Abstract
Non-alcoholic fatty liver disease (NAFLD) is strongly associated with dyslipidemia, and the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) is a more comprehensive indicator of lipids. This study aimed to investigate the association between NHHR and hepatic steatosis and liver fibrosis. The 2017–2020 national health and nutrition examination survey (NHANES) dataset was used for the cross-sectional survey. NHHR was calculated by lipid profiling, and the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) were determined by vibration-controlled transient elastography (VCTE). Multiple linear regression models were used to test the linear association between NHHR and hepatic steatosis and liver fibrosis. Fitted smoothing curves and threshold effect analysis were used to describe the nonlinear relationships. This population-based study included 6575 adults (≥ 18 years). After adjusting for covariates, we found a U-shaped association between NHHR and hepatic steatosis, with a breakpoint of 1.26. There was a negative association on the left side of the breakpoint (OR [95% CI] − 24.31 [− 43.92, − 4.70]) and a positive association on the right side of the breakpoint (OR [95% CI] 3.82 [2.05, 5.59]). There was no significant association between NHHR and liver fibrosis. In addition, subgroup analyses and interaction tests showed stable results. In summary, NHHR has a U-shaped association with hepatic steatosis and no significant association with liver fibrosis. Keeping NHHR below 1.26 may be an effective option to reduce the risk of hepatic steatosis. NHHR is a more efficient and cost-effective marker for NAFLD surveillance that can be utilized in future clinical practice.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a globally prevalent chronic liver disease affecting more than 25% of the world’s population and one of the leading causes of serious liver disease, with annual healthcare costs in the United States directly attributable to NAFLD amounting to $100 billion1,2,3,4,5. Hepatic steatosis is considered to be one of the main features of NAFLD, and prolonged and abnormal accumulation of fat in the liver cytoplasm may lead to severe destruction of hepatocytes, which may develop into liver fibrosis, cirrhosis, and even hepatocellular carcinoma6,7. Hepatic steatosis and liver fibrosis are two fundamental aspects of the physiopathological progression of NAFLD and are important factors in analyzing the course of NAFLD. Therefore, assessing the degree of hepatic fibrosis and steatosis is crucial for the clinical prognosis and evaluation of patients with NAFLD8,9. VCTE is a noninvasive test with good accuracy and is now widely used for screening NAFLD10. CAP and LSM are used to assess hepatic steatosis and liver fibrosis, respectively11,12,13.
NAFLD is closely associated with dyslipidemia, and in previous studies of dyslipidemia in NAFLD, only some of the lipids have often been detected or elucidated14,15,16, resulting in conflicting results regarding the role of a single lipid species in promoting hepatic fat accumulation17,18. In recent years, many studies have explored the relationship between nontraditional lipid markers such as non-HDL-C and NAFLD and found them to be strongly correlated19,20,21.
NHHR is a newly discovered index for assessing atherosclerotic lipid combinations, and non-HDL-C is defined as total cholesterol (TC) minus HDL-C. Compared with traditional lipid parameters, it explains the dual role of HDL-C and non-HDL-C and avoids the limitations of previous monolipid studies. NHHR is associated with and has predictive value for a variety of diseases including diabetes, nephropathy, and cardiovascular disease22,23,24. A study in a Japanese population indicated that NHHR was independently associated with NAFLD25. A study in a Chinese population also showed that NHHR was an independent predictor of NAFLD26. Another study compared non-HDLC with NHHR and found that NHHR was a better predictor in Chinese patients with NAFLD27. These studies suggest that NHHR is a tool with higher diagnostic and predictive value for NAFLD compared with traditional lipid markers, but most of these studies only explored the association between NHHR and the risk of developing NAFLD, without exploring changes in hepatic steatosis and liver fibrosis.
Therefore, this study, based on findings from previous studies, aims to explore the association between NHHR and hepatic steatosis and liver fibrosis in US adults, providing new perspectives for the assessment and management of NAFLD.
Methods
Study population
This study used data from NHANES, and the protocol of the NHANES study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, with each study participant signing an informed consent form. All detailed NHANES study designs and data are publicly available at www.cdc.gov/nchs/nhanes/.
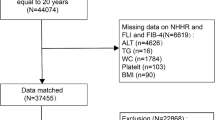
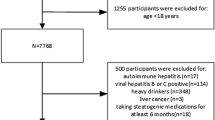
This study included data from 2017 to 2020. A total of 15,560 participants were sampled for the study, from which 5862 subjects with missing CAP or LSM data, 1074 heavy drinkers who drank 4 or more drinks per day, 198 patients with Hepatitis B or C, 660 subjects with missing NHHR data, and 1191 subjects under the age of 18 were excluded. Finally, 6575 participants were included in this study (Fig. 1).
Study variables
Definition of NHHR
As previously described in the literature22,28, NHHR represents the ratio of non-HDL-C to HDL-C. Non-HDL-C is determined by subtracting HDL-C from TC levels.
Assessment of hepatic steatosis and liver fibrosis
A simple, noninvasive ultrasound technique called vibration-controlled transient elastography (VCTE) is used to evaluate the degree of hepatic steatosis and fibrosis in patients. LSM is used to assess the degree of liver stiffness, with higher LSM values suggesting more liver fibrosis, and CAP is used to assess liver fat content, with higher CAP values suggesting more hepatic steatosis.
Based on several previous studies, we concluded that a CAP ≥ 274 dB/m is indicative of hepatic steatosis, and a CAP ≥ 302 dB/m is severe hepatic steatosis29,30. Fibrosis grade was determined by LSM, and fibrosis grades ≥ F2 (8.2 kPa) were considered clinically significant hepatic fibrosis, ≥ F3 (9.7 kPa) advanced fibrosis, and ≥ F4 (13.6 kPa) cirrhosis31,32.
Selection of covariates
The following covariates were included in this study: age, gender, race, marital status, Socioeconomic status, physical activity (< 450 MET⋅min/week, ≥ 450 MET⋅min/week), smoking status, depression status, body mass index (BMI), and waist circumference (WC). Laboratory parameters included triglyceride(TG), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Chronic diseases include diabetes, hypertension, and serious cardiovascular disease (CVD), defined as yes or no. CVD was based on a physician’s diagnosis of coronary heart disease, heart attack, congestive heart failure, angina, and stroke.
Statistical analysis
Statistical studies were conducted using the statistical computing and graphing software R (version 4.1.3) and EmpowerStats (version: 4.2). Appropriate NHANES sampling weights were used for all statistical analyses. Means ± standard deviation (SD) were used for continuous variables and percentages were used for categorical variables. β values and 95% confidence intervals between NHHR and CAP and LSM were calculated using multiple linear regression analysis. Multivariate regression analyses were constructed using three models: model 1: unadjusted for variables; model 2: adjusted for gender, age, and race; and model 3: adjusted for all covariates. For further sensitivity analyses, we categorized NHHR into tertiles to assess its robustness. Smooth curve fitting was used to address nonlinearities, and a threshold effects analysis model was used to determine the presence of thresholds and breakpoints (K). Finally, subgroup analyses and interaction effect tests were performed to see if the findings were heterogeneous. Results were considered statistically significant at P < 0.05.
Results
Baseline characteristics of participants
After screening a total of 6575 adults ≥ 18 years of age were included in this study. The mean age of the participants was 48.49 ± 17.78, with 54.70% females and 45.30% males. 63.53% were non-Hispanic whites. The mean ± SD for CAP, LSM, and NHHR were 262.61 ± 62.59 (dB/m), 5.76 ± 4.56 (kPa), and 2.71 ± 1.41, respectively.
Supplementary Materials 1 lists the clinical characteristics of participants with NHHR tertile as the stratification variable. All clinical characteristics were statistically significant (P < 0.05) except socioeconomic status. Participants in the highest tertile of NHHR were more likely to be male and to have higher values of CAP, LSM, ALT, AST, HbA1c, FBG, TG, WC, and BMI than participants in the lowest tertile.
Association between NHHR and CAP
Table 1 shows the association between NHHR and CAP. We found a significant positive association between NHHR and CAP in Model 1, Model 2, and Model 3. In the fully adjusted Model 3, for every 1 unit increase in NHHR, CAP rises by 3.2 units (β [95% CI]: 3.20 [1.48, 4.92]). When we transformed NHHR into a categorical variable (tertiles), we found that the positive association between NHHR and CAP persisted (β [95% CI]: 11.78 [7.05, 16.51]).
In addition, we performed a smoothed curve-fitting test, which showed a nonlinear association between NHHR and CAP (Fig. 2). A threshold analysis was then performed, and a breakpoint of 1.26 was calculated. On the left side of the breakpoint, NHHR was negatively correlated with CAP (β[95% CI]: -24.31 [-43.92, -4.70]), while on the right side of the breakpoint, it showed a positive correlation (β[95% CI]: 3.82 [2.05, 5.59]) (Table 2).
Association between NHHR and LSM
Table 3 shows the association between NHHR and LSM. We found a significant positive association between NHHR and LSM in Model 1 and Model 2, but no statistically significant correlation between the two in the fully adjusted Model 3. When we transformed NHHR into a categorical variable (tertiles), NHHR in the highest tertile remained positively correlated with LSM in Model1 and Model 2, but the opposite result occurred in Model 3, where NHHR and LSM were negatively correlated. As NHHR increased, LSM values in the highest tertile decreased compared to the lowest tertile (β[95% CI]: -0.57 [-1.00, -0.14]).
The smoothed curve fitting test showed a nonlinear association between NHHR and LSM (Fig. 3). Threshold analysis calculated a breakpoint of 2.69. On the left side of the breakpoint, NHHR was negatively correlated with LSM (β[95% CI]: -0.62 [-0.93, -0.31]), whereas on the right side of the breakpoint, the two were not statistically significant (β[95% CI]: 0.17 [-0.03, 0.38]) (Table 4).
Subgroup analysis
To assess whether the association between NHHR and CAP, LSM was influenced by a particular baseline characteristic. We performed subgroup analyses and interaction tests stratified by gender, age, race, degree of hepatic steatosis, and degree of liver fibrosis. The results showed that none of these covariates had a significant effect on the association between NHHR and CAP and LSM (P for interaction > 0.05). It is worth noting that the association between NHHR and CAP and LSM remained stable after we graded hepatic steatosis and liver fibrosis in detail (Supplementary material 2).
Discussion
In this study, we found the following results: (1) There is a U-shaped correlation between NHHR and hepatic steatosis with a breakpoint of 1.26. On the left side of the breakpoint, NHHR is negatively correlated with CAP, while on the right side of the breakpoint, it is positively correlated. (2) There was no significant association between NHHR overall and LSM. However, the threshold analysis identified a breakpoint (2.69) where NHHR was negatively correlated with LSM on the left side of the breakpoint, while on the right side, it showed an increasing trend, but not statistically significant. (3) Subgroup analyses and interaction tests showed that the associations of NHHR with CAP and LSM were not affected by confounders and the results were stable.
Our findings identified a U-shaped association between NHHR and CAP. Notably, the breakpoint 1.26 might be the critical value for the occurrence of hepatic steatosis, and when NHHR exceeded 1.26, the value of CAP subsequently increased, which suggests that NHHR can be an effective predictor of the risk of hepatic steatosis and suggests that keeping our NHHR below 1.26 might be a good choice to reduce the risk of hepatic steatosis. In addition, we also found that there is a nonlinear correlation between NHHR and LSM. To the left of the breakpoint 2.69, an increase in NHHR is associated with a decrease in LSM, and this negative correlation stops when NHHR exceeds 2.69, and LSM begins to show a non-significant upward trend. Although we cannot conclude from this result that 2.69 is the turning point of LSM from decreasing to increasing, it seems to suggest that the value of 2.69 may have a special meaning that is worth noting.
The pathogenesis of NAFLD is complex, and it is now generally accepted that disturbances in the balance of cholesterol metabolism are the main cause of the development of NAFLD, and dyslipidemia due to abnormalities in hepatic cholesterol metabolism is an extremely important risk factor33,34,35,36. High cholesterol can lead to activation of liver X receptors (LXR), which promotes very low density lipoprotein (VLDL) secretion, increases low density lipoprotein (LDL) levels, and induces hepatic steatosis37. A clinical study showed that abnormalities in fatty acid oxidation and VLDL secretion can induce severe lipid oxidation and oxidative DNA damage and lead to the development of NAFLD38. It has also been found that non-HDL-C better reflects pro-atherosclerotic lipoproteins containing apolipoprotein B compared to VLDL, MDI, and LDL39, and a multidimensional study demonstrated that lowering non-HDL-C and LDL reduces the prevalence of non-alcoholic steatohepatitis and fibrosis40. In addition, insulin resistance is also a non-negligible part of the pathogenesis of NAFLD. Hyperinsulinemia and hyperglycemia state will cause adipocytes to release more free fatty acids, promote a large amount of hepatic VLDL synthesis and secretion, and accelerate hepatic fat accumulation41,42. These findings suggest that the pathogenesis of NAFLD is inextricably linked to the abnormalities of various components of blood lipids and that attention to lipid profiles is of great significance in the monitoring of NAFLD.
NHHR identifies a full range of lipid disorders and is also a better predictor of cardiovascular, metabolic syndrome, and insulin resistance43,44, and can be easily obtained simply by measuring lipid profiles. In this study, we found that there is a significant association between NHHR and CAP, and regulating NHHR may affect the development process of NAFLD. NHHR can be regulated by a variety of factors, and some studies have found that vitamin D deficiency increases the risk of dyslipidemia, and the vitamin D level in the high NHHR group was significantly lower than that of the low NHHR group, which suggests that taking care of supplementation with a variety of vitamin D in daily life may have a positive effect on NHHR and thus on liver disease45,46. Combined with previous studies25,26,27, we can infer that NHHR might be a more efficient and cost-effective indicator for NAFLD monitoring, which could provide a new approach to assessing and managing NAFLD.
Finally, we performed subgroup analyses and interaction tests, which showed that NHHR was not affected by factors such as gender, age, race, degree of hepatic steatosis, and liver fibrosis, and the results obtained were stable. This demonstrates that NHHR is a stable index for assessing the association of lipids with hepatic steatosis and liver fibrosis.
Our study has several strengths. First, we explored the association between NHHR and hepatic steatosis and hepatic fibrosis in U.S. adults, the first known study to do so, which is innovative. Second, we included a large sample size and adjusted for known confounding covariates, strengthening the generalizability and reliability of the findings. Third, we performed subgroup analyses and interaction tests to verify the stability of the results. However, this study has some limitations. First, the cross-sectional design of this study limits the causal inference between NHHR and hepatic steatosis and liver fibrosis, and more rigorous randomized controlled trials are needed to further validate the exploration. Second, although we adjusted for many important covariates, we could not eliminate the effects of other possible confounding variables. Third, although we chose the currently widely recognized VCTE for the diagnosis of NAFLD, the accuracy is still not as good as the liver histopathology diagnosis. Our findings should be interpreted with caution.
Conclusion
In summary, NHHR has a U-shaped association with hepatic steatosis and no significant association with liver fibrosis. Keeping NHHR below 1.26 may be an effective option to reduce the risk of hepatic steatosis. NHHR is a more efficient and cost-effective marker for NAFLD surveillance that can be utilized in future clinical practice.
Data availability
The datasets supporting the conclusions of this article are available in the NHANES repository, https://www.cdc.gov/nchs/nhanes/.
References
Lonardo, A., Leoni, S., Alswat, K. A. & Fouad, Y. History of nonalcoholic fatty liver disease. Int. J. Mol. Sci. 21, 5888. https://doi.org/10.3390/ijms21165888 (2020).
Ayonrinde, O. T. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD - Reconciling the present with the past. JHEP Rep.: Innovat. Hepatol. 3, 100261. https://doi.org/10.1016/j.jhepr.2021.100261 (2021).
Tamaki, N., Ajmera, V. & Loomba, R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat. Rev. Endocrinol. 18, 55–66. https://doi.org/10.1038/s41574-021-00584-0 (2022).
Youssef, S. S. & Hamdy, N. M. SOCS1 and pattern recognition receptors: TLR9 and RIG-I; novel haplotype associations in Egyptian fibrotic/cirrhotic patients with HCV genotype 4. Adv. Virol. 162, 3347–3354. https://doi.org/10.1007/s00705-017-3498-7 (2017).
Ali, N. A., Hamdy, N. M., Gibriel, A. A. & El Mesallamy, H. O. Investigation of the relationship between CTLA4 and the tumor suppressor RASSF1A and the possible mediating role of STAT4 in a cohort of Egyptian patients infected with hepatitis C virus with and without hepatocellular carcinoma. Adv. Virol. 166, 1643–1651. https://doi.org/10.1007/s00705-021-04981-8 (2021).
Brunt, E. M., Janney, C. G., Di Bisceglie, A. M., Neuschwander-Tetri, B. A. & Bacon, B. R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 94, 2467–2474. https://doi.org/10.1111/j.1572-0241.1999.01377.x (1999).
Long, M. T. et al. Hepatic fibrosis associates with multiple cardiometabolic disease risk factors: The framingham heart study. Hepatology (Baltimore, Md.) 73, 548–559. https://doi.org/10.1002/hep.31608 (2021).
Díaz, L. A. et al. The establishment of public health policies and the burden of non-alcoholic fatty liver disease in the Americas. Lancet Gastroenterol. Hepatol. 7, 552–559. https://doi.org/10.1016/s2468-1253(22)00008-5 (2022).
Ginès, P. et al. Liver cirrhosis. Lancet (London, England) 398, 1359–1376. https://doi.org/10.1016/s0140-6736(21)01374-x (2021).
Petzold, G. Role of ultrasound methods for the assessment of NAFLD. J. Clin. Med. 11, 4581. https://doi.org/10.3390/jcm11154581 (2022).
Eddowes, P. J. et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 156, 1717–1730. https://doi.org/10.1053/j.gastro.2019.01.042 (2019).
Ciardullo, S., Monti, T. & Perseghin, G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 44, 519–525. https://doi.org/10.2337/dc20-1778 (2021).
Siddiqui, M. S. et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol.: Off. Clin. Practice J. Am. Gastroenterol. Associat. 17, 156-163.e152. https://doi.org/10.1016/j.cgh.2018.04.043 (2019).
Hamaguchi, M. et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. internal Med. 143, 722–728. https://doi.org/10.7326/0003-4819-143-10-200511150-00009 (2005).
Omagari, K. et al. Fatty liver in non-alcoholic non-overweight Japanese adults: Incidence and clinical characteristics. J. Gastroenterol. Hepatol. 17, 1098–1105. https://doi.org/10.1046/j.1440-1746.2002.02846.x (2002).
DeFilippis, A. P. et al. Nonalcoholic fatty liver disease and serum lipoproteins: The multi-ethnic study of atherosclerosis. Atherosclerosis 227, 429–436. https://doi.org/10.1016/j.atherosclerosis.2013.01.022 (2013).
Neuschwander-Tetri, B. A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology (Baltimore, Md.) 52, 774–788. https://doi.org/10.1002/hep.23719 (2010).
Zelber-Sagi, S. et al. Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver Int.: Off. J. Int. Associat. Study Liver 34, e128-135. https://doi.org/10.1111/liv.12318 (2014).
Lee, J. H., Kim, J., Kim, J. O. & Kwon, Y. J. Association of non-high-density lipoprotein cholesterol trajectories with the development of non-alcoholic fatty liver disease: An epidemiological and genome-wide association study. J. Transl. Med. 21, 435. https://doi.org/10.1186/s12967-023-04291-4 (2023).
Zou, Y., Hu, C., Kuang, M. & Chai, Y. Remnant cholesterol/high-density lipoprotein cholesterol ratio is a new powerful tool for identifying non-alcoholic fatty liver disease. BMC Gastroenterol 22, 134. https://doi.org/10.1186/s12876-022-02216-x (2022).
Chen, Z., Qin, H., Qiu, S., Chen, G. & Chen, Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: A retrospective cohort research. Lipids Health Dis. 18, 162. https://doi.org/10.1186/s12944-019-1104-6 (2019).
Tan, M. Y. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio with type 2 diabetes mellitus: Recent findings from NHANES 2007–2018. Lipids Health Dis. 23, 151. https://doi.org/10.1186/s12944-024-02143-8 (2024).
Wen, J. et al. Association of the TG/HDL-C and non-HDL-C/HDL-C ratios with chronic kidney disease in an adult Chinese population. Kidney Blood Pressure Res. 42, 1141–1154. https://doi.org/10.1159/000485861 (2017).
Sheng, G. et al. Utility of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio in evaluating incident diabetes risk. Diabetes, Metabol. Syndrome Obesity: Targets Therapy 15, 1677–1686. https://doi.org/10.2147/dmso.S355980 (2022).
Lu, S. et al. Utility of traditional and non-traditional lipid indicators in the diagnosis of nonalcoholic fatty liver disease in a Japanese population. Lipids Health Dis. 21, 95. https://doi.org/10.1186/s12944-022-01712-z (2022).
Gao, S., Ramen, K., Yu, S. & Luo, J. Higher non-HDL-cholesterol to HDL-cholesterol ratio is linked to increase in non-alcoholic fatty liver disease: Secondary analysis based on a longitudinal study. Int. J. Clin. Exp. Pathol. 13, 2569–2575 (2020).
Wang, K. et al. Non-HDL-cholesterol to HDL-cholesterol ratio is a better predictor of new-onset non-alcoholic fatty liver disease than non-HDL-cholesterol: A cohort study. Lipids Health Dis. 17, 196. https://doi.org/10.1186/s12944-018-0848-8 (2018).
Qi, X. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: A cross-sectional NHANES study. J. Affect. Disorders 344, 451–457. https://doi.org/10.1016/j.jad.2023.10.064 (2024).
Xie, R. et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front. Immunol. 13, 925690. https://doi.org/10.3389/fimmu.2022.925690 (2022).
Yang, N., Lu, Y., Cao, L. & Lu, M. The association between non-alcoholic fatty liver disease and serum ferritin levels in American adults. J. Clin. Lab. Anal. 36, e24225. https://doi.org/10.1002/jcla.24225 (2022).
Ciardullo, S. & Perseghin, G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabol. Clin. Exp. 121, 154752. https://doi.org/10.1016/j.metabol.2021.154752 (2021).
Roulot, D. et al. Liver stiffness values in apparently healthy subjects: Influence of gender and metabolic syndrome. J. Hepatol. 48, 606–613. https://doi.org/10.1016/j.jhep.2007.11.020 (2008).
Fon Tacer, K. & Rozman, D. Nonalcoholic Fatty liver disease: Focus on lipoprotein and lipid deregulation. J. Lipids 2011, 783976. https://doi.org/10.1155/2011/783976 (2011).
Speliotes, E. K. et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham Heart Study. Hepatology (Baltimore, Md.) 51, 1979–1987. https://doi.org/10.1002/hep.23593 (2010).
Katsiki, N., Mikhailidis, D. P. & Mantzoros, C. S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabol. Clin. Exp. 65, 1109–1123. https://doi.org/10.1016/j.metabol.2016.05.003 (2016).
Li, H., Yu, X. H., Ou, X., Ouyang, X. P. & Tang, C. K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Progr. Lipid Res. 83, 101109. https://doi.org/10.1016/j.plipres.2021.101109 (2021).
Ducheix, S., Montagner, A., Theodorou, V., Ferrier, L. & Guillou, H. The liver X receptor: A master regulator of the gut-liver axis and a target for non alcoholic fatty liver disease. Biochem. Pharmacol. 86, 96–105. https://doi.org/10.1016/j.bcp.2013.03.016 (2013).
Fujita, K. et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology (Baltimore, Md.) 50, 772–780. https://doi.org/10.1002/hep.23094 (2009).
Brunner, F. J. et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: Results from the Multinational Cardiovascular Risk Consortium. Lancet (London, England) 394, 2173–2183. https://doi.org/10.1016/s0140-6736(19)32519-x (2019).
Ayada, I. et al. Dissecting the multifaceted impact of statin use on fatty liver disease: A multidimensional study. EBioMedicine 87, 104392. https://doi.org/10.1016/j.ebiom.2022.104392 (2023).
Muzurović, E., Mikhailidis, D. P. & Mantzoros, C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabol. Clin. Exp. 119, 154770. https://doi.org/10.1016/j.metabol.2021.154770 (2021).
Browning, J. D. & Horton, J. D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investigat. 114, 147–152. https://doi.org/10.1172/jci22422 (2004).
Lamprea-Montealegre, J. A. et al. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: The ARIC study. Atherosclerosis 234, 42–46. https://doi.org/10.1016/j.atherosclerosis.2014.02.006 (2014).
Taskinen, M. R. et al. Ability of traditional lipid ratios and apolipoprotein ratios to predict cardiovascular risk in people with type 2 diabetes. Diabetologia 53, 1846–1855. https://doi.org/10.1007/s00125-010-1806-9 (2010).
Chen, Y. C. et al. Effect of vitamin D supplementation on primary dysmenorrhea: A systematic review and meta-analysis of randomized clinical trials. Nutrients 15, 2830. https://doi.org/10.3390/nu15132830 (2023).
Zhen, R. et al. The relationship between non-HDL-C /HDL-C Ratio (NHHR) and vitamin D in type 2 diabetes mellitus. Diabetes, Metabol. Syndrome Obesity: Targets Therapy 16, 2661–2673. https://doi.org/10.2147/dmso.S414673 (2023).
Acknowledgements
Thanks to all the authors for their contributions.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Xiaoxian Yang conceptualized the study. Statistical analysis was done by Xiaoxian Yang, Haiyi Yan and Rui Guo. The manuscript was reviewed by Yan Chen. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This is an observational study. The studies involving human participants were reviewed and approved by the NCHS Ethics Review Board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, X., Yan, H., Guo, R. et al. Association between the NHHR and hepatic steatosis and liver fibrosis: a population-based study. Sci Rep 15, 8462 (2025). https://doi.org/10.1038/s41598-025-90818-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90818-2
Keywords
This article is cited by
-
Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and short-term outcomes in hepatitis B virus-related acute-on-chronic liver failure
BMC Infectious Diseases (2026)
-
The HbA1c/HDL-C ratio as a screening indicator of NAFLD in U.S. adults: a cross-sectional NHANES analysis (2017–2020)
BMC Gastroenterology (2025)
-
Development and validation of a risk predictive nomogram for carotid intima-media thickening in patients with type 2 diabetes
Acta Diabetologica (2025)