Abstract
Sleep apnea (SA) is common in Down syndrome (DS), but its impact on daytime autonomic control is poorly described. The aim of this study was to assess the impact of SA on autonomic control of heart rate (HR) and blood pressure (BP) during a Head-Up Tilt test (HUTT) in adults with DS. Ancillary cross-sectional analysis of an observational study conducted on 28 adults with DS (31.7 ± 7.4 years; 32% female) free from congenital cardiopathy, thyroid disorders, or medication that impact autonomic control were included. Full-night polysomnography and HUTT were performed. Temporal and spectral analyses were performed on the signals of RR intervals, systolic and diastolic blood pressures (SBP and DBP). SBP and DBP min-max variations were specifically analyzed during the initial 3 min post-tilting (3minUp). 21 (75%) subjects presented moderate-severe SA (Apnea-Hypopnea Index ≥ 15/h). During the 3minUp, moderate-severe SA group showed reduced min-max variations in systolic and diastolic blood pressures (40.0 ± 10.2 vs. 51.7 ± 18.7 mmHg and 27.0 ± 7.2 vs. 39.9 ± 17.4 mmHg, all p < 0.05) with blunted vascular sympathetic activity during the entire orthostatic phase (LFSBP; 24.3 ± 11.7 vs. 28.6 ± 9.8 nu; p < 0.05). SA-related intermittent hypoxia, rather than sleep fragmentation was associated with changes in SBP during the 3minUp. The baroreflex sensitivity and heart rate variability parameters were similar in both groups. SA was associated with a blunted autonomic control of BP during HUTT, without changes in HR variability parameters. SA-related intermittent hypoxia, rather than sleep fragmentation seems to play a key role in these alterations.
Similar content being viewed by others
Introduction
Down syndrome (DS) is the most common aneuploidy, with an estimated prevalence in Europe of 4.9 per 10,000 individuals1. DS is caused by a trisomy of chromosome 21 leading to morphological anomalies, cardiovascular comorbidities, and physiological disorders that cause autonomic cardiovascular dysfunctions2.
In adults with DS, sleep apnea (SA) is a common comorbidity with an estimated prevalence ranging from 78 to 94%, depending on patient characteristics and scoring criteria used during polysomnography3,4,5. SA consequences (i.e., intermittent hypoxia, sleep fragmentation and variations in intrathoracic pressure) disrupt autonomic cardiovascular control in individuals with or without DS6,7. However, SA in DS is linked to different alterations in cardiovascular autonomic control during sleep7,8 exhibiting blunted heart rate (HR) and sympathetic responses to both arousal or respiratory events during sleep6,9,10. The SA-associated hypertension seen in non-DS population11, does not appear in individuals with DS3. Interestingly, HR dipping is lower in individuals with DS, despite similar blood pressure (BP) dipping comparing to those without DS, suggesting that SA may have a more focused impact on the autonomic control of HR or BP12. The daytime consequences of SA on the autonomic control of HR and BP remain unknown8.
Several studies have investigated cardiovascular adaptations in adults with DS during the Head-Up Tilt Test (HUTT)13, the gold standard for exploring autonomic cardiovascular control14. Adults with DS exhibit reduced vagal withdrawal and blunted sympathetic activation during the HUTT15,16,17,18. SA, despite its frequency in DS and its consequences in autonomic dysfunctions, has never been assessed during daytime sympathovagal stresses. The weight load of comorbidities in DS may explain the conflicting results obtained so far16 and has led to guidelines indicating the need to consider SA and DS comorbidities to assess autonomic cardiovascular control19.
We assessed the impact of SA on autonomic control of HR and BP by comparing cardiovascular responses to HUTT in DS adults with no/mild SA versus those with moderate/severe SA. We hypothesized that SA severity alters the cardiovascular response to the HUTT, with a specific focus on the autonomic control of HR or BP.
Results
Population
40 adults with DS (40% female, 29.7 ± 7.5 years; range to 18 to 46 years) non-smokers, were initially included in the study. 12 participants were excluded from the HUTT analysis (Fig. 1) due to hypothyroidism (n = 4), hyperthyroidism (n = 1), congenital heart disease (n = 5), aortic valve insufficiency (n = 1), and discomfort (n = 1). The anthropometric, polysomnographic and biological data did not differ between the entire SYNAPSO.T21 population and the ancillary subset (Supplemental Table 1).
Seven adults (25%) had no or mild SA (AHI < 15 events/h), while 21 (75%) had moderate (n = 4) to severe (n = 17) SA (AHI ≥ 15 events/h). There were no significant differences in anthropometric and biological measures between SA groups, and biological measures were within normal values (Table 1).
Epworth scores were comparable between SA groups and all participants were considered non-sleepy. By design, the group with moderate to severe SA exhibited significantly higher central AHI, ODI3 and arousal index compared to their counterparts with no or mild SA (p < 0.001, p < 0.001 and p = 0.009 respectively). The T90 did not significantly differ between the groups. The time in bed, total sleep period, total sleep time, and wake after sleep onset did not differ between the groups. The sleep efficiency index was comparable between the groups and below the 85% threshold, and the sleep continuity index remained consistent across the groups (Table 1).
Head-up tilt
Blood pressure variability
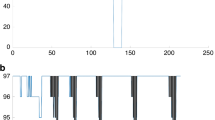
In the supine position, adults with moderate to severe SA displayed significantly lower SBP (-11mmHg), and DBP (-7mmHg), compared to those with mild or no SA (p = 0.035 and p = 0.028, respectively). Both groups exhibited similar αLF and LFSBP in the supine position (Fig. 2).
During the 3minUp, the moderate to severe SA group presented significantly lower min-max variations in SBP (-12mmHg) and DBP (-13mmHg) compared to the no or mild SA group (p = 0.023 and p = 0.018, respectively; Fig. 3). Male gender and ODI3 were associated with a lower min-max variation of SBP during the 3minUp (p = 0.021 and p = 0.019, respectively) whereas T90 was associated with a higher min-max variation of SBP during the 3minUp (p = 0.016). Age, BMI, TSH, mean duration of obstructive events (apneas and hypopneas) and arousal index were not associated with SBP variations during the 3minUp. Overall model or each specific variables were not significantly associated with min-max variations of DBP during the 3minUp (Table 2).
Blood pressure variability during the head-up tilt test. The panels represent SBP (a), DBP (b), LFBP (c), and αLF (d) measured in supine and orthostatic positions. The numerical values were presented as mean ± standard deviation with assumption checks in supplemental Table 2. DBP diastolic blood pressure, LF vascular sympathetic modulations, SASBP sleep apnea, SBP systolic blood pressure, αLF baroreflex sensitivity index.
During the complete orthostatic period, SBP and LFBP were significantly lower (-7mmHg and − 5 nu respectively) in the moderate to severe SA group compared to those with mild or no SA (p = 0.038 and p = 0.022, respectively). Both groups exhibited similar αLF in the orthostatic position (Fig. 2).
Heart rate variability
In the supine position, RR intervals and derived variables in the time domain (HR, RMSSD, pNN50) or frequency domain (LFRR, HFRR, LFRR/HFRR) did not significantly differ between groups. These parameters also did not differ in the orthostatic position (Table 3).
Discussion
In this cross-sectional study, we assessed the impact of SA on autonomic control of HR and BP during a HUTT in adults with DS. We showed that DS adults with moderate-severe SA exhibited lower SBP and DBP in the supine position, reduced variations of SBP and DBP during the 3minUp, and reduced vascular sympathetic activity and SBP in the orthostatic position. In contrast, moderate-severe SA did not impact the autonomic control of HR variability. Rather than the arousal index, markers of hypoxia, as well as male gender, both seem to play a key role in the variations of SBP during the 3minUp, supporting previous hypotheses about gender impact in autonomic cardiovascular control in DS18.
In our study, 21 out of 28 participants (75%) presented moderate-severe SA (AHI > 15 events/h), consistent with previous polysomnographic studies and highlighting the high prevalence of undiagnosed SA in adults with DS3,4,5. Consistent with an earlier study5, the mean BMI in our population was close to the normal range (25.5 ± 4.3 kg.m− 2) and did not differ between the two groups of SA severity, indicating BMI might not primarily indicate SA in this population. Interestingly, sleep efficiency did not differ between our two groups of SA severity, supporting previous findings from a polysomnographic study comparing the impact of SA (AHI threshold of 5 events/hour) on sleep efficiency in adults with DS4.
In adults with DS, blunted daytime vascular sympathetic activity and BP responses to the HUTT have already been described16. However, SA was not assessed despite its high prevalence and potential implication in the autonomic control alterations during sleep in this population6,9,10. In supine position, moderate-severe SA was associated with significantly lower SBP and DBP. Adversely, the vascular sympathetic modulations, assessed by LF power of the BP variability spectrum (LFSBP), and baroreflex sensitivity (αLF) were comparable in both groups. Our results showed opposite autonomic responses compared to non-DS adults with SA, who present heightened BP and sympathetic responses during the verticalization phase of the HUTT20,21. Studies comparing resting BP and baroreflex sensitivity between adults with or without DS were conflicting22,23 mostly due to the lack of adjustment for comorbidities, especially SA16. Thus, our data suggest that SA may partly contribute to these discrepancies. During sleep, Horne et al. (n = 44 patients with DS) observed that DS participants with SA showed higher HR, lower parasympathetic activity (HFRR), and higher sympathovagal balance (LFRR/HFRR) associated with greater oxygen desaturation6. We observed, rather, effects on the autonomic control of BP. Together, these data suggest a persisting impact of moderate-severe SA on autonomic cardiovascular daytime control in individuals with DS.
Both groups showed a response to passive verticalization in the physiological range for the non-DS population, with a modest HR rise (10–20 bpm) and a slight SBP fall (< 10 mmHg), thus making direct comparison between our results and the general population challenging14. However, the moderate-severe SA group showed reduced SBP and LFBP, not explained by altered baroreflex sensitivity, which was similar in both groups. Physiologically, during the first 3 min of verticalization of the HUTT (3minUp), gravitational blood volume shifts induce pressure variations, activating the baroreceptors and leading to heart vagal withdrawal and a net increase in sympathetic activity14. During this period, we observed reduced min-max variations in SBP and DBP in the moderate-severe SA group, suggesting a lack of baroreceptor stimulation, possibly explaining the blunted responses of SBP and LFBP to verticalization. Our multivariable model has revealed that hypoxia markers (i.e., ODI3 and T90) were significant predictors of SBP variations during the 3minUp, whereas the arousal index (a marker of sleep fragmentation), initially included in the equation, was not. However, in the non-DS population, both SA-related hypoxia and sleep fragmentation trigger periodic spikes in cardiovascular activity, altered baroreceptor sensitivity, and increased daytime sympathetic activity and BP7,24. In healthy young adults, 14 nights of intermittent hypoxia exposure mimicking severe SA-related intermittent hypoxia, impaired baroreflex sensitivity and increased daytime BP and sympathetic activity25,26. In their studies, O’Driscoll et al.9 found that young participants with DS (n = 10) and obstructive SA (OAHI = 10.7 ± 3.3 events/h) exhibited reduced HR responses during spontaneous arousal in both sleep states when compared to obstructive SA severity-matched non-DS participants (n = 10). In a more consistent sample (n = 32 DS participants with obstructive SA and n = 32 non-DS participants, matched for obstructive SA severity) DS participants showed reduced HR responses and delayed reoxygenation after obstructive events in non-rapid eye movement sleep, alongside blunted overnight sympathetic activity indicated by urinary catecholamine levels10. These findings suggest reduced cardiovascular spikes in individuals with DS in response to the pathophysiological intermediary mechanisms of obstructive SA, potentially explaining the distinct impact of SA on autonomic cardiovascular control compared to non-DS individuals. In our model, higher ODI3 values (intermittent hypoxia) being associated with reduced SBP variation during the 3minUp, while higher values of T90 (hypoxic load) were associated with increased SBP variations during the 3minUp. These results warrant further investigation, as only a few subjects presented long T90 durations and we did not control for the level of hypercapnia following the respiratory events. Therefore, altered peripheral CO2 chemosensitivity in individuals with DS was recently demonstrated27. In fine, these alterations in BP control, characterized by lower values of BP both at rest and in response to verticalization, should not be considered as beneficial, lower resting BP values being associated with comorbidities in DS, including Moyamoya syndrome and leukemia28.
We showed a specific impact of moderate-severe SA on the daytime autonomic control of BP, contrasting with no HR parameter changes. This finding differs from the results of another polysomnographic study (n = 18 DS patients, compared to n = 18 controls without DS matched for age, gender, and SA severity) who observed similar BP dipping in all sleep stages except N2, whereas HR dipping was consistently lower in DS patients12. These discrepancies suggest that SA can differentially affect autonomic control of HR and/or BP, depending on physiological stresses, and highlight the need to control this common comorbidity in DS. Supporting this, previous HUTT studies without controlling for SA have also shown conflicting results regarding HR and BP kinetics15,16. Fernhall et al. (n = 19 DS adults) reported a blunted HR with similar BP responses in DS adults, suggesting a compensatory BP mechanism15. In contrast, Agiovlasitis et al. (n = 26 DS adults) showed blunted vascular sympathetic activity and BP responses to HUTT with comparable HR responses16. Our results contribute to clarify the ambiguities surrounding autonomic control alterations related to DS and underscore the complex and distinct autonomic regulation in DS, with or without SA.
Perspectives
Medical knowledge
SA status should be considered when assessing autonomic cardiovascular control in individuals with DS, consistently with the latest recommendations19.
Outlook
Future study should decipher whether chemoreflex or endothelial function (i.e. other mechanisms involved in BP control) underlie the alterations in autonomic control of BP related to SA.
Limitations
Our study, with discussed strengths, has limitations. While being prospective, its observational nature and small sample size limits the applicability of our results. However, despite the low prevalence of DS and rigorous non-inclusion and exclusion criteria, our dataset represents the largest sample of HUTT in DS population. We did not control for breathing rate, which can impact HR variability at rest to some extent, but this was deemed unfeasible in previous studies on adults with DS16,18.
Conclusion
In DS, moderate-severe SA reduces the BP adaptations in response to HUTT, without affecting the autonomic control of HR. Intermittent hypoxia, rather than sleep fragmentation, appears to contribute to the SBP variations immediately after the orthostatic stress.
Methods
Study design and population
An ancillary cross-sectional analysis of an observational study at the Grenoble Alpes University Hospital (France; SYNAPSO.T21). Forty participants were initially included in the SYNAPSO.T21 study. Inclusion criteria were: aged ≥ 18 years, diagnosed with DS, without comorbid diabetes, leukemia, asthma, other respiratory infections, or pharmacological treatments that could impact autonomic adaptations during the HUTT or the severity of SA. Participants were also not included if they had contraindications to engaging in physical exercise. For the present analysis, and in line with recent recommendations for HR variability and BP variability analysis in DS19, patients with congenital heart diseases, as well as cardiovascular and thyroid disorders known before medical consultation were not included.
The study was approved by the National Ethics Committee (France; n°2017-AOI914-49) registered on Clinical trial (NCT03445962) and was conducted according to the principles of the Declaration of Helsinki. Oral and written informed consent was obtained from each participant.
Clinical and biological assessments
A medical consultation was conducted to record anthropometric characteristics (Table 1). Participants fulfilled an Epworth Sleepiness Scale to assess daytime sleepiness. Each participant fasted before a blood sample was taken from the arm between 8 a.m. and 9 a.m. while in a semi-recumbent position to assess biological variables (Table 1).
Head-up tilt test
The HUTT is the gold standard method to assess the capacity of the autonomic nervous system to regulate cardiovascular responses during postural changes, mediated by baroreflex activity14.
The HUTT was performed in standardized conditions in a dark, noise-free room at a constant temperature (~ 20 °C), according to international recommendations14,19,29. Participants were secured on the motorized examination table using straps before the tilt process. The HUTT consisted of three distinct periods: (i) supine position, at rest for 10 min (ii) passive straightening within 30 s to a 70-degree incline, and (iii) upright position for 10 min. Continuous measurements were conducted during these periods. For BP measurements, we completed our analyzes with a specific focus on the initial three minutes post-tilting (3minUp) as they influenced autonomic cardiovascular adaptations in response to the change in body position from supine to upright14.
Autonomic BP modulations were calculated by continuous BP monitoring with a finger photoplethysmography positioned at the level of the second phalanx of the middle finger (Nexfin HD-BMeye, Finapres, Amsterdam, Netherlands). Systolic and diastolic BP (SBP, DBP), along with LF power of the SBP variability spectrum (LFSBP; 0.07–0.15 Hz, reflecting vascular sympathetic modulations) were assessed in both supine and orthostatic positions before and after passive tilting. During the 3minUp, the min-max variations of SBP and DBP were calculated (i.e., the difference in mmHg between the highest and lowest values). These variations served as indicators of baroreceptor stimulation in response to blood volume redistribution following passive straightening14. Baroreflex sensitivity (αLF), representing the reactivity of the baroreflex to a given pressure variation was computed using the formula (αLF= √(LFRR/LFSBP)) in both positions30.
An electrocardiogram (ECG, Nexfin HD-BMeye, Finapres, Amsterdam, Netherlands) continuously recorded beat-to-beat HR using six-lead configuration to determine successive RR intervals (1000 Hz). Additional parameters were calculated to investigate autonomic cardiac modulation during the HUTT, including the root mean square of successive differences in RR intervals (RMSSD; measured in ms², reflecting vagal modulation) and the proportion of differences exceeding 50ms between consecutive RR intervals (pNN50; expressed as a percentage, depicting vagal modulation). In the frequency domain, the Fourier transform method decomposed the RR signal into distinct components. Low frequencies (LFRR; 0.04–0.15 Hz bands) represent an amalgam of sympathetic and parasympathetic activities. High frequencies (HFRR; 0.15–0.40 Hz bands) reflect vagal cardiac modulations. The LFRR and HFRR indices are standardized (u.n) and expressed as values relative to the total spectral power minus the very low frequency (VLF) component. The LFRR/HFRR ratio provides information about sympathovagal balance.
Artefacts and ectopic checking (firstly by visual inspection) and spectral analysis was performed on the Kubios Heart Rate Variability (V.2.0, http://kubios.uef.fi) HR variability software according to recommendations29.
Polysomnography
Participants underwent overnight polysomnography (Deltamed, NATUS, France) following international recommendations31. Electrophysiological signals included electroencephalogram (Fz-A+, Cz-A+, and Pz-A + of the international 10–20 Electrode Placement System), right and left electro-oculogram, submental electromyogram, right and left tibial electromyogram, ECG and overnight video monitoring. Airflow was measured with nasal pressure prongs, along with the sum of oral and nasal thermistor signals. Peripheral oxygen saturation levels (SpO2) were measured using a pulse oximeter. Respiratory efforts were assessed using thoracic and abdominal movements and pulse transit time.
Sleep respiratory events were manually scored by certified sleep specialists following the American Academy of Sleep Medicine guidelines32. Apnea was defined as the complete cessation or a reduction of at least 90% of airflow for at least 10 s. Hypopnea was defined as a reduction of at least 30% in the nasal pressure signal associated with either oxygen desaturation of at least 3% or an electroencephalogram arousal, both lasting for at least 10 s. Respiratory-effort-related arousal was scored as events lasting at least 10 s, characterized by a flattening of the inspiratory portion of the nasal pressure which did not meet the flow criteria for an apnea or hypopnea associated with an increase in respiratory effort followed by an arousal.
Participants were stratified based on the apnea-hypopnea index (AHI, defined as the total number of apnea and hypopnea per hour of total sleep time) into nor or mild SA (AHI < 15 events/h) and moderate to severe SA (AHI ≥ 15 events/h). The apnea was classified as obstructive, central or mixed, according to the presence or absence of respiratory effort. The hypopnea classification was based on the thoraco-abdominal band signal, the shape of the respiratory nasal pressure curve (flow limited aspect or not), and the presence or absence of respiratory effort on the pulse transit time signal. Other parameters, including the respiratory disturbance index (the sum of AHI and respiratory-effort-related arousal), the frequency of 3% oxygen desaturation per hour of sleep (ODI3), the time with an SpO2 ≤ 90% per hour of total sleep time (T90) and mean duration of obstructive respiratory events were used to characterize the severity of SA.
Sleep efficacy and fragmentation were assessed based on wake after sleep onset (defined as the duration of wakefulness in minutes during the total sleep period) and the respiratory arousal index (calculated as the total number of respiratory event-related arousal per hour of sleep), respectively. Sleep efficiency index (ratio of total sleep time to time in bed) and sleep continuity index (ratio of total sleep time to total sleep period time) were calculated.
Statistical analysis
Results are expressed as mean ± standard deviation for continuous variables and as numbers and percentages for categorical variables. To assess the comparability of the ancillary population (n = 28) relative to the entire SYNAPSO.T21 population (n = 40), we first compared clinical, biological, and polysomnographic data (Supplemental Table 1). For the present analysis (n = 28), we evaluated differences between the moderate to severe SA and no or mild SA groups. Independent samples T-test (for parametric data), Mann-Whitney tests (for non-parametric data), and χ2 test (for categorical variables) were used.
To assess the impact of SA on autonomic cardiovascular control, we performed ANCOVA analyzes adjusted for age, body mass index (BMI), gender and thyroid-stimulating hormone (TSH), factors affecting autonomic cardiovascular control. The distribution of data was checked before analysis, and decimal logarithmic transformation was applied when necessary to meet ANCOVA requirements.
Backward multivariable regression analyzes were conducted to identify associative factors of SBP and DBP min-max variations during the 3minUp. Clinical parameters described as influencing autonomic adaptations (i.e., gender, age, BMI and TSH), mean duration of obstructive apneas and hypopneas, T90, ODI3 along with the arousal index were included in the model. The assumption checks for the model are presented in supplemental Fig. 1.
Statistical analyses were performed using Jamovi (V.2.3.28, https://www.jamovi.org/) and RStudio (V.4.2.1, https://download1.rstudio.org/electron/windows/RStudio-2024.12.0-467.exe) softwares. The significance level was set at p < 0.05.
Data availability
Data are available on reasonable request from the corresponding author.
Abbreviations
- AHI:
-
Apnea-hypopnea index
- DS:
-
Down syndrome
- HF:
-
High frequency
- HUTT:
-
Head-up tilt test
- LF:
-
Low frequency
- ODI3:
-
Oxygen desaturation index than ≥ 3%
- SA:
-
Sleep apnea
- T90:
-
Time with a SpO2 ≤ 90% per hour of total sleep time
- 3minUp:
-
The initial 3 minutes post-tilting
References
Antonarakis, S. E. et al. Down syndrome. Nat. Rev. Dis. Primers 6 (1), 9. https://doi.org/10.1038/s41572-019-0143-7 (2020).
Dimopoulos, K. et al. For down syndrome international (DSi). Cardiovascular complications of down syndrome: scoping review and expert consensus. Circulation 147 (5), 425–441. https://doi.org/10.1161/CIRCULATIONAHA.122.059706 (2023).
Trois, M. S. et al. Obstructive sleep apnea in adults with down syndrome. J. Clin. Sleep Med. 5 (4), 317–323. https://doi.org/10.1002/ajmg.a.61853 (2009).
Cornacchia, M., Sethness, J., Alapat, P., Lin, Y. H. & Peacock, C. The prevalence of OSA among an adult population with down syndrome referred to a medical clinic. Am. J. Intellect. Dev. Disabil. 124 (1), 4–10. https://doi.org/10.1352/1944-7558-124.1.4 (2019).
Giménez, S. et al. Prevalence of sleep disorders in adults with down syndrome: a comparative study of self-reported, actigraphic, and polysomnographic findings. J. Clin. Sleep. Med. 14 (10), 1725–1733. https://doi.org/10.5664/jcsm.7382 (2018).
Horne, R. S. C. et al. Children with Down syndrome and sleep disordered breathing have altered cardiovascular control. Pediatr. Res. 90 (4), 819–825. https://doi.org/10.1038/s41390-020-01285-6 (2021).
Tamisier, R., Weiss, J. W. & Pépin, J. L. Sleep biology updates: Hemodynamic and autonomic control in sleep disorders. Metabolism 84, 3–10. https://doi.org/10.1016/j.metabol.2018.03.012 (2018).
Horne, R. S., Wijayaratne, P., Nixon, G. M. & Walter, L. M. Sleep and sleep disordered breathing in children with down syndrome: effects on behaviour, neurocognition and the cardiovascular system. Sleep. Med. Rev. 44, 1–11. https://doi.org/10.1016/j.smrv.2018.11.002 (2019).
O’Driscoll, D. M. et al. The heart rate response to spontaneous arousal from sleep is reduced in children with down syndrome referred for evaluation of sleep-disordered breathing. Am. J. Physiol. Heart Circ. Physiol. 298 (6), H1986–H1990. https://doi.org/10.1152/ajpheart.00701.2009 (2010).
O’Driscoll, D. M. et al. Cardiac and sympathetic activation are reduced in children with down syndrome and sleep disordered breathing. Sleep 35 (9), 1269–1275. https://doi.org/10.5665/sleep.2084 (2012).
Xia, W. et al. Relationship between obstructive sleep apnoea syndrome and essential hypertension: a dose-response meta-analysis. Sleep Med. 47, 11–18. https://doi.org/10.1016/j.sleep.2018.03.016 (2018).
Bassam, A. et al. Nocturnal dipping of heart rate is impaired in children with down syndrome and sleep disordered breathing. Sleep Med. 81, 466–473. https://doi.org/10.1016/j.sleep.2021.03.020 (2021).
de Carvalho, T. D. et al. Heart rate variability in individuals with down syndrome - A systematic review and meta-analysis. Auton. Neurosci. 213, 23–33. https://doi.org/10.1016/j.autneu.2018.05.006 (2018).
Aponte-Becerra, L. & Novak, P. Tilt test: a review. J. Clin. Neurophysiol. 38 (4), 279–286. https://doi.org/10.1097/WNP.0000000000000625 (2021).
Fernhall, B. et al. Blunted heart rate response to upright tilt in people with down syndrome. Arch. Phys. Med. Rehabil. 86 (4), 813–818. https://doi.org/10.1016/j.apmr.2004.10.027 (2005).
Agiovlasitis, S. et al. Autonomic response to upright tilt in people with and without down syndrome. Res. Dev. Disabil. 31 (3), 857–863. https://doi.org/10.1016/j.ridd.2010.03.002 (2010).
Agiovlasitis, S., Baynard, T., Pitetti, K. H. & Fernhall, B. Heart rate complexity in response to upright tilt in persons with down syndrome. Res. Dev. Disabil. 32 (6), 2102–2107. https://doi.org/10.1016/j.ridd.2011.08.015 (2011).
Bunsawat, K. et al. Normal HR with tilt, yet autonomic dysfunction in persons with down syndrome. Med. Sci. Sports Exerc. 47 (2), 250–256. https://doi.org/10.1249/MSS.0000000000000411 (2015).
Gąsior, J. S. et al. Heart rate variability in individuals with down syndrome: a scoping review with methodological considerations. Int. J. Environ. Res. Public Health 20 (2), 941. https://doi.org/10.3390/ijerph20020941 (2023).
Veale, D., Pépin, J. L. & Lévy, P. A. Autonomic stress tests in obstructive sleep apnea syndrome and snoring. Sleep 15 (6), 505–513. https://doi.org/10.1093/sleep/15.6.505 (1992).
Veale, D., Pépin, J. L., Wuyam, B. & Lévy, P. A. Abnormal autonomic stress responses in obstructive sleep apnoea are reversed by nasal continuous positive airway pressure. Eur. Respir J. 9 (10), 2122–2126. https://doi.org/10.1183/09031936.96.09102122 (1996).
Iellamo, F. et al. Altered autonomic cardiac regulation in individuals with down syndrome. Am. J. Physiol. Heart Circ. Physiol. 289 (6), H2387–H2391. https://doi.org/10.1152/ajpheart.00560.2005 (2005).
Heffernan, K. S. et al. Baroreflex sensitivity during static exercise in individuals with down syndrome. Med. Sci. Sports Exerc. 37 (12), 2026–2031. https://doi.org/10.1249/01.mss.0000179217.59831.41 (2005).
Hamilton, G. S., Solin, P. & Naughton, M. T. Obstructive sleep apnoea and cardiovascular disease. Intern. Med. J. 34 (7), 420–426. https://doi.org/10.1111/j.1445-5994.2004.00596.x (2004).
Tamisier, R. et al. A new model of chronic intermittent hypoxia in humans: effect on ventilation, sleep, and blood pressure. J. Appl. Physiol. 107 (1), 17–24. https://doi.org/10.1152/japplphysiol.91165.2008 (2009).
Tamisier, R. et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur. Respir J. 37 (1), 119–128. https://doi.org/10.1183/09031936.00204209 (2011).
Bokov, P., Boujemla, I., Dahan, J., Dudoignon, B. & Delclaux, C. Obstructive sleep apnea in children with down syndrome: contribution of upper airway and chemosensitivity. Pediatr. Res. 94 (6), 1990–1997. https://doi.org/10.1038/s41390-023-02718-8 (2023).
Santoro, J. D. et al. Diminished blood pressure profiles in children with down syndrome. Hypertension 75 (3), 819–825. https://doi.org/10.1161/HYPERTENSIONAHA.119.14416 (2020).
Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065 (1996).
Barbieri, R., Parati, G. & Saul, J. P. Closed- versus open-loop assessment of heart rate baroreflex. IEEE Eng. Med. Biol. Mag. 20 (2), 33–42. https://doi.org/10.1109/51.917722 (2001 Mar-Apr).
Kapur, V. K. et al. Clinical practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 13 (3), 479–504. https://doi.org/10.5664/jcsm.6506 (2017).
Berry, R. B. et al. AASM scoring manual updates for 2017 (version 2.4). J. Clin. Sleep. Med. 13 (5), 665–666. https://doi.org/10.5664/jcsm.6576 (2017).
Acknowledgements
We thank Jacob Paterno for editing the manuscript.
Funding
This research received co-funding from the AFRT (French Association for Research on Trisomy 21), Grenoble Alpes Hospital and Harmonie Mutuelle.
Author information
Authors and Affiliations
Contributions
SC: played a major role in analyzing and interpreting data; drafted the initial manuscript. SB: conducted medical consultations; interpreted data; drafted the manuscript; diagnosed and managed sleep apnea. SD and MG: conducted medical consultations; interpreted data. RT and JLP: interpreted data; diagnosed and managed sleep apnea. VB: conceived and designed the research; performed experiments; analyzed and interpreted data; drafted the manuscript. All authors edited and revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Covain, S., Baillieul, S., Doutreleau, S. et al. Sleep apnea is associated with reduced daytime blood pressure variability in adults with Down syndrome. Sci Rep 15, 11484 (2025). https://doi.org/10.1038/s41598-025-90455-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90455-9