Abstract
Hypertension and diabetes are prevalent among older people and may be associated with cancer. Although several network analyses have been conducted to visualize the associations between diseases and relevant factors, to the best of our knowledge, none have focused on visualizing the associations between cancer and other diseases. We conducted a network analysis to explore the associations between cancer, hypertension, and diabetes. This study used a large-scale clinical dataset of 1,026,305 hospitalized patients aged ≥ 65 years, collected between April 2008 and December 2020. Diseases were categorized using the International Classification of Diseases-10 (2019 version) codes. The analysis focused on diseases with a prevalence of ≥ 1%. A multimorbidity network was constructed for the entire patient cohort, and the same analysis was applied specifically to cancer patients. Hypertension (degree centrality: 58/61) and diabetes (degree centrality: 56/61) were connected to several diseases, indicating significant multimorbidity in the cohort. The associations (observed-to-expected ratio) between digestive system cancers and hypertension and diabetes were relatively stronger than those between the diseases and other cancers. Type 2 diabetes and essential hypertension may be risk factors of cancers at multiple digestive system sites. Early treatment of these conditions could prevent or delay the progression of digestive system cancers.
Similar content being viewed by others
Introduction
The rapid growth of the older population is a significant challenge to modern society. The global population of individuals ≥ 65-years-old, which was 771 million (9.7%) in 2022, is projected to more than double, reaching 1.6 billion (16.4%) by 2050—exceeding the number of children aged < 5 years. In Europe and North America, one in four people is expected to be ≥ 65 years by 2050. In Asia, the population of older adults is growing at a rate above the global average, with the proportion of individuals aged ≥ 65 in East and Southeast Asia expected to double from 12.7% in 2022 to 25.7% by 20501. This trend is especially prominent in Japan, where approximately 28.9% of the total population was ≥ 65-years-old in 2022, making it one of the most aged societies worldwide2.
These rapid demographic changes have significantly altered disease epidemiology, including cancer. Cancer remains a leading cause of death worldwide, with approximately 10 million deaths reported in 2020. The most common cancers include breast, lung, colorectal, and prostate cancers3. In Japan, cancer is the leading cause of death, with deaths from malignant neoplasms increasing annually, reaching approximately 380,000 in 20224. Mortality rates for lung, colorectal, pancreatic, breast, and prostate cancers continue to rise annually5. Additionally, the global prevalence of chronic diseases such as hypertension and diabetes is increasing. An estimated 1.28 billion adults aged 30–79 worldwide have hypertension6, whereas approximately 422 million individuals had diabetes in 20147. In Japan, approximately 43 million individuals have hypertension8, and 11.5 million have diabetes9, representing significant proportions of its 120 million population. Considering the long-term progression of these conditions, early detection and prevention of cancer, hypertension, and diabetes are critical and represent a significant health challenge in an aging society.
Hypertension and diabetes are suggested risk factors for cancer. Diabetes has been shown to increase the risk of several cancer types10,11, and hypertension has also been associated with cancer12,13,14,15. Understanding these associations in older patients with multiple conditions could facilitate early treatment and potentially reduce cancer risk. Network analysis, a method for visualizing relationships between diseases and other factors16,17,18, has been used in recent studies. However, to the best of our knowledge, no network analysis has yet to explore the associations between cancer and other diseases. Therefore, this study aimed to use network analysis to visualize the associations between cancer, hypertension, and diabetes using a large-scale clinical dataset of > 1 million patients ≥ 65-years-old.
Methods
Data source
This cross-sectional study utilized data from approximately 1 million patients randomly selected from the Diagnosis Procedure Combination (DPC) database provided by Medical Data Vision Co. The dataset includes information on hospitalized patients collected between April 2008 and December 2020, with each patient contributing 1 year of data. No long-term follow-up of patients was conducted; instead, a cross-sectional analysis was performed using the data collected within this period. As of February 2024, the company’s clinical database contained information on approximately 46.74 million patients. The DPC system, used in Japanese hospitals for coding and aggregating diagnostic and treatment processes, collates data based on this framework and includes it in the dataset19. Data anonymity is strictly maintained, and data management adheres to international ethical standards.
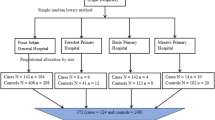
The classification of diseases for this study was based on the blocks in Chapters II to XIX of the 10th Revision of the International Classification of Diseases (ICD-10; 2019 version). A total of 185 blocks of diseases represented by three-digit disease codes (C00–C14 to S90–S99) were analyzed. The initial analysis targeted 1,055,407 patients. The study focused on patients aged 65–99 years who had diseases with a ≥ 1% prevalence. Based on this criterion, 1,026,305 patients were included in analysis dataset A. Patients with diseases having a prevalence < 1% were excluded from the analysis. Specifically, patients with both diseases having a prevalence ≥ 1% and diseases with a prevalence < 1% were excluded to eliminate ambiguity in determining relationships between the diseases.
To comprehensively examine the associations between malignant neoplasms of the digestive system and essential hypertension or type 2 diabetes, the analysis included 14 malignant neoplasms of the digestive system (C15–C25), type 2 diabetes (E11), unspecified diabetes mellitus (E14), and essential hypertension (I10). To avoid duplication, corresponding blocks (C15–C26, E10–E14, I10–I15) of disease names and patients were removed from the original dataset. Patients with diseases other than E11, E14, and I10 who had the included and excluded conditions were also excluded to ensure a more accurate evaluation of the associations. Therefore, analysis dataset B was created, comprising 81 diseases and 1,026,124 patients. Patients with unspecified diabetes mellitus (E14) were included because many patients did not have their diabetes type (type 1 or 2) specified.
Network analysis
Network analysis was conducted using datasets A and B. The analysis was further refined to focus on patients with cancer, specifically examining multimorbidity associations within this group. Diseases with a prevalence < 1% were excluded. In dataset A, the cancer group comprised 170,384 patients with 62 distinct diseases, whereas the non-cancer group included 855,921 patients with 63 diseases. Similarly, in dataset B, the cancer group comprised 170,365 patients with 73 diseases (Fig. 1).
Construction of the multimorbidity network
We constructed a network graph wherein disease names based on the ICD-10 (2019 version) served as nodes, and their co-occurrence relationships were represented as edges18. The observed-to-expected Ratio (OER) was used to quantify the relationships and interactions between diseases within the network16,17,18. The OER is calculated as the ratio of the observed frequency of the co-occurrence of disease i and disease j in the same patient to the expected frequency if the diseases occurred independently. The formula for OER is as follows.
where N represents the total number of patients in the study population, \({c}_{ij}\) indicates the number of patients with disease i and disease j, and \({c}_{i}\) and \({c}_{j}\) represent the number of patients with disease i and disease j, respectively. An OER > 1 indicates that the disease pair co-occurred more frequently in the same patient than expected by chance, whereas an OER < 1 suggests mutual exclusivity between the diseases. For this study, disease pairs were included in the network if the upper and lower bounds of the 95% confidence interval (95% CI) of the OER, calculated based on a normal distribution, were both > 1. The OER represents the strength of association between specific disease pairs, with larger OER values indicating stronger associations. Conversely, an OER close to or < 1 suggests weak or no association. Although the network graph is commonly referred to as a disease co-occurrence network, phenotypic disease network, comorbidity network, or disease comorbidities network, we designated it the “multimorbidity network” in this study17.
Visualization of the multimorbidity network
The multimorbidity network graph was created using the Fruchterman–Reingold algorithm20. This algorithm optimizes the overall network structure by balancing the attractive and repulsive forces between nodes. The attractive force \({F}_{attr}(d)\) acts between directly connected nodes, whereas the repulsive force \({F}_{rep}(d)\) adjusts the layout based on node distances. The forces are calculated as follows.
where d represents the distance between nodes and k is the spring constant, scaled appropriately based on the display size. The algorithm balances the network by applying attractive forces between nodes directly connected by edges (disease pairs) and repulsive forces between all node pairs.
In the multimorbidity network graph, the inverse of the OER value was used as the weight of each edge, allowing the OER value to directly influence node placement. Edges with smaller weights (stronger associations) exerted stronger forces, bringing nodes closer together visually. This layout enabled nodes with stronger disease associations to be positioned closer, effectively visualizing co-occurrence patterns. However, as the number of nodes increases, it becomes challenging to perfectly reflect all node distances. Particularly in cases of complex disease associations, some distances may deviate from the ideal layout. This occurs because the algorithm minimizes overall energy, prioritizing the overall balance of the network rather than the exact lengths of individual edges. Notably, the graph’s visual representation reflects only one aspect of disease associations and may not be entirely accurate. For a more accurate interpretation, the OER values should be referenced.
Structure of the multimorbidity network
To assess the structural characteristics of the network graph, we used the OER as a measure of the strength of the association between specific disease pairs, along with two additional network metrics: network density and degree centrality. Network density represents the proportion of edges in the network and indicates how closely diseases are associated with each other. A high network density suggests strong associations between diseases. Degree centrality measures a node’s connectivity based on the number of edges it has, expressed as a percentage (%).
All analyses and visualizations were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerations
The study protocol adhered to relevant guidelines and regulations, including the Declaration of Helsinki. It was approved by the Ethics Committee of the Graduate School of Medicine, Juntendo University (approval number: E21-0264), and informed consent was waived by the Ethics Committee of the Graduate School of Medicine, Juntendo University due to the retrospective nature of the research. Patient privacy and rights were prioritized, and all data used in the study were fully anonymized.
Results
Baseline characteristics of patients
In analysis dataset A, which included 1,026,305 patients (Fig. 1), the average age was 76.16 years, with a mean of 3.18 comorbidities per patient. Among these patients, 533,280 (52.0%) were female (average age = 76.9 years; average number of comorbidities = 3.09), and 493,025 (48.0%) were male (average age = 75.3 years; average number of comorbidities = 3.28). The age distribution showed that 477,455 patients (46.5%) were aged between 65 and 74 years (average age = 69.5 years; average number of comorbidities = 2.90), whereas 548,850 patients (53.5%) were aged ≥ 75 years (average age = 82.0 years; average number of comorbidities = 3.43; Table 1).
Regarding disease prevalence, 225,614 patients (6.91%) had malignant neoplasms; 644,153 patients (19.71%) had diseases of the circulatory system, including essential hypertension and type 2 diabetes; and 486,917 patients (14.90%) had diseases of the digestive system (Fig. 2).
Multimorbidity network
The network analysis results for dataset A revealed a network density of 0.52, with 70 nodes and 1,258 edges (Fig. 3).
Hypertension (I10–15) was connected to 50 other diseases, with a degree centrality of 72.5% (50/69), ranking 14th among 70 diseases. Among the malignant neoplasms associated with hypertension, one was identified: malignant neoplasm of the digestive organs (Fig. 4). The association (OER) between hypertension and malignant neoplasms of the digestive organs was 1.17 (95% CI 1.15–1.19).
Diabetes mellitus (E10–14) was connected to 55 other diseases, with a degree centrality of 79.7% (55/69), ranking 6th among the 70 diseases. Three malignant neoplasms were associated with diabetes mellitus: malignant neoplasms of the digestive organs, urinary system, and ill-defined or unknown sites, including secondary sites (Fig. 4). The associations (OER) were as follows: malignant neoplasms of the digestive organs, 1.43 (95% CI 1.41–1.46); urinary system, 1.07 (95% CI 1.01–1.12); and ill-defined or unknown sites, including secondary sites, 1.18 (95% CI 1.14–1.22). The highest association was observed for malignant neoplasms of the digestive organs. Notably, the association (OER) with malignant neoplasms of the digestive organs was stronger for diabetes mellitus than for hypertension.
Malignant neoplasms of the digestive organs (C15–26) were connected to 26 diseases, with the highest association (OER) observed with malignant neoplasms of ill-defined or unknown sites, including secondary sites (C76–80), at 6.34 (95% CI 6.18–6.51). This was followed by associations with hemolytic anemia (D50–53), at 3.49 (95% CI 3.40–3.57), and other diseases of the digestive system (K90–93), at 3.26 (95% CI 3.13–3.39).
The cancer group in analysis dataset A included 170,384 patients with an average age of 75.6 years and average of 3.96 comorbidities. Of these, 73,158 (42.9%) were female (average age = 75.8 years; average number of comorbidities = 3.75) and 97,226 (57.1%) were male (average age = 75.4 years; average number of comorbidities = 4.11). Among these patients, 82,462 (48.4%) were aged between 65–74 years (average age = 69.6 years; average number of comorbidities = 3.59), whereas 87,922 (51.6%) were aged ≥ 75 years (average age = 81.3 years; average number of comorbidities = 4.30).
The cohort’s major disease distributions were as follows: malignant neoplasms of digestive organs (C15–C26), 63,822 (37.5%) patients; malignant neoplasms of respiratory and intrathoracic organs (C30–C39), 21,016 (12.3%) patients; malignant neoplasm of breast (C50), 13,679 (8.0%) patients; malignant neoplasms of male genital organs (C60–C63), 19,802 (11.6%) patients; malignant neoplasms of urinary tract (C64–C68), 11,671 (6.8%) patients; malignant neoplasms of ill-defined, secondary, and unspecified sites (C76–C80), 23,121 (13.6%) patients; neoplasms of uncertain or unknown behavior (D37–D48), 48,918 (28.7%) patients; diabetes mellitus (E10–E14), 26,416 (15.5%) patients; and hypertensive diseases (I10–I15), 33,267 (19.5%) patients. The network analysis (Fig. 5) revealed a network density of 0.81, with 62 nodes and 1,526 edges (Table 2).
The multimorbidity network showed that hypertension (I10–15) was connected to 58 diseases, with a degree centrality of 58/61 (95.1%), the fifth highest among the 62 diseases. Four types of malignant neoplasms were associated with hypertension: malignant neoplasms of digestive organs (OER = 1.30; 95% CI 1.28–1.33); malignant neoplasms of ill-defined, secondary, and unspecified sites (OER = 1.11; 95% CI 1.08–1.15); malignant neoplasms of respiratory and intrathoracic organs (OER = 1.08; 95% CI 1.05–1.12); and malignant neoplasms of urinary tract (OER = 1.06; 95% CI 1.01–1.11; Fig. 4). The strongest association was observed with malignant neoplasms of the digestive organs.
Diabetes mellitus (E10–14) was connected to 56 other diseases, with a degree centrality of 56/61 (91.8%), the 13th highest among the 62 diseases. Two types of malignant neoplasms were associated with diabetes: malignant neoplasms of digestive organs (OER = 1.35; 95% CI 1.32–1.38) and ill-defined, secondary, and unspecified sites (OER = 1.11; 95% CI 1.07–1.15; Fig. 4). The association between diabetes and malignant neoplasms of digestive organs was stronger than that between diabetes and malignant neoplasms of ill-defined, secondary, and unspecified sites. Additionally, the association (OER) between malignant neoplasms of digestive organs and diabetes was stronger than that between these cancers and hypertension.
Malignant neoplasms of digestive organs (C15–26) were connected to 32 other diseases. The association between malignant neoplasms of the digestive organs and other diseases of the digestive system (K90–93) was the strongest (OER = 2.13; 95% CI 2.03–2.23), followed by those between malignant neoplasms of digestive organs and diseases of the gallbladder, biliary tract, and pancreas (K80–87; OER = 1.90; 95% CI 1.86–1.96) and nutritional anemias (D50–53; OER = 1.76; 95% CI 1.72–1.81).
Detailed multimorbidity network
Analysis dataset B includes a total of 14 diseases: 11 malignant neoplasms of the digestive organs (C15–C25), type 2 diabetes (E11), unspecified diabetes mellitus (E14), and essential hypertension (I10). The network analysis results indicated a network density of 0.47, with 81 nodes and 1,536 edges (Figure S3).
Essential hypertension (I10) was connected to 58 other diseases, with a degree centrality of 72.5% (58/80), ranking 12th among the 81 diseases. It was associated with eight types of malignant neoplasms of the digestive organs, including malignant neoplasms of the stomach, colon, rectosigmoid junction, rectum, liver and intrahepatic bile ducts, gallbladder, other and unspecified parts of the biliary tract, and pancreas. The strongest association (OER) was between essential hypertension and malignant neoplasms of the liver and intrahepatic bile ducts, at 1.54 (95% CI 1.47–1.62).
Type 2 diabetes (E11) was connected to 59 other diseases, with a degree centrality of 73.8% (59/80), ranking 10th among the 81 diseases. Malignant neoplasms of the digestive organs connected to type 2 diabetes included nine diseases: malignant neoplasms of the stomach, small intestine, colon, rectosigmoid junction, rectum, liver and intrahepatic bile ducts, gallbladder, other and unspecified parts of the biliary tract, and pancreas. In other words, type 2 diabetes was connected to all malignant neoplasms of the digestive organs, excluding those of the esophagus and anus/anal canal. The strongest association (OER) was between type 2 diabetes and pancreatic malignant neoplasm, at 2.59 (95% CI 2.43–2.77).
The cancer group in analysis dataset B comprised 170,365 patients. The average age of the patients was 75.6 years, with an average of 4.00 comorbidities. Regarding sex distribution, 73,147 (42.9%) of the patients were female (average age = 75.8 years; average number of comorbidities = 3.78), and 97,218 (57.1%) were male (average age = 75.4 years; average number of comorbidities = 4.34). By age group, 82,449 (48.4%) patients were aged 65–74 years (average age = 69.6 years; average number of comorbidities = 3.62), whereas 87,916 (51.6%) were aged ≥ 75 years (average age = 81.3 years; average number of comorbidities = 4.34).
The distribution of major diseases in the cohort was as follows: malignant neoplasm of esophagus (C15), 4,910 (2.9%) patients; malignant neoplasm of stomach (C16), 21,703 (12.7%) patients; malignant neoplasm of small intestine (C17), 466 (0.3%) patients; malignant neoplasm of colon (C18), 18,330 (10.8%) patients; malignant neoplasm of rectosigmoid junction (C19), 671 (0.4%) patients; malignant neoplasm of rectum (C20), 7,741 (4.5%) patients; malignant neoplasm of anus and anal canal (C21), 168 (0.1%) patients; malignant neoplasm of liver and intrahepatic bile ducts (C22), 6,030 (3.5%) patients; malignant neoplasm of gallbladder (C23), 1,070 (0.6%) patients; malignant neoplasm of other and unspecified parts of biliary tract (C24), 2,333 (1.4%) patients; malignant neoplasm of pancreas (C25), 5,123 (3.0%) patients; type 2 diabetes (E11), 67,108 (7.8%) patients; unspecified diabetes mellitus (E14), 64,655 (7.6%) patients; and essential hypertension (I10), 188,621 (22.0%) patients.
The network analysis (Fig. 6) revealed a network density of 0.68, with 73 nodes and 1,775 edges.
The multimorbidity network revealed that essential hypertension (I10) was connected to 68 diseases, with a degree centrality of 68/72 (94.4%), the highest among the 73 diseases. Essential hypertension was associated with 10 types of malignant neoplasms of the digestive organs: esophagus, stomach, small intestine, colon, rectosigmoid junction, rectum, liver and intrahepatic bile ducts, gallbladder, other and unspecified parts of the biliary tract, and pancreas, excluding only the anus and anal canal. The associations (OER) between essential hypertension and the neoplasms are listed in Table 3. Malignant neoplasms of the liver and intrahepatic bile ducts had the strongest association with essential hypertension (OER = 1.72; 95% CI 1.64–1.81).
Type 2 diabetes (E11) was connected to 66 other diseases, with a degree centrality of 66/72 (91.7%), the fourth highest among the 73 diseases. Nine types of malignant neoplasms of the digestive organs were linked to type 2 diabetes: stomach, small intestine, colon, rectosigmoid junction, rectum, liver and intrahepatic bile ducts, gallbladder, other and unspecified parts of the biliary tract, and pancreas, excluding only the esophagus and the anus and anal canal. The associations (OER) between type 2 diabetes and neoplasms are summarized in Table 3. Malignant neoplasms of the pancreas had the strongest association with type 2 diabetes (OER = 2.45; 95% CI 2.29–2.62).
Malignant neoplasms of the digestive organs were connected to each other as shown in Fig. 7. Malignant neoplasms of the colon had the most connections to other diseases (38), followed by those of the liver and intrahepatic bile ducts (31), and the rectum (26). Malignant neoplasms of the anus/anal canal had the fewest connections (6), followed by those of the esophagus (14) and gallbladder (17).
Discussion
In this study, we conducted a network analysis to examine the associations between cancer, hypertension, and diabetes in a large-scale clinical dataset of > 1 million patients aged ≥ 65 years. The data analysis from 170,384 patients with cancer showed high network density, suggesting complex disease interactions. Hypertension and diabetes exhibited high degrees of centrality, indicating significant multimorbidity among the patients in the cohort. This finding suggests that prevention and early treatment of hypertension and diabetes may contribute to the prevention and management of other diseases. The associations (OER) between hypertension, diabetes, and digestive system cancers were stronger than those with other cancers. Additionally, the association (OER) between digestive system cancers and diabetes was stronger than that between digestive system cancers and hypertension, suggesting that diabetes is associated with a higher risk of digestive system cancers than hypertension.
This study found a strong association between diabetes and malignant neoplasms of the digestive organs. In the multimorbidity network, type 2 diabetes was connected to all malignant neoplasms of the digestive organs, excluding those of the esophagus and anus/anal canal. The strongest associations (OER) were observed between diabetes and pancreatic and liver neoplasms, including intrahepatic bile duct neoplasms.
The risk factors for pancreatic cancer include smoking, obesity, heavy alcohol consumption, and diabetes. Patients with diabetes for > 3 years have been reported to have a 1.5- to twofold increased risk for pancreatic cancer21,22. In diabetes, insulin resistance leads to hyperinsulinemia, which may promote the growth of pancreatic cancer cells, potentially contributing to its etiology23,24. Additionally, insulin-like growth factor and its receptor can enhance proliferation, angiogenesis, and apoptosis regulation, further contributing to pancreatic cancer development25. In the present study, diabetes showed a strong association (OER) with pancreatic cancer, supporting the findings of previous studies. However, diabetes may develop as a consequence of pancreatic cancer.
The risk factors for liver cancer include chronic hepatitis B or C infection, heavy alcohol consumption, metabolic diseases such as obesity and diabetes, and aflatoxin exposure26. Non-alcoholic steatohepatitis has also been reported as a cause of liver cancer and is closely associated with metabolic syndrome, including diabetes27. Excessive intake of simple sugars, especially fructose, can cause metabolic disorders such as insulin resistance, obesity, and non-alcoholic fatty liver disease, potentially leading to hepatocellular carcinoma28. In the present study, diabetes was strongly associated (OER) with liver cancer, second only to pancreatic cancer. Therefore, managing diabetes in patients with chronic liver disease is crucial for reducing the risk of liver cancer.
The risk factors for colorectal cancer include obesity, low physical activity, consumption of processed meats and a Western diet, alcohol consumption, and smoking29. A meta-analysis found that patients with impaired fasting glucose levels, glucose intolerance, or diabetes have a 29% increased risk of developing colorectal cancer30. Additionally, insulin resistance plays a role in the association between colorectal cancer and metabolic syndrome31. Insulin resistance may directly contribute to colorectal cancer by acting as an energy reservoir for cancer cell proliferation32. The present study revealed a correlation between type 2 diabetes and malignant neoplasms of the colon, indicating that managing metabolic syndrome, including diabetes, is essential for mitigating colorectal cancer risk.
Previous studies on the association between hypertension and cancer report that in men, hypertension is positively associated with the overall incidence of cancer, including non-melanoma skin cancer and cancers of the oropharynx, colon, rectum, lung, bladder, and kidney33. Although no study has revealed an association between hypertension and overall cancer incidence in women, positive associations have been reported between hypertension and liver, pancreatic, cervical, and endometrial cancers, as well as melanoma33. These findings suggest a potential association between hypertension and various cancers. Among all the malignant neoplasms analyzed in the present study, hypertension showed the strongest association (OER) with malignant neoplasms of the digestive organs. Essential hypertension was linked to all malignant neoplasms of the digestive organs, except for those of the anus/anal canal, with the strongest association (OER) observed for malignant neoplasms of the liver and intrahepatic bile ducts.
A meta-analysis of five prospective studies on the association between hypertension and liver cancer indicated a significant association (relative risk [RR], 1.23; 95% CI, 1.16–1.30; I2, 0%)14. Similarly, a meta-analysis of 13 prospective studies on the association between hypertension and colorectal cancer showed a positive association, with patients having hypertension exhibiting an 11% increased risk of colorectal cancer (RR 1.11; 95% CI, 1.01–1.21; I2, 69.3%)14. Although the mechanisms linking hypertension and cancer remain unclear, tissue hypoxia and the inhibition of apoptosis caused by hypertension are suggested to increase cancer risk34.
Helicobacter pylori infection accounts for approximately 90% of all distal gastric cancer cases. Other established risk factors for gastric cancer include excessive body fat, smoking, and diets high in salt and processed meat35. Although the association between gastric cancer and hypertension is not well-established, high salt intake has been associated with an increased risk of gastric cancer risk (RR = 1.68; 95% CI 1.17–2.41), particularly in Japan36. Animal studies suggest that in a high-salt environment, H. pylori infection increases tyrosine phosphorylation of CagA, enhances interleukin-8 secretion by epithelial cells, activates signaling pathways, and upregulates inducible nitric oxide synthase and cyclooxygenase-2, potentially promoting gastric carcinogenesis37,38. This study also found a connection between essential hypertension and malignant neoplasms of the stomach, suggesting a potential association between gastric cancer and hypertension.
Notably, although essential hypertension was linked to malignant neoplasms of the esophagus in this study, the association (OER) was weak. This may reflect differences between the Japanese and Western populations. In Western countries, risk factors for esophageal adenocarcinoma, which is more common in Western populations, include obesity and gastroesophageal reflux39,40. A meta-analysis of four prospective studies on the relationship between hypertension and esophageal adenocarcinoma revealed a significant positive association14. In contrast, esophageal squamous cell carcinoma, which is more prevalent in Japan, is mainly associated with smoking and alcohol consumption41. Considering that this study used a Japanese database, the high prevalence of esophageal squamous cell carcinoma in the cohort may have contributed to the weaker observed association with hypertension.
This study has several notable strengths. First, it was conducted using a large-scale clinical dataset of older patients aged ≥ 65 years, with 53.5% of them aged ≥ 75 years. Second, by conducting a network analysis, we demonstrated the multimorbidity relationships between hypertension, diabetes, and cancer. This study explored the associations between malignant neoplasms in various parts of the digestive system and essential hypertension and type 2 diabetes. The approach adopted in this study provides a better understanding of the roles of essential hypertension and type 2 diabetes as risk factors for digestive system cancers. The results suggest that effective prevention and management of hypertension and diabetes could contribute to reducing the risk of digestive system cancers.
This study has some limitations. First, although random sampling was used, the possibility of selection bias cannot be entirely eliminated. Second, the analysis relied solely on disease diagnoses, making it difficult to accurately capture the patients’ complete disease histories or current conditions, as a diagnosis may remain in a patient’s records even after recovery. Additionally, the potential for misclassification of disease diagnoses cannot be excluded. Third, this study is limited by the nature of the DPC data, which do not include information on physical measurements such as weight and height, the duration of chronic conditions including diabetes, or other major risk factors such as smoking and alcohol consumption. Moreover, medication use and the control status of blood pressure and blood glucose levels were not considered in this analysis. Similarly, the occurrence of hypertension and diabetes as side effects of anticancer treatments was not identified or excluded. Fourth, longitudinal data were not utilized, preventing the tracking of temporal progression in disease occurrence and development. Consequently, this study did not aim to establish causality or examine associations with common cancer risk factors, such as smoking, alcohol consumption, and obesity. Furthermore, hypertension and type 2 diabetes are highly correlated, and the combined impact of these conditions on other diseases and cancer was not analyzed. Indirect associations arising from combinations of multiple diseases were also not considered.
As numerous disease pairs were evaluated, there is a risk of false positives due to multiple comparisons; however, no correction for multiple comparisons was performed. Finally, network validation and robustness assessments were not conducted, necessitating cautious interpretation of the network structure and disease associations.
These limitations should be carefully considered when interpreting this paper’s results. Addressing these factors in future research is expected to provide a more comprehensive and accurate understanding of disease associations.
In conclusion, this large-scale network analysis of > 1 million older adults aged ≥ 65 years found a strong association between hypertension, diabetes, and digestive system cancers compared with other cancer types. The findings suggest that type 2 diabetes and essential hypertension are significant risk factors for various digestive system cancers, including liver, pancreatic, gastric, and colorectal cancers. Early treatment and effective management of type 2 diabetes and essential hypertension could help prevent and manage these cancers. Future research should focus on clarifying these associations and developing strategies to reduce the incidence of digestive system cancers.
Data availability
The data supporting the findings of this study are available from Medical Data Vision Co. However, restrictions apply to their availability, as the data were used under license for this study and are not publicly accessible. Data may be available from the corresponding author upon reasonable request and with permission from Medical Data Vision Co. Please note that if raw data are shared, they must be de-identified to protect patient privacy unless explicit consent for publication has been obtained from all patients.
References
United Nations. World Population Prospects 2022. United Nations Organizations https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (2022).
Cabinet Office Japan. Annual Report on the Ageing Society [Summary] FY2022 https://www8.cao.go.jp/kourei/english/annualreport/2022/pdf/2022.pdf (2022).
World Health Organization. Cancer. World Health Organization https://www.who.int/news-room/fact-sheets/detail/cancer (2022).
Ministry of Health, Labour and Welfare. Trends in leading causes of death. Ministry of Health, Labour and Welfare https://www.mhlw.go.jp/english/database/db-hw/populate/dl/E03.pdf.
Director-General for Statistics, Information, Policy, and Industrial Relations, Ministry of Health, Labour and Welfare. Vital Statistics of Japan 2019. Ministry of Health, Labour and Welfare https://www.mhlw.go.jp/english/database/db-hw/index.html (2019).
World Health Organization. Hypertension. World Health Organization https://www.who.int/news-room/fact-sheets/detail/hypertension (2023).
World Health Organization. Diabetes. World Health Organization https://www.who.int/news-room/fact-sheets/detail/diabetes (2023).
Umemura, S. et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens. Res. 42, 1235–1481 (2019).
Nutrition NIoHa. Health Japan 21 (the second term) Analysis and Assessment Project. Annual changes in current data. Nutrition NIoHa https://www.nibiohn.go.jp/eiken/kenkounippon21/en/kenkounippon21/genjouchi.html (2024).
Wang, C. G. et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: A systematic review and meta-analysis of cohort studies. Int. J. Cancer 130, 1639–1648 (2012).
Giovannucci, E. et al. Diabetes and cancer: A consensus report. Diabetes Care 33, 1674–1685 (2010).
Hisamatsu, T. et al. High blood pressure and colorectal cancer mortality in a 29-year follow-up of the Japanese general population: Nippon DATA80. Hypertens. Res. 47, 206–214 (2024).
Kaneko, H. et al. Untreated hypertension and subsequent incidence of colorectal cancer: Analysis of a nationwide epidemiological database. J. Am. Heart Assoc. 10, e022479 (2024).
Seretis, A. et al. Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 9, 8565 (2019).
Petrelli, F. et al. Effects of hypertension on cancer survival: A meta-analysis. Eur. J. Clin. Invest. 51, e13493 (2021).
Zhou, D. J., Wang, L. Y., Ding, S. H., Shen, M. H. & Qiu, H. Phenotypic disease network analysis to identify comorbidity patterns in hospitalized patients with ischemic heart disease using large-scale administrative data. Healthcare (Basel) 10, 80 (2022).
Fränti, P., Sieranoja, S., Wikström, K. & Laatikainen, T. Clustering diagnoses from 58 million patient visits in Finland between 2015 and 2018. JMIR Med. Inform. 10, e35422 (2022).
Hidalgo, C. A., Blumm, N., Barabási, A. L. & Christakis, N. A. A dynamic network approach for the study of human phenotypes. PLOS Comput. Biol. 5, e1000353 (2009).
Hayashida, K., Murakami, G., Matsuda, S. & Fushimi, K. History and profile of Diagnosis Procedure Combination (DPC): Development of a real data collection system for acute inpatient care in Japan. J. Epidemiol. 31, 1–11 (2021).
Fruchterman, T. M. J. & Reingold, E. M. Graph drawing by force-directed placement. Softw. Pract. Exp. 21, 1129–1164 (1991).
Klein, A. P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 18, 493–502 (2021).
Bosetti, C. et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 25, 2065–2072 (2014).
Fisher, W. E., Boros, L. G. & Schirmer, W. J. Insulin promotes pancreatic cancer: Evidence for endocrine influence on exocrine pancreatic tumors. J. Surg. Res. 63, 310–313 (1996).
Ding, X. Z., Fehsenfeld, D. M., Murphy, L. O., Permert, J. & Adrian, T. E. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas 21, 310–320 (2000).
Pollak, M. Insulin and insulin-like growth factor signaling in neoplasia. Nat. Rev. Cancer 8, 915–928 (2008).
El-Serag, H. B. & Rudolph, K. L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007).
Llovet, J. M. et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 20, 487–503 (2023).
Laguna, J. C., Alegret, M. & Roglans, N. Simple sugar intake and hepatocellular carcinoma: Epidemiological and mechanistic insight. Nutrients 6, 5933–5954 (2014).
Keum, N. & Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 16, 713–732 (2019).
Esposito, K. et al. Colorectal cancer association with metabolic syndrome and its components: A systematic review with meta-analysis. Endocrine 44, 634–647 (2013).
Giovannucci, E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am. J. Clin. Nutr. 86, s836–s842 (2007).
Trevisan, M. et al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol. Biomark. Prev. 10, 937–941 (2001).
Stocks, T. et al. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension 59, 802–810 (2012).
Hamet, P. Cancer and hypertension: A potential for crosstalk?. J. Hypertens. 15, 15731577 (1997).
Thrift, A. P., Wenker, T. N. & El-Serag, H. B. Global burden of gastric cancer: Epidemiological trends, risk factors, screening, and prevention. Nat. Rev. Clin. Oncol. 20, 338–349 (2023).
D’Elia, L., Rossi, G., Ippolito, R., Cappuccio, F. P. & Strazzullo, P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin. Nutr. 31, 489–498 (2012).
Toyoda, T. et al. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol. Histopathol. 23, 593–599 (2008).
Loh, J. T., Torres, V. J. & Cover, T. L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 67, 4709–4715 (2007).
Hoyo, C. et al. Body mass index in relation to esophageal and esophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int. J. Epidemiol. 41, 1706–1718 (2012).
Cook, M. B. et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: A pooled analysis from the Barrett’s and esophageal adenocarcinoma consortium (BEACON). Plos One 9, e103508 (2014).
GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of esophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 582–597 (2020).
Acknowledgements
We also thank Editage (www.editage.jp) for the English language editing.
Author information
Authors and Affiliations
Contributions
HH, SN, and YN conceived and designed the study. HH, SN, and TT conducted the literature review. HH and WU extracted the data and performed the statistical analysis. HH, SN, TT, and YN interpreted the findings. HH and TT drafted the article, and YN, TT, AN, and SA critically revised it for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hashimoto, H., Nojiri, S., Takeda, T. et al. Examining associations of digestive system cancer with hypertension and diabetes using network analysis in older patients. Sci Rep 15, 6458 (2025). https://doi.org/10.1038/s41598-025-90734-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90734-5