Abstract
This study focuses on preparing composite hierarchical porous carbon nanofiber film that includes ZnO and MnO2. Using electrospinning technology, hierarchical porous structure was introduced into the nanofibers, enhancing energy density through the synergistic effect of zinc oxide and manganese dioxide. The zinc-manganese dioxide co-modified hierarchical porous carbon nanofiber film (ZnMnO-HPC) exhibits outstanding electrochemical performance when used as supercapacitor electrode, with a specific capacity reaching 401.77 C/g at 0.5 A/g, and 201.29 C/g at high current density of 5 A/g. ZnMnO-HPC also exhibits remarkable energy density when assembled with activated carbon electrode into asymmetric capacitor, reaching 38.37 Wh/kg at a power density of 407 W/kg and 19.5 Wh/kg at a power density of 12,800 W/kg, indicating promising applications in the high-energy-density supercapacitor field.
Similar content being viewed by others
Introduction
The Introduction section, of referenced text1 expands on the background of the work (some overlap with the Abstract is acceptable). The introduction should not include subheadings.
Supercapacitors exhibit exceptional power density, fast charging and discharging rate capabilities, as well as prolonged cycle life. Given the global emphasis on reducing carbon emissions, supercapacitors have gained significant attention and are widely employed as eco-friendly energy storage devices1,2,3,4. However, the constrained energy density of supercapacitors limits their potential applications, and enhancing the energy density has emerged as a prominent research focus5,6,7,8.
Pore size distribution of carbon significantly impacts the charge transport, storage, and release in supercapacitors9,10. The microporous structure enhances adsorption capacity, providing a large specific surface area and facilitating capacity improvement11. Additionally, macropores and mesoporous pores with larger sizes not only store charge but also facilitate electrolyte ion transport/migration under high load conditions12. Therefore, the optimization of pore distribution is crucial for enhancing the capacity and amplification performance of carbon-based supercapacitors. The nanocarbon fibers possess high surface area, facilitating the adjustment of pore structure and exhibiting excellent structural stability. Consequently, nanocarbon fibers are widely employed in supercapacitors due to its high capacitance and exceptional cyclic stability, making it a promising candidate for raw material in supercapacitor electrodes. Electrospinning offers flexible control over the morphology and pore structure of nanofibers at both micro and nano scales, rendering it a common technique for fabricating electrode materials used in nanofiber supercapacitors.
The self-supporting flexible electrode for the supercapacitor can be directly prepared through the technique of electrospinning. Additionally, electrospinning technology allows for adjustment of the diameter, composition, and shape of one-dimensional nanomaterials13,14. Consequently, high-performance supercapacitor electrode materials can be fabricated from nanofibers produced via the electrospinning method. Electrospinning has become a straight forward and widely employed approach for synthesizing electrode substrates in supercapacitors15,16. Advancements in structural optimization, including effective morphology control, high porosity, and a favorable ion diffusion rate, make electrostatic spinning technology promising for generating flexible electrodes with enhanced power density and energy density17,18.
The electrospinning method allows for the preparation of carbon nanofibers from a single polymer, characterized by a simple structure, small specific surface area, and relatively low capacitance19,20. To overcome these limitations, porous carbon nanofibers were fabricated by incorporating easily pyrolyzed polymers (such as polyvinyl alcohol (PVA), polymethyl methacrylate (PMMA), polyvinyl pyronidone (PVP), etc.) into polyacrylonitrile (PAN) matrix21,22,23,24,25. These added polymers facilitate the formation of short ion transport channels, well-developed pore structures, and enhanced electrolyte wettability.
However, the intrinsic characteristics of carbon-based supercapacitors impose limitations on their energy density, and achieving a substantial increase in energy density while maintaining power density remains an immense challenge. To further enhance the energy density of supercapacitors, metal oxides like RuO226,27, ZnO28,29, CuO30,31, MnO232,33, Co3O434, V2O535, etc., are incorporated onto carbon materials to facilitate rapid REDOX reactions and achieve high specific capacitance. Despite some progress, these efforts have not effectively achieved a balance between overall performance, synthesis methods, and costs. Therefore, the exploration of electrode materials with excellent performance, easy synthesis and low cost remains a research hotspot in the field of supercapacitor electrode materials.
Among the electrode materials utilized in transition metal oxide supercapacitors, manganese dioxide stands out due to its remarkable high specific capacitance (1370 F/g), abundant reserves, low cost, and environmental friendliness. These properties render it a promising candidate for high-performance supercapacitor electrode materials36. However, the reversible specific capacitance and cyclic stability of the manganese dioxide electrode are limited by its low conductivity and high charge mass transfer resistance. To enhance the conductivity and charge transfer efficiency of the MnO2 electrode, traditional methods can be categorized into two approaches: one involves incorporating other metal elements to improve conductivity and charge storage capacity by generating more vacancies and charge carriers; another approach entails depositing ultra-thin, highly conductive transition metal oxides onto the surface of MnO2 to accelerate electron transport rates without increasing the amount of MnO237,38,39. Nevertheless, while modifying MnO2 electrodes with transition metal oxides can enhance their performance or improve their electrochemical properties to some extent, certain modifications may result in a substantial increase in cost or only offer limited improvement in electrode material properties40,41,42,43. Therefore, there are still significant limitations facing the application of manganese dioxide electrodes.
The present study explores how varying PAN and PMMA ratios impact the morphology and pore size of ZnO composite hierarchical porous carbon nanofibers carbon nanofibers (ZnO-HPCs) prepared via electrospinning. Additionally, MnO2 is incorporated through a hydrothermal method to synthesize hierarchical porous carbon nanofibers co-modified with ZnO and MnO2 self-supporting flexible electrode (ZnMnO-HPC). The ZnMnO-HCP as the electrode of supercapacitor exhibits superior overall performance in terms of specific capacitance, energy density and cycle stability. The hierarchical porous structure of ZnMnO-HPC, along with the synergistic effect of ZnO and MnO2, results in a high specific capacity (401.77 C/g at 0.5 A/g) and high energy density (38.37 Wh/kg at 407 W/kg) when assembled with activated carbon electrode into asymmetric capacitor.
Results and discussion
Figure 1 shows the typical scanning electron microscope (SEM) images of ZnO-HPC1, ZnO-HPC2, ZnO-HPC3, ZnO-HPC4 and ZnO-HPC5 respectively. The SEM images demonstrate that ZnO-HPC1 to ZnO-HPC5 (Fig. 1a–e) exhibit well-preserved nanofiber structures, suggesting the feasibility of producing carbon nanofibers by electrospinning. The SEM images reveal that the hollow structure of nanofibers becomes more prominent with increasing PMMA to PAN mass ratio, ranging from 0:10 (ZnO-HPC1) to 2:8 (ZnO-HPC3). The SEM image of ZnO-HPC1 (Fig. 1f) reveals that the fiber exhibits a solid cross-sectional structure when PAN is the only component. However, at a mass ratio of 1:9 for PMMA to PAN, a hollow structure starts to emerge in the nanofiber’s cross section (ZnO-HPC2, Fig. 1g). At a mass ratio of 2:8, the hollow structure becomes more pronounced (ZnO-HPC3, Fig. 1h). Nevertheless, as the mass ratio further increases to 3:7 (ZnO-HPC4, Fig. 1i) and 4:6 (ZnO-HPC5, Fig. 1j) respectively, the cross-sectional hollow structure of nanofibers becomes increasingly evident. This evidence indicates that utilizing the optimal PMMA to PAN ratio enables the fabrication of hollow nanofibers through electrospinning. The structure of ZnO-HPC1 comprises solely of electrospun PAN fibers lacking any pore architecture to accommodate ZnO, resulting in the precipitation of ZnO crystal particles on the surface of ZnO-HPC1 fibers. In contrast, the hollow porous fibers formed by PMMA and PAN in ZnO-HPC2 to ZnO-HPC5 effectively encapsulate ZnO, thereby preventing noticeable particle precipitation on the fiber surface.
TGA and DSC curves are shown in Fig. 2a,b. The weight loss prior to 120 oC primarily results from the physical adsorption of water on the surface of the fibers. With the temperature increase, PMMA gradually undergoes pyrolysis, resulting in the formation of abundant voids within the fibers and inducing hollow structures. The exothermic peaks observe at around 238 °C in the DSC curves indicate the thermal decomposition of zinc acetate [Zn(C2H3O2)2∀ZnO + 2CH3COOH], which facilitates the formation of a hierarchical porous structure and loading of ZnO particles into the fibers26. At temperatures ranging from 300 °C to 450 °C, PAN undergoes further decomposition into carbon fibers and ammonia. Subsequently, at temperatures between 450 °C and 650 °C, PAN continues to disintegrate into carbon fibers, carbon monoxide, and nitric oxide, throughout this process, the degree of fiber carbonization gradually intensifies25.
Raman spectroscopy was performed to investigate the carbon structure of ZnO-HCPs (Fig. 2c). The low-frequency peak observed at approximately 1350 cm-1 in the Raman spectra corresponds to the D-band, indicating a disordered sp3 hybrid carbon structure resulting from defects or impurities. The G-band, located near 1580 cm-1, originates from vibrations associated with sp2 hybrid carbon-carbon bonds within the carbon material. The intensity ratio ID/IG represents the relative abundance of D-peak and G-peak. A higher ID/IG ratio suggests a greater presence of defects within the crystal lattice of C-atoms, while a lower ID/IG ratio indicates a more ordered carbon structure. Increased disorder in carbon materials results in a higher presence of defects, facilitating rapid ion storage. Increasing the PMMA proportion enhances the degree of ordered carbon structure of ZnO-HCPs, suggesting that PMMA facilitates the formation of well-organized carbon structure in ZnO-HCPs carbon fibers.
The BET-specific surface area and pore structure of ZnO-HCPs were analyzed. Figure 2d shows the N2 adsorption isotherms of ZnO-HCPs, and the specific pore structure parameters of ZnO-HCP shave been summarized in Table S1. The N2 adsorption isotherms of ZnO-HCPs conform to the typical IUPAC Type II classification. Low-pressure N2 adsorption predominantly occurs in micropores, while high-pressure N2 adsorption predominantly takes place in mesopores, as widely acknowledged. The nitrogen adsorption isotherms and pore structure information indicate that the fibers obtained by electrospinning possess both micropores and mesoporous structures. Increasing the PMMA content resulted in a trend of first increasing and then decreasing specific surface area and average pore diameter in ZnO-HCPs. This trend is consistent with the observed change in fiber structure from solid to hollow in the SEM images, gradually closing with higher PMMA content. Among them, ZnO-HCP3 stands out with the largest BET-specific surface area and average pore size, along with the highest micropore volume and a more suitable micropore proportion Combined with the results of Raman testing, it is demonstrated that an appropriate ratio of disordered/ordered carbon structure enables ZnO-HCPs to achieve a higher specific surface area and a more reasonable pore size distribution. In comparison, ZnO-HCP3, characterized by an appropriate disordered/ordered carbon structure, the highest specific surface area and more reasonable pore size distribution, was selected for subsequent preparation of ZnMnO-HPC composite with MnO2.
Figure 2e shows the schematic diagram illustrates of ZnO-HCPs. The surface tension disparity between PAN and PMMA results in the formation of distinct fiber sheath and core layers during electrospinning, with the lower surface tension of PAN favoring its sheath formation, while the core layer is formed by PMMA. The pyrolysis of PMMA in the core layer results in the formation of a hollow structure within the fibers, while the thermal decomposition of PAN leads to the development of carbon skeleton and facilitates the generation of abundant voids within the fibers. Additionally, zinc acetate decomposition enables high specific capacity ZnO incorporation onto the fibers, further enhancing pore structure of ZnO-HCPs fibers. Based on SEM images of ZnO-HCPs, an increase followed by a decrease in fiber pore diameter is observed with increasing proportion of PMMA. The presence of zinc acetate in the solution can be attributed to this phenomenon, as an excessively high PMMA content during the electrospinning process prevents the formation of a core layer, resulting in the absence of a hollow structure in the fibers.
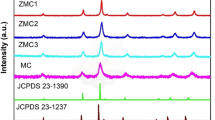
The XRD patterns of ZnMnO-HPC (Fig. 3a) display characteristic peaks corresponding to the crystal faces (002), (101), and (103) of ZnO, and the crystal faces (200), (310), and (521) of MnO2. These findings indicate that ZnMnO-HPC possesses a composite structure comprising both ZnO and MnO2. Figure 3b shows the N2 adsorption and desorption isotherms of ZnMnO-HPC. The BET method reveals that the specific surface area of ZnMnO-HPC is 269.76 m2/g, indicating a 196.38% increase compared to that of ZnO-HPC3. This result suggests that the incorporation of MnO2 nanosheets further enhances the specific surface area.
The SEM images of ZnMnO-HPC at various magnifications are depicted in Fig. 3c,d. SEM images of ZnMnO-HPC reveal the uniform attachment of MnO2 nanosheets to the surface of ZnO-HCP3 fibers after hydrothermal treatment. The enlarged cross-section topography reveals that the ZnMnO-HPC fibers maintain a well-preserved hollow structure simultaneously. Furthermore, TEM images (Fig. 3e,f) reveal that the MnO2 nanosheets exhibited a compact and uniform attachment to the surface of ZnMnO-HPC fibers. The cross-sectional magnification further confirms the hollow nature of ZnMnO-HCP nanofibers. Elemental analysis (Fig. 3g) reveals even distribution of Zn, Mn, and O elements in ZnMnO-HPC, confirming its high homogeneity. The digital photograph of ZnMnO-HPC electrode and flexible bending test are presented in Figure S1. It can be inferred that ZnMnO-HPC self- supporting electrode exhibits excellent flexibility and resistance to bending.
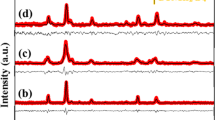
The element composition and bonding structure of ZnMnO-HPC were characterized using XPS, as depicted in Fig. 4a. The wide-scan XPS spectrum exhibits distinct characteristic peaks at approximately 285 eV, 532 eV, 642 eV, 654 eV, 1022 eV, 1046 eV, corresponding to C1s, O1s, Mn2p3/2, Mn2p1/2, Zn2p3/2, Zn2p1/2, respectively. The C 1s spectrum in Fig. 4b exhibits excellent fitting to the peaks of C-C/C = C/C-H (284.7 eV), C-O (286.1 eV) and C = O (288.5 eV), providing evidence for the presence of carbon structure in ZnMnO-HPC. Figure 4c gives an overview the XPS spectra of the O1s region, which can be divided into OIII (lattice oxygen, 529.7 eV), OII (oxygen vacancy/defect, 531.1 eV) and OI (chemisorbed oxygen, 532.2 eV) peaks. The lattice oxygen can actively participate in REDOX reactions and serve as a catalyst to enhance the capacity of ZnMnO-HPC20. The lattice oxygen and chemisorbed oxygen primarily originate from ZnO and MnO2. The high-resolution XPS spectra of Zn2p (Fig. 4d) exhibit two symmetrical peaks, with one peak centered at 1022.3 eV corresponding to Zn2p3/2 and the other peak centered at 1046.3 eV corresponding to Zn2p1/2, indicating the presence of normal Zn2+ ions in bulk ZnO nanostructures28. The XPS spectra of Mn2p (Fig. 4e) shows peaks of Mn2p3/2 and Mn2p1/2 centered on 642.2 eV and 654.0 eV, respectively. And Mn2p3/2 peak can be fitted from Mn4+ and Mn3+ respectively17.
The CV curves of ZnMnO-HPC at various scan rates are depicted in Fig. 5a. The CV curves exhibit approximate rectangles within potential range of -0.1 V to 0.8 V, indicating excellent electrochemical reversibility and capacitance characteristics of MnO2-loaded composite fibers. Increasing the scan rate to 100 mV/s maintains the curve shape of the original CV of 10 mV/s, indicating remarkable capacitive performance, fast reaction kinetics, and a highly reversible electrochemical process. Furthermore, ZnMnO-HCP electrode demonstrates a rapid response to changes in scanning direction, implying low internal resistance and fast charge storage capability. Figure 5b shows galvanostatic charge-discharge profiles of ZnMnO-HCP at different current densities. The GCD profiles of ZnMnO-HCP present symmetric isosceles triangle, indicating favorable capacitance characteristics. At current densities of 0.5 A/g, 1 A/g, 2 A/g, 3 A/g, 4 A/g and 5A/g, the specific capacity of ZnMnO-HCP is 401.77 C/g, 343.56 C/g, 318.07 C/g, 240.73 C/g, 217.96 C/g and 201.29 C/g, respectively, demonstrating excellent rate performance (Fig. 5c).
The optimal mass ratio of the electrochemically active material in the positive and negative electrodes can be described by equation S4 to achieve charge balance and maximize the electrochemical performance of asymmetric supercapacitors. The positive electrode (ZnMnO-HCP) and negative electrode (activated carbon, AC) are assembled to asymmetric supercapacitor ZnMnO-HCP//AC. The optimal mass ratio of ZnMnO-HCP electrode to AC electrode (activated carbon, AC) is 1:1.32 at current density of 1 A/g. Figure 6a illustrates the specific capacity matching curves for electrodes with the current density of 1 A/g. The voltage windows of the positive and negative electrode are overlapped, resulting in the voltage window range of 0–1.6 V for ZnMnO-HCP//AC. CV curves of ZnMnO-HCP//AC symmetric supercapacitors at different scanning rates are depicted in Fig. 6b. It can be observed that the CV curves exhibit rectangular shape, indicating excellent capacitance characteristics and enhanced electrochemical reversibility of the assembled ZnMnO-HCP//AC asymmetric supercapacitor even when the scanning rate is decreased.
(a) Capacity matching of ZnMnO-HCP electrode and AC electrode at the current density 1 A/g, (b) CV curves of ZnMnO-HCP//AC at different scan rates, (c) galvanostatic charge and discharge profiles of ZnMnO-HCP//AC at different current densities, (d) cycle performance of ZnMnO-HCP//AC at the current density of 2 A/g, (e) EIS profiles of ZnMnO-HCP//AC before and after cycles, (f) Ragone plots based on ZnMnO-HCP//AC.
The GCD curves of ZnMnO-HCP//AC asymmetric supercapacitor under different current densities are illustrated in Fig. 6c. It can be observed that the charging time and discharge time are nearly equal, indicating excellent electrochemical reversibility and high coulomb efficiency of the assembled ZnMnO-HCP//AC asymmetric capacitor. The specific capacity is determined based on the discharge time from the GCD curve. At the current density of 0.5 A/g, the specific capacity of the asymmetric supercapacitor reaches 77.2 C/g. Furthermore, at the current density of 10 A/g, the specific capacity decreases to 53.87 C/g of ZnMnO-HCP//AC asymmetric supercapacitor. Notably, with the twenty-fold increase in current density, there is still a remarkable retention rate of 69.78% for specific capacity, demonstrating favorable rate performance exhibited by the experimental assembly of ZnMnO-HCP//AC asymmetric capacitors. The practicability of ZnMnO-HCP//AC was further investigated by conducting 10,000 constant current charge and discharge tests at the current density of 2 A/g. Test results revealed that after the completion of 10,000 cycles, the specific capacity of ZnMnO-HCP//AC still maintained 92.61% of its initial value (Fig. 6d). This demonstrates the excellent reversibility and cycle stability exhibited of ZnMnO-HCP//AC asymmetric supercapacitor.
Figure 6e shows the electrochemical impedance spectroscopy (EIS) profiles of ZnMnO-HCP//AC asymmetric supercapacitor before and after cycles, and the embedded diagram is EIS equivalent circuit diagram, and the frequency range is 10− 2 Hz to 105 Hz. In comparison, the resistance of ZnMnO-HCP//AC after cycling remains nearly unchanged from its initial value, thereby providing further evidence for the excellent cycle stability of ZnMnO-HCP as the electrode of supercapacitor. The Ragone plots illustrate energy density and power density of ZnMnO-HCP//AC asymmetric supercapacitor (Fig. 6f). Remarkably, asymmetric supercapacitors based on ZnMnO-HCP electrode demonstrate an exceptional energy density of 38.37 Wh/kg power density of 407 W/kg and 19.5 Wh/kg at power density of 12,800 W/kg. The results demonstrate that ZnMnO-HCP electrode significantly enhances the energy density and power characteristics of supercapacitor. In comparison with numerous previously reported manganese ZnO - MnOx based devices, it exhibits superior competitiveness, as illustrated in Fig. 6e; Table 1.
The ZnMnO-HCP//AC asymmetric capacitor after cycling was disassembled, the ZnMnO-HCP electrode exhibited no apparent cracking deformation after cycling. Following multiple washes with deionized water and subsequent drying, SEM testing was conducted, revealing that the morphology of the ZnMnO-HCP fiber remained intact without any structural changes, which further confirms the exceptional cycle stability of ZnMnO-HCP as supercapacitor electrode (Figure S2).
Conclusions
The prepared ZnO and MnO2 co-modified hierarchical porous carbon nanofiber films (ZnMnO-HCP) serve as electrodes for supercapacitors by combining electrospinning technology and hydrothermal method. ZnMnO-HCP exhibits a high specific capacity (401.77 C/g at 0.5 A/g) and high energy density (38.37 Wh/kg at 407 W/kg) owing to the synergistic effect of the electrospinning-induced hierarchical porous structure and ZnO and MnO2. These findings highlight the great potential of ZnMnO-HCP as electrode materials for achieving elevated energy density.
Methods
Synthesis of ZnO-HPCs and ZnMnO-HPC
1.2 g of zinc acetate (Zn(C2H3O2)2) was dissolved in 12 mL of N, N-Dimethylformamide (DMF) and magnetically stirred for 3 h to yield a solution with a concentration of 10% (wt/v%). Subsequently, 1.2 g of polyacrylonitrile (PAN, MW = 150000) and different mass ratios of polymethyl methacrylate (PMMA) were weighed and added to the transparent mixed solution, which was stirred at 50 oC for 12 h using magnetic stirring until light yellow mixed solution was obtained. The solution was utilized to prepare composite nanofiber films precursorvia electrospinning, employing following experimental conditions. The inner diameter of the needle is 0.4 mm, the voltage is 15 kV, the distance from the needle to the collecting board is 17 cm, the pushing speed is 0.006 mm/s, and the receiving device is aluminum foil. The composite nanofiber film precursor was subsequently subjected to vacuum drying at 60 oC to eliminate the residual solvent DMF.
The precursor was placed into Muffle furnace and heated to 250 oC at a temperature ramp rate of 2 oC/min, followed by a 2-hour pre-carbonization in the atmosphere of nitrogen to achieve precarbonization of the nanofiber film. Subsequently, it was further heated under the nitrogen atmosphere at a rate of 5 oC/min to reach 750 oC for 2 h, resulting in the formation of a hierarchical porous carbon nanofiber film which combined with ZnO (ZnO-HPCs), The ZnO-HPCs samples denoted as ZnO-HPC1, ZnO-HPC2, ZnO-HPC3, ZnO-HPC4 and ZnO-HPC5 respectively, were prepared by the mass ratio of PMMA to PAN as 0:10, 1:9, 2:8, 3:7 and 4:6. Schematic of the fabrication procedure of ZnO-HPC and ZnMnO-HPC is exhibited in Fig. 7.
ZnO-HPCs were immersed in a 5 mmol/L KMnO4 solution and subsequently subjected to an 80 oC water bath for 8 h. The mass ratio of potassium permanganate to ZnO-HPC was 1:1. Afterwards, it underwent multiple cycles of alternating washing with deionized water and ethanol before being dried to yield the MnO2-introduced composite hierarchical porous carbon nanofiber film (ZnMnO-HPC).
Electrochemical characterization
The ZnMnO-HCP carbon fiber film, prepared through electrospinning, exhibits a certain degree of flexibility. The pre-cut self-supporting fiber film (2 cm*2 cm) with the mass loading of 2.5 mg/cm2 was securely fastened onto the electrode clamp as the working electrode, with a platinum (Pt) sheet serving as the counter electrode and Hg/HgO mercury acting as the reference electrode, 2 M KOH aqueous solution served as the electrolyte for conducting electrochemical tests on the electrochemical workstation (CHI760E, Shanghai Chenhua Instrument Co., Ltd.). For the Mn-based (or Zn-based) electrode materials, the capacity(C, C/g) for the electrode over the desired operating regime is more suitable to show the supercapacitive performances than specific capacitance(F, F/g) at the selected potential windows, owing to the fact that the SC will obviously not be constant throughout the whole potential window .The mass-specific capacity was calculated by equation S1 and the calculation of specificenergy (E, Wh⋅kg[-1)and specificpower (P, W⋅kg[-1)of the supercapattery device were accomplished via the equations S2 and S3.
Activated carbon (AC), conductive agent (Super P), and adhesive (PVDF) were uniformly mixed with NMP as the solvent in an 8:1:1 ratio and coated onto a carbon cloth substrate. The mass loading of activated carbon was controlled by adjusting the coating thickness, the activated carbon electrode was prepared after drying. The ZnMnO-HCP self-supporting electrode was cut into a 16 mm diameter wafer as the positive electrode, while the activated carbon electrode of the same size was utilized as the negative electrode. The electrodes were matched according to equation S4 and subsequently assembled into the CR2032 button asymmetric capacitor (ZnMnO-HCP//AC) for electrochemical performance testing.
Data availability
All data is provided within the manuscript or supplementary information files.
References
Asare, K., Hasan, M. F., Shahbazi, A. & Zhang, L. A comparative study of porous and hollow carbon nanofibrous structures from electrospinning for supercapacitor electrode material development. Surf. Interfaces 26, 101386 (2021).
Zhai, Z. et al. A review of carbon materials for supercapacitors. Mater. Design, 111017 (2022).
Forouzandeh, P., Kumaravel, V. & Pillai, S. C. Electrode materials for supercapacitors: a review of recent advances. Catalysts 10, 969 (2020).
Zhang, J., Zhu, L., Jia, H., Wei, K. & Wen, L. Microreactor facilitated preparation and Ni-doping of MnO2 nanoparticles for supercapacitors. J. Alloys Compd. 889, 161772 (2021).
Wang, F. et al. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem. Soc. Rev. 46, 6816–6854 (2017).
Huang, J. et al. Rational design of electrode materials for advanced supercapacitors: from lab research to commercialization. Adv. Funct. Mater. 33, 2213095 (2023).
Ma, C. et al. ZnO-assisted synthesis of lignin-based ultra-fine microporous carbon nanofibers for supercapacitors. J. Colloid Interface Sci. 586, 412–422 (2021).
Xiong, C. et al. Polyaniline@ cellulose nanofibers multifunctional composite material for supercapacitors, electromagnetic interference shielding and sensing. J. Materiomics (2024).
Tian, W. et al. Porous carbons: structure-oriented design and versatile applications. Adv. Funct. Mater. 30, 1909265 (2020).
Meng, Z. et al. Micro/nano metal–organic frameworks meet energy chemistry: a review of materials synthesis and applications. eScience 3, 100092 (2023).
Yin, J., Zhang, W., Alhebshi, N. A., Salah, N. & Alshareef, H. N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods 4, 1900853 (2020).
Sun, L., Gong, Y., Li, D. & Pan, C. Biomass-derived porous carbon materials: synthesis, designing, and applications for supercapacitors. Green Chem. 24, 3864–3894 (2022).
Lu, X., Wang, C., Favier, F. & Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: designed architectures and electrochemical performance. Adv. Energy Mater. 7, 1601301 (2017).
Joshi, B. et al. Review of recent progress in electrospinning-derived freestanding and binder-free electrodes for supercapacitors. Coord. Chem. Rev. 460, 214466 (2022).
Liu, J. et al. Structure and electrochemistry comparison of electrospun porous carbon nanofibers for capacitive deionization. Electrochim. Acta 210, 171–180 (2016).
Tao, B. et al. Designing a carbon nanofiber-encapsulated iron carbide anode and nickel-cobalt sulfide-decorated carbon nanofiber cathode for high-performance supercapacitors. J. Colloid Interface Sci. 621, 139–148 (2022).
Nie, G. et al. Fiber-in-tube and particle-in-tube hierarchical nanostructures enable high energy density of MnO2-based asymmetric supercapacitors. J. Colloid Interface Sci. 582, 543–551 (2021).
Fan, P., Ye, C. & Xu, L. One-dimensional nanostructured electrode materials based on electrospinning technology for supercapacitors. Diam. Relat. Mater. 109803 (2023).
Zhu, J. et al. Robust N-doping porous carbon nanofiber membranes with inter-fiber cross-linked structures for supercapacitors. Carbon 202, 13–25 (2023).
He, H., Lian, J., Chen, C., Xiong, Q. & Zhang, M. Super hydrophilic carbon fiber film for freestanding and flexible cathodes of zinc-ion hybrid supercapacitors. Chem. Eng. J. 421, 129786 (2021).
Amiri, A., Conlee, B., Tallerine, I., Kennedy, W. J. & Naraghi, M. A novel path towards synthesis of nitrogen-rich porous carbon nanofibers for high performance supercapacitors. Chem. Eng. J. 399, 125788 (2020).
Li, X. et al. Highly conductive, hierarchical porous ultra-fine carbon fibers derived from polyacrylonitrile/polymethylmethacrylate/needle coke as binder-free electrodes for high-performance supercapacitors. J. Power Sources 521, 230943 (2022).
Wang, H. et al. Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance. J. Power Sources 482, 228986 (2021).
Huang, C. L., Wei, T. H., Peng, S. Y. & Lee, K. M. Study of electrospun polyacrylonitrile fibers with porous and ultrafine nanofibril structures: Effect of stabilization treatment on the resulting carbonized structure. J. Appl. Polym. Sci. 136, 48218 (2019).
Luo, G. et al. Highly conductive, stretchable, durable, breathable electrodes based on electrospun polyurethane mats superficially decorated with carbon nanotubes for multifunctional wearable electronics. Chem. Eng. J. 451, 138549 (2023).
Zhang, Q. et al. Energy release from RuO2//RuO2 supercapacitors under dynamic discharge conditions. Electrochim. Acta 367, 137455 (2021).
Han, Z. J. et al. RuO 2-coated vertical graphene hybrid electrodes for high-performance solid-state supercapacitors. J. Mater. Chem. A 5, 17293–17301 (2017).
Kim, C. H. & Kim, B. H. Electrochemical behavior of zinc oxide-based porous carbon composite nanofibers as an electrode for electrochemical capacitors. J. Electroanal. Chem. 730, 1–9 (2014).
Duan, G. et al. Pyrolysis of zinc salt-treated flax fiber: hierarchically porous carbon electrode for supercapacitor. Diam. Relat. Mater. 129, 109339 (2022).
Zhan, Y. et al. Facile synthesis of biomass-derived porous carbons incorporated with CuO nanoparticles as promising electrode materials for high-performance supercapacitor applications. J. Alloys Compd. 885, 161014 (2021).
Liu, X., Huang, Y., Zhao, X., Yan, J. & Zong, M. Flexible N-doped carbon fibers decorated with Cu/Cu2O particles for excellent electromagnetic wave absorption. J. Colloid Interface Sci. 616, 347–359 (2022).
Fan, Z. et al. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 21, 2366–2375 (2011).
Liu, P. et al. Rational construction of bowl-like MnO2 nanosheets with excellent electrochemical performance for supercapacitor electrodes. Chem. Eng. J. 350, 79–88 (2018).
Pallavolu, M. R. et al. A novel hybridized needle-like Co3O4/N-CNO composite for superior energy storage asymmetric supercapacitors. J. Alloys Compd. 908, 164447 (2022).
Zhang, B. et al. Opening tubular structure polyimide/polyvinyl chloride based carbon nanofibers for supercapacitor. Mater. Sci. Eng. B 288, 116169 (2023).
Dai, Z. et al. Highly enhanced electrochemical cycling stabilities of hierarchical partially-embedded MnO/carbon nanofiber composites as supercapacitor electrodes. Mater. Sci. Eng. B 262, 114684 (2020).
Diantoro, M. et al. Potential of MnO2-based composite and numerous morphological for enhancing supercapacitors performance. Int. J. Appl. Ceram. Technol. (2023).
Zhang, Q. Z., Zhang, D., Miao, Z. C., Zhang, X. L. & Chou, S. L. Research progress in MnO2–carbon based supercapacitor electrode materials. Small 14, 1702883 (2018).
Yue, T., Shen, B. & Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: a review. Renew. Sustain. Energy Rev. 158, 112131 (2022).
Wang, L. et al. Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density. Chem. Eng. J. 334, 1–9 (2018).
Zhang, Y. et al. Versatile electrochemical activation strategy for high-performance supercapacitor in a model of MnO2. J. Mater. Chem. A 7, 21290–21298 (2019).
Hu, B. et al. Structure-tunable Mn3O4-Fe3O4@ C hybrids for high-performance supercapacitor. J. Colloid Interface Sci. 581, 66–75 (2021).
Chen, L. M. et al. Simple synthesis of flower-like manganese dioxide nanostructures on cellulose nanocrystals for high-performance supercapacitors and wearable electrodes. ACS Sustain. Chem. Eng. 7, 11823–11831 (2019).
Li, S. et al. G.Three-dimensional MnO2 nanowire/ZnO nanorod arrays hybrid nanostructure for high-performance and flexible supercapacitor electrode. J. Power Sources 256 206 – 211. (2014).
Yu, M. et al. Hierarchical Al-doped and hydrogenated ZnO nanowire@MnO2 ultra-thin nanosheet core/shell arrays for high-performance supercapacitor electrode. Int. J. Electrochem. Sci. 8, 2313–2329 (2013).
Raj, C. J. et al. .Two-dimensional planar supercapacitor based on zinc oxide/manganese oxide core/shell nano-architecture. Electrochim. Acta 247, 945–957 (2017).
Samuel, E., Joshi, B., Kim, Y., Aldalbahi, A. & Rahaman, M. Yoon, SS. ZnO/MnOx nanoflowers for high performance supercapacitor. ACS Sustain. Chem. Eng. 8 (3697 – 3708), (2020).
Rashid, A. R. et al. Inductive effect in Mn-doped ZnO nanoribon arrays grown on ni foam: a promising key for boosted capacitive and high specific energy supercapacitors. Ceram. Int. 47, 28338–28347 (2021).
Wu, J. H. et al. J. Zn-doped MnO2 ultrathin nanosheets with rich defects for high performance aqueous supercapacitors. Electrochim. Acta 418, 140339 (2022).
Yang, Q. et al. Rationally designed hierarchical MnO2-shell/ZnO-nanowire/carbon fabric for high-performance supercapacitor electrodes. J. Power Sources 272, 654–660 (2014).
Yu, D. et al. Liu, X., the synthesis of hierarchical ZnCo2O4 @MnO2 core–shell nanosheetarrays on ni foam for high-performance all-solid-state asymmetric supercapacitors. Inorg. Chem. Front. 5, 597–604 (2017).
Acknowledgements
This research was funded by National Natural Science Foundation of China (52274260, 52164017, 52074096, 52263004), Guizhou Provincial Science and Technology Projects GCC ([2023] 017), Innovative young scientific and technological talents training projects of Guiyang City (Zhu Ke He Tong [2024] 2–12), Guizhou Provincial Science and Technology Projects (Qian Ke He Ji Chu-ZK [2022] Yi Ban 179).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.L.; methodology, Y.L.; software, W.L.; validation, X.L.; formal analysis, Y.L.; investigation, J.L.; resources, C.C.; data curation, Y.L.; writing and original draft preparation, Y.L.; review and editing, Y.L.; supervision, W.L.; project administration, Y.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Y., Li, J., Chen, C. et al. ZnO-MnO2 co-modified hierarchical porous carbon nanofiber film electrodes for high-energy density supercapacitors. Sci Rep 15, 6393 (2025). https://doi.org/10.1038/s41598-025-90747-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90747-0
This article is cited by
-
Boosting energy storage performance of ZnCoTe@NiCoSe2 with core-shell structure as an efficient positive electrode for fabrication of high-performance hybrid supercapacitors
Scientific Reports (2025)
-
Engineering NiO/g-C₃N₄ and NiO/rGO composites for dual applications in electrochemical water splitting and energy storage
Scientific Reports (2025)
-
MnO2 nanoflower decorated on ZnO/Co3O4 derived from ZIF-8@ZIF-67 for high-performance supercapacitor electrodes
Ionics (2025)