers for Disease Control and Prevention, which is responsible for providing the nation with vital and health statistics. NHANES interviews include demographic, socioeconomic, dietary, and health-related issues. The screening component includes medical, dental, and physiologic measurements, as well as laboratory tests performed by trained medical personnel. The results of this survey are used to determine the prevalence of major diseases and risk factors for disease, and to assess nutritional status and its relationship to health promotion and disease prevention.
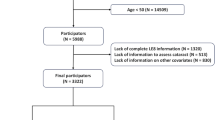
There were a total of 70,190 subjects in these 7 cycles. First, we excluded 35,840 individuals who were unable to evaluate bladder function and 8,500 individuals who had insufficient information to calculate LE8. In addition, 2,663 individuals with missing covariate information were also excluded. Finally, a total of 23,187 people were included in the study, of which 11,765 were women and 11,422 were men (Fig. 1).
Diagnosis of overactive bladder
In this paper, the diagnosis of OAB follows the flowchart of OAB diagnosis based on overactive bladder syndrome score first proposed by Yunfei Xiao et al.7. The nocturia frequency of 0, 1, 2, and greater than or equal to 3 times received scores of 0, 1, 2, and 3, respectively. Urge incontinence was given scores of 0, 1, 2, and 3 based on how frequently it occurred—never, once a month, once a week, and once a day, respectively. By summing the two scores, the Overactive Bladder Syndrome Symptom Score (OABSS) was calculated. Individuals who had an overall OABSS of ≥ 3 were diagnosed with OAB.
Life’s essential 8 (LE8)
The American Heart Association (AHA) released revised cardiovascular health guidelines in the August 2022 issue of Circulation. Because a history of inadequate sleep has been linked to an increased risk of death from all causes in numerous epidemiologic studies. Based on the 2010 AHA publication (Life’s Simple 7), the guidelines add the behavior “sleep duration” and rename the most recent health guideline " LE8” to better support individuals with healthier sleep patterns in managing risks related to weight, blood pressure, or diabetes. The "LE8” Cardiovascular Health Guidelines include maintaining a healthy weight, blood pressure, blood lipid, and blood glucose levels, engaging in physical activity, abstaining from tobacco, and maintaining a nutritious diet. The specific algorithm for the LE8 scores for each indicator for the NHANES data can be found in Supplementary Tables 1–2.
Statistical analysis
Sample weights from various study years were pooled in accordance with the NHANES website criteria. Baseline data were grouped according to whether OAB was diagnosed or not, continuous variables were expressed as “Mean ± SD” deviation, and t-test with ANOVA was used to test for baseline differences between the two groups. Categorical information was expressed as percentages, and the x2 test was utilized to check for baseline differences. We first analyzed the relationship between LE8 and OAB incidence by stepwise adjustment of covariates using a generalized linear model (GLM). In the crude model, no covariates were adjusted. In the Model 1, adjustments were made for age, gender and race. In the Model 2, we further adjusted education level, poverty income ratio (PIR), alcohol intake based on Model 1. Next, we used restricted cubic spline curves (RCS) to explore whether there was a nonlinear association between the fully adjusted model and OAB. Finally, we used WQS regression to explore the overall effect of LE8 scores on the incidence of OAB and to assess the weight share of each indicator in LE8. The R package “Survey” combined weights in the analysis above.
Results
Baseline characteristics of the study population
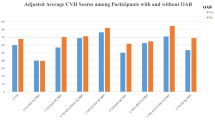
In the primary cohort of this study, a total of 23,187 eligible individuals were examined, comprising 11,422 males and 11,765 females. Based on the OAB diagnosis status of these people, two separate groups were formed (Table 1). In this study, 18,617 controls and 4,570 OAB patients were enrolled. Specifically, those individuals afflicted with OAB exhibited relatively lower scores across several vital parameters within the LE8 framework, as follows: Physical Activity (PA) score (Mean ± SE, 56.897 ± 46.072), Nicotine Exposure Score (Mean ± SE, 68.633 ± 38.827),Slee p Health Score (Mean ± SE, 76.438 ± 28.636), Body Mass Index (BMI) Score (Mean ± SE, 50.219 ± 34.399), Glucose Score (Mean ± SE, 71.565 ± 30.394), and BP Score (Mean ± SE, 54.260 ± 32.972) of the LE8. These observations underscore a noteworthy association between lower LE8 scores and the presence of OAB, implying an inverse correlation between LE8 scores and the prevalence of OAB. People with high LE8 scores (80–100, 7.81%) are less likely to get OAB. In addition, OAB patients were more likely to be female(59.15%),older (≥ 65, 41.20%), Non-Hispanic Black (27.16%), low high school (31.82%), abstaining or never drinkers (40.02%).
Relationship between model-adjusted LE8 score and the risk of OAB by the generalized linear model
We employed three separate GLM models to step-wise adjust for the covariates because the baseline data indicated that the two groups differed statistically on certain factors (Table 2). In the crude model, no covariates were adjusted. In the Model 1, adjustments were made for age, gender and race. In the Model 2, we further adjusted education level, PIR, alcohol intake based on Model 1. Using the low LE8 subgroup as a reference, we plotted a forest plot (Fig. 2) for each model and labeled the odds ratio (OR) of the moderate LE8 score to the high LE8 score and the corresponding 95% confidence intervals (CIs) in the plots.
Forest plot for the association between Life’s Essential 8 Score and overactive Bladder. Crude Model: no covariates were adjusted. Model 1: adjusted for age; gender and race. Model 2: adjusted for age; gender; race; education level; poverty income ratio and alcohol intake. Low LE8 score: 0~49; Moderate LE8 score: 50~79; High LE8 score: 80~100.
Compared with the crude model, the ORs increased progressively (ORs < 1) after gradual adjustment of the covariates. Even after adjusting for these covariates, LE8 was a protective factor for overactive bladder syndrome. The ORs for high LE8 scores were lower in all three models compared to the ORs for medium LE8 scores, indicating a negative correlation between high LE8 scores and the incidence of OAB and supporting the idea that high LE8 scores may prevent the development of OAB.
Relationship between model-adjusted LE8 score and the risk of OAB by the restricted cubic spline
We applied the restricted cubic spline model8 to fit the association between the LE8 score and the incidence of OAB for the purpose to discover whether there is a nonlinear relationship between the two. (Fig. 3). When age, gender, race, education level, PIR and alcohol intake were taken into account, the model showed a non-linear association between the LE8 score and the incidence of OAB (P-non-linear = 0.043). However, as shown in Fig. 3, no clear U-shaped or inverted U-shaped trend was observed. Instead, the incidence of OAB consistently decreased with increasing LE8 scores. Therefore, we hypothesize that an increase in the LE8 score is associated with a reduced risk of OAB.
Restricted cubic spline plots of the association between LE8 score and incidence of OAB. RCS regression was adjusted for age; gender; race; education level; poverty income ratio and alcohol intake. The red solid line represents ORs. Histogram represents the distribution of people with different LE8 scores.
The association between OAB and model-adjusted LE8 score by the weighted quantile sum regression model
WQS regression9 is a statistical model for multiple regression of high-dimensional datasets often used in environmental exposures. In our study, we employ WQS regression to analyze the individual contributions of the LE8 score’s eight evaluation measures as well as to quantify the overall effect of the score on OAB risk (Fig. 4). The LE8 score derived from the fully adjusted WQS regression model was inversely associated with the risk of OAB (OR: 0.24, 95% CI: 0.21 to 0.28, P < 0.001). This statistical observation suggests that the LE8 score is protective against the development of OAB. Furthermore, a number of the LE8 assessors made a substantial contribution to the final result and were given a lot of weight in the WQS model. The largest impact was found in blood glucose (28.85%), followed by BMI (21.92%), nicotine exposure (13.45%), blood pressure (13.18%) and sleep health (13.13%).
Discussion
This study demonstrates an inverse association between LE8 score, a composite metric of cardiovascular health, and risk of OAB10. Higher LE8 scores were associated with progressively lower odds of OAB in a dose-response manner11. This relationship persisted in multivariate regression models adjusting for potential demographic and socioeconomic confounders, suggesting it is independent of these factors.
WQS regression further identified blood glucose, BMI, nicotine exposure, BP and sleep health as the key LE8 components contributing to its inverse link with OAB. Of these, blood glucose exhibited the strongest individual association, aligning with prior evidence that diabetes can result in bladder dysfunction6. Taken together, these findings indicate that improving LE8 metrics may confer protective effects against OAB susceptibility12. This highlights novel opportunities for lifestyle-based OAB prevention strategies by targeting optimization of cardiovascular health factors13.
Among the top LE8 metrics, many diabetic metabolites (e.g. monosodium urate, HMGB1, etc.) can trigger bladder inflammation and overactive bladder by activating NLRP3 inflammatory vesicles in the urine epithelium5,6. Excess weight predisposes to OAB potentially via direct pressure effects on the bladder and neuropathy associated with metabolic conditions14. Nicotine can elicit lower urinary tract symptoms (LUTS) through decreased bladder blood flow and uroepithelial hypoxia15,16, Optimal blood pressure control reduces risk of bladder ischemia and oxidative stress that can precipitate detrusor overactivity13. Finally, adequate sleep optimizes hormonal balance and neural pathways regulating urination, whereas sleep deprivation has been linked to overactivity of bladder afferent pathways17. Elucidating these mechanisms provides biological plausibility linking LE8 components to lower OAB susceptibility.
This study reveals an important link between cardiovascular health as measured by LE8 and susceptibility to overactive bladder syndrome18. The findings have significant public health implications in illuminating lifestyle optimization as a promising strategy for OAB prevention. Improving diet quality, increasing physical activity, achieving healthy weight status, and optimizing sleep, cholesterol, BP and glucose levels may confer collateral benefits for bladder health14. Population-level interventions targeting LE8 metrics could potentially help curb the rising tide of OAB and related healthcare burden.
On an individual patient level, the results provide a rationale for clinicians to consider LE8 status in OAB risk assessment and counsel patients on lifestyle changes for better bladder control12. This represents a shift from compartmentalized care focusing only on the bladder, towards whole-person preventive health19. Realizing such integrated care models will require cross-disciplinary collaboration between urologists, primary care providers, and other specialists20.
Ultimately, research on LE8 scores helps move the field towards prevention-oriented management21. Further studies elucidating lifestyle, behavioral and cardiovascular factors influencing OAB susceptibility will be instrumental in this paradigm shift22.
This study has several innovative aspects advancing our understanding of modifiable OAB risk factors. First, it represents the first analysis linking the novel LE8 cardiovascular health score, representing a constellation of lifestyle, clinical and biological factors, to OAB susceptibility23. This moves beyond conventional risk factors to a more integrated metric encompassing overall wellbeing. Second, the use of robust statistical techniques including RCS and WQS regression provides rigorous delineation of the relationship between LE8 and OAB. The dose-response curve and identification of key LE8 drivers are significant methodological strengths.Third, the dissection of individual contributions of LE8 components is highly innovative, revealing glycemic control as the most impactful element5. This granular analysis of a composite risk metric is novel in the OAB literature and generates actionable insights for targeted preventive approaches12. Overall, the analytical strategies overcome limitations of previous observational research and provide high-quality evidence to motivate lifestyle optimization for maintaining bladder health19. This study exemplifies the type of innovative methodology required to advance our understanding of modifiable determinants of OAB21.
This study has certain limitations intrinsic to cross-sectional analyses that warrant acknowledgement. First, the cross-sectional design precludes causal inference, and reverse causation remains possible if OAB leads to decreased physical activity and subsequent worsening of LE8 metrics24. To establish temporality, large-scale longitudinal cohorts tracking LE8 status and onset of OAB over time are needed25. Second, self-reported data on OAB symptoms and LE8 components like diet and exercise carries potential for recall bias. However, NHANES uses well-validated instruments administered by trained staff to maximize accuracy26. Third, using a composite of multiple predictors to define a multifactorial syndrome like OAB can complicate result interpretation. Our goal was to explore how integrating diverse factors could provide a more comprehensive understanding of OAB. Additionally, we employed Weighted Quantile Sum (WQS) regression to assess the individual contributions of these factors, aiming to generate actionable insights for real-world application. We anticipate further research to refine and enhance its broader implementation. Finally, one-time assessment of LE8 may not fully reflect long-term exposure to poor cardiovascular health markers, which would be better captured through repeated measurements27.
This research motivates several fruitful avenues for future investigation. Longitudinal cohort studies tracking LE8 status over years or decades could elucidate whether optimal cardiovascular health metrics assessed earlier in life predict lower OAB incidence with aging. Large, multi-center trials are needed to determine if lifestyle interventions targeting LE8 improvement can reduce downstream OAB risk28. Studies in more diverse populations can evaluate generalizability of associations across racial/ethnic groups. Finally, research on pathologic mechanisms is warranted to explain observed epidemiological links between cardiovascular health metrics and OAB susceptibility21.
Conclusions
This study provides evidence of an inverse relationship between cardiovascular health as assessed by LE8 metrics and risk of developing overactive bladder syndrome. The findings point to lifestyle optimization as a promising strategy for OAB prevention, building on past research demonstrating links between glycemic control, BMI, nicotine exposure and urinary symptoms. This work advances a conceptual shift from compartmentalized urologic care towards integrated models leveraging lifestyle, behavioral and cardiovascular factors to promote bladder health. Additional research in large, diverse populations confirming the preventive potential of ideal cardiovascular health behaviors is still needed to translate these observational data into effective OAB prevention policies and clinical recommendations.
Data availability
Publicly available datasets were analyzed in this study. These data can be found at: www.cdc.gov/nchs/nhanes/.
References
Abrams, P. et al. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 61(1), 37–49. https://doi.org/10.1016/s0090-4295(02)02243-4 (2003).
Irwin, D. E. et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur. Urol. 50(6), 1306–1315. https://doi.org/10.1016/j.eururo.2006.09.019 (2006).
Nazir, J., Hakimi, Z., Jacobson, M. & Al-Hothi, H. Overactive bladder: Impact on quality of life and adherence in patients prescribed anticholinergics. Int. J. Clin. Pract. 68(11), 1340–1346. https://doi.org/10.1111/ijcp.12443 (2014).
American Heart Association. Guideline for cardiovascular health and risk reduction in children and adolescents. Circulation 145(8), e997–e1023. https://doi.org/10.1161/CIR.0000000000001073 (2022).
Francis, M. et al. Todd purves; NLRP3 promotes diabetic bladder dysfunction and changes in symptom-specific bladder innervation. Diabetes 1 Febr. 68(2), 430–440. https://doi.org/10.2337/db18-0845 (2019).
Hughes, F. M. Jr, Allkanjari, A., Odom, M. R., Jin, H. & Purves, J. T. Diabetic bladder dysfunction progresses from an overactive to an underactive phenotype in a type-1 diabetic mouse model (Akita female mouse) and is dependent on NLRP3. Life Sci. 299, 120528. https://doi.org/10.1016/j.lfs.2022.120528 (2022).
Xiao, Y. et al. A positive association between the prevalence of circadian syndrome and overactive bladder in United States adults. Front. Public. Health 11, 1137191 (2023).
Durrleman, S. & Simon, R. Flexible regression models with cubic splines. Stat. Med. 8(5), 551–561 (1989).
Carrico, C., Gennings, C., Wheeler, D. C. & Factor-Litvak, P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat. 20(1), 100–120 (2015).
Irwin, D. E. et al. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: Impact of overactive bladder. Eur. Urol. 56(1), 14–20 (2009).
Coyne, K. S. et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: Results from the epidemiology of LUTS (EpiLUTS) study. BJU Int. 104(3), 352–360 (2009).
Nazarko, L. Lifestyle choices for better bladder health. Br. J. Community Nurs. 23(9), 438–442 (2018).
Duralde, E. R. et al. Cardiovascular risk factors and incident urinary incontinence in middle-aged women. Obstet. Gynecol. 127(6), 949–957 (2016).
Hannestad, Y. S., Rortveit, G. & Hunskaar, S. Help-seeking and associated factors in female urinary incontinence. The Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. Scand. J. Prim. Health Care. 20(2), 102–107 (2002).
Nagai, T. et al. Nicotine-induced hypoxia in rat urothelium deteriorates bladder storage functions. Neurourol. Urodyn. 38(6), 1560–1570. https://doi.org/10.1002/nau.24050 (2019).
Maserejian, N. N., Kupelian, V., Miyasato, G., McVary, K. T. & McKinlay, J. B. Are physical activity, smoking and alcohol consumption associated with lower urinary tract symptoms in men or women? Results from a population based observational study. J. Urol. 188(2), 490–495. https://doi.org/10.1016/j.juro.2012.03.128 (2012).
Kupelian, V. et al. Association of Nocturia and disturbed sleep: Results from the Boston Area Community Health Survey. J. Urol. 184(2), 613–619 (2010).
Coyne, K. S. et al. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: Results from EpiLUTS. BJU Int. 108(7), 1059–1071 (2011).
Lightner, D. J., Gomelsky, A., Souter, L. & Vasavada, S. P. Diagnosis and treatment of overactive bladder (Non-Neurogenic) in adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 201(5), 787–798 (2019).
Oelke, M. et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including Benign Prostatic obstruction. Eur. Urol. 64, 118–140 (2013).
Malmsten, U. G., Molander, U., Peeker, R., Irwin, D. E. & Milsom, I. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: A longitudinal population-based survey in men aged 45–103 years. Eur. Urol. 58(1), 149–156 (2010).
Coyne, K. S. et al. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77(5), 1081–1087 (2011).
Looker, H. C. et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Diabetes Care 38(12), 2288–2296 (2015).
Nambiar, A. K. et al. EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur. Urol. 73(4), 596–609 (2018).
Pakgohar, M., Sabetghadam, S., Vasegh, M. & Kazemnezhad, E. Effect of pelvic floor muscle exercises on the overactive bladder syndrome and quality of life in Postmenopausal women: A systematic review and Meta-analysis. J. Family Med. Prim. Care. 8(1), 5–15 (2019).
Buntinx, F. et al. Use of the center for epidemiological studies depression scale (CES-D) to identify elderly subjects with depressive symptoms. Psychol. Health Med. 2(3), 219–231 (1997).
Vaughan, C. P., Goode, P. S., Burgio, K. L. & Markland, A. D. Obesity and urinary incontinence in women. Female Pelvic Med. Reconstr. Surg. 17(5), 264–269 (2011).
Hannestad, Y. S., Rortveit, G., Daltveit, A. K. & Hunskaar, S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT study. BJOG 110(3), 247–254 (2003).
Author information
Authors and Affiliations
Contributions
Z.H.L: Data curation, Conceptualization, Methodology, Formal analysis, Validation, Writing – original draft, Writing – review & editing X.L.L: Data curation, Conceptualization, Methodology, Writing – original draft, Writing – review & editing Y.G.L: Data curation, Conceptualization, Methodology, Software, Formal analysis X.Q.C: Conceptualization, Writing – review & editing Z.M.L: Conceptualization, Writing – review & editing X.X.G: Conceptualization, Writing – review & editing J.W.C: Writing – review & editing, Supervision, Conceptualization, Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Z., Liu, X., Li, Y. et al. Association between cardiovascular health and overactive bladder. Sci Rep 15, 5760 (2025). https://doi.org/10.1038/s41598-025-90438-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90438-w