Abstract
Coronary artery disease (CAD) is a leading cause of morbidity and mortality globally, highlighting the need for improved risk assessment tools. Traditional measures like body mass index (BMI) do not fully capture the risk associated with body fat distribution. The weight-adjusted waist index (WWI) has emerged as a potential anthropometric measure that may provide a more precise evaluation of central adiposity and its cardiovascular risks. We analyzed data from 49,240 participants in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2020. Participants were stratified into quartiles based on WWI: Q1 (WWI < 10.43), Q2 (10.43 ≤ WWI < 10.99), Q3 (10.99 ≤ WWI < 11.56), and Q4 (WWI ≥ 11.56). The primary outcome was self-reported coronary artery disease (CAD). Multivariate logistic regression, generalized additive models, and stratified analyses were conducted. The predictive performance of WWI versus body mass index (BMI) for CAD was assessed using receiver operating characteristic (ROC) curves, with differences in area under the curve (AUC) evaluated by DeLong’s test. Multivariate logistic regression analysis showed a positive association between WWI and CAD prevalence, which persisted after adjusting for confounding variables. Higher WWI was associated with increased risk of CAD, with Group 4 showing the highest risk compared to Group 1 (OR 2.06, 95% CI 1.37–3.09, P < 0.001). This relationship was not observed between BMI and CAD risk. Subgroup analysis revealed that the association between WWI and CAD prevalence was more pronounced in participants aged ≤ 60 years (P for interaction < 0.001). ROC curve analysis demonstrated that WWI had superior predictive ability for CAD compared to BMI (AUC 0.694 vs. 0.547, De-long test P < 0.001). WWI demonstrated a stronger association with CAD prevalence compared to BMI in the US population, with this relationship particularly pronounced among younger adults. Longitudinal studies are needed to establish this relationship.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) remains a significant global health concern, characterized by high morbidity and mortality rates1. According to recent epidemiological data, cardiovascular diseases, predominantly heart disease and stroke, accounted for approximately 17.7 million deaths worldwide in 20192. The socioeconomic impact of CAD is substantial, encompassing direct healthcare costs, productivity losses, and reduced quality of life. Patients with CAD face adverse outcomes, including recurrent hospitalizations, decreased functional capacity, and an elevated risk of severe complications such as heart failure and sudden cardiac death3.
Traditionally, body mass index (BMI) has been widely utilized as an anthropometric measure to assess cardiovascular risk4. However, BMI exhibits notable limitations in accurately reflecting body fat distribution and predicting CAD risk. Research has demonstrated that central obesity, rather than overall adiposity, is more closely associated with cardiovascular risk factors and outcomes5. Considering these limitations, the weight-adjusted waist index (WWI) has been proposed as a novel anthropometric measure more correlated with central adiposity6. WWI is calculated by dividing waist circumference (WC) by the square root of body weight, aiming to provide a more precise assessment of central adiposity and its associated risks7. Recent studies have explored the potential of WWI in predicting cardiovascular outcomes. Fang et al. found that higher WWI levels were associated with increased odds of cardiovascular disease in US adults, with stronger associations observed in individuals under 50 years of age8. Han et al. demonstrated that elevated WWI levels were independently associated with higher risks of cardiovascular and all-cause mortality in a prospective cohort study9. Furthermore, Qin et al. reported a positive association between WWI and abdominal aortic calcification scores in adults aged ≥ 40 years, suggesting WWI’s potential as a biomarker for subclinical atherosclerosis10.
Despite these advances, the association between WWI and CAD risk in the general population remains largely unexplored. This study aimed to investigate the relationship between WWI and CAD prevalence using data from the National Health and Nutrition Examination Survey (NHANES) spanning 1999 to 2020. The predictive capacities of WWI and BMI for CAD risk were compared. The findings of this study may contribute to improving cardiovascular risk assessment and inform the development of more targeted public health interventions. While the cross-sectional nature of this study inherently limits causal inference, these preliminary findings provide valuable insights that may guide future research endeavors.
Methods
Participants and study design
The data for this study was derived from NHANES database, a nationally representative survey program conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). It aimed to comprehensively assess the health and nutritional status of non-institutionalized civilian residents in the United States. NHANES interviews covered various domains, including demographic, socioeconomic, dietary, and health-related factors. The NHANES research plan received ethical approval from the National Center for Health Statistics ethics review board. All participants provided written informed consent prior to enrollment in the study. Further details regarding the study protocol and procedures can be found in the National Health and Nutrition Examination Survey documentation (www.cdc.gov/nchs/nhanes/index.htm).
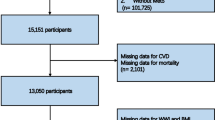
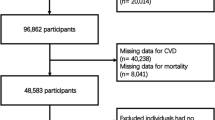
We accessed NHANES data spanning 1999 to 2020 and collected data on 116,876 individuals initially. Exclusion criteria were applied as follows: age below 20 years or at least 85 years, pregnancy or cancer, missing CAD data, and incomplete data on weight, height or waist preventing calculation of the WWI. Ultimately, a total of 49,240 patients were included in the analysis. The detailed patient selection and screening process is illustrated in Fig. 1.
Assessment of weight-adjusted-waist index
WWI was designed as an exposure variable in our study. WWI was an anthropometric index on WC and weight to estimate obesity, calculated using the formula7: \(\:\text{W}\text{W}\text{I}\:(\text{c}\text{m}/\sqrt{kg})\:=\:\text{W}\text{C}\:\left(\text{c}\text{m}\right)\:/\:\sqrt{\text{W}\text{e}\text{i}\text{g}\text{h}\text{t}}\left(\sqrt{\text{k}\text{g}}\right)\). The body measures data about WC and weight were collected in the MEC by trained health technicians.
Assessment of coronary artery disease
The primary outcome was the prevalence of CAD. The CAD data were derived from the NHANES questionnaire interviews, which were carried out by well-trained health professionals, and every question was standardized. Participants were asked the following questions: “Have you ever been told by a doctor or other health professional that you have CAD?”, “Have you been told by a doctor or other health professional that you have angina pectoris?”, and “Have you been told by a doctor or other health professional that you have a myocardial infarction?. If they answered “yes” to one or more of these questions, they were considered to have CAD7.
Data collection
The following patient information was also collected: Demographic data included age, sex, and race. Life Signs comprised BMI, weight, waist, systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse. Laboratory parameters were obtained from fasting blood samples and consisted of lipid profile (triglycerides [TG], low-density lipoprotein [LDL], high-density lipoprotein [HDL], total cholesterol [TC]), and metabolic indicators (fating plasma glucose [FPG] ), liver function markers (albumin, alanine aminotransferase [ALT], and aspartate aminotransferase [AST]), renal function markers (blood urea nitrogen [BUN] and creatinine [Cr]), complete blood count (white blood cell [WBC], lymphocyte [LY], monocyte [MONO], neutrophil [NE], red blood cells [RBC], hemoglobin [Hb], hematocrit [HCT], red cell distribution width [RDW], and platelets [PLT]). Questionnaire data encompassed smoking status and comorbidities status, including CAD, hypertension, diabetes, hypercholesterolemia, congestive heart failure (HF), stroke, and chronic pulmonary disease. Smoking was categorized as having smoked over 100 cigarettes within a year. All detailed measurement processes of these variables were publicly available at www.cdc.gov/nchs/nhanes/.
Statistical analysis
All experiments were adjusted for weighted variables and survey design to ensure that the included population was nationally representative. The data weight of 1999–2002 was obtained by multiplying the WTMEC4YR by 4/21.2. The data weight of 2017–2020 was obtained by multiplying the WTMECPRP by 3.2/21.2, while for other cycles, the WTMEC2YR was multiplied by 2/21.2.
Continuous variables were expressed as means with 95% confidence intervals (95% CI) and categorical variables were expressed as percentiles with 95% CI to illustrate variability and statistical reliability based on the data distribution. All participants were divided into four groups according to WWI quartiles: Q1 (WWI < 10.43, n = 12,310), Q2(10.43 < WWI < 10.99, n = 12,310), Q2(10.99 < WWI < 11.56, n = 12,310), Q4 (WWI > 11.56, n = 12,310). Differences across groups of WWI quartiles were examined for categorical or continuous variables using weighted t-tests or weighted chi-square tests. Three weighted logistic regression models were utilized to analyze the association between WWI and CAD. The results were expressed as odds ratios (OR) with 95% CI. Model 1 was unadjusted, while Model 2 accounted for sex, age, and race. Model 3 was further adjusted for additional parameters, including diabetes, stroke, smoke, albumin, ALT, BUN, Cr, MONO, NE, RBC, PLT. The selection of covariates in Model 3 were chosen based on clinical experience and stepwise regression, and only those variables with a significance level of p < 0.05 were retained. Subgroup analysis was conducted to estimate the consistency of the effect in different groups including sex, age (≤ 60, > 60years), race, BMI (≤ 28, > 28 kg/m2), SBP (≤ 140, > 140mmHg), WBC (≤ 10, > 10 × 10⁹/L), albumin (≤ 35, > 35 g/L), smoke, diabetes and stroke. These subgroups were categorized based on commonly used clinical thresholds. Shapes describing the association of WWI and BMI with prevalence of CAD were generated using the generalized additive model (GAM) and smoothed curve fitting. Receiver operative characteristic (ROC) analysis curves were performed to further evaluate the predictive efficacy of the WWI and BMI. The area under the curve (AUC) values were calculated and compared using the DeLong test. All statistical analyses were performed using the R software environment (Version 4.3.2; The R Foundation; available at http://www.R-project.org). A two-tailed p-value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the participants
49,240 individuals met the eligibility criteria and were included in the analysis (Fig. 1). There were 49.69% males and 50.31% females. Totally, 1,749 individuals experienced CAD, accounting for 2.96% of all participants.
The baseline characteristics were shown in Table 1. In higher quartiles of WWI, a notably higher rate of CAD was observed (P<0.001). Furthermore, individuals in these higher WWI groups tended to be females and had advanced age as well as higher levels of BMI, weight, waist, SBP, DBP, pulse, TG, LDL, TC, FPG, ALT, AST, BUN, WBC, LY, MONO, NE, RDW, PLT, CAD, hypertension, diabetes, hypercholesterolemia, congestive HF, stroke, chronic pulmonary disease and smoke, but lower levels of HDL, albumin, Cr, RBC, Hb, HCT than those in the lower WWI groups (all P < 0.001).
Relationship between WWI and CAD prevalence
In Model 1, using the Q1 group as the reference, a significant positive association between WWI and CAD was observed, with OR values of 2.86 (95% CI 2.11 to 3.88), 5.17 (95% CI 3.77 to 7.09), and 9.30 (95% CI 6.74 to 12.82) for the Q2, Q3, and Q4 groups respectively (P for trend < 0.001). In Model 2, after adjusting for sex, age and race, WWI still showed a positive correlation with CAD (Q4 vs. Q1: OR (95% CI): 2.49 (1.72, 3.59), p < 0.001, P for trend < 0.001). In Model 3, the WWI was still independently related to the increased risk of CAD (Q4 vs. Q1: OR (95% CI): 2.06 (1.37, 3.09), p = 0.001, P for trend < 0.001). Furthermore, when WWI was considered as a continuous variable in the model for analysis, we observed that for each unit increase in the WWI, the probability of developing CAD increased by 156% in Model 1 (p < 0.001), 56% in Model 2 (p < 0.001), 34% in Model 3 (p < 0.001) respectively (Table 2).
GAM and smoothed curve fitting for the association of WWI and CAD prevalence
The GAM and smoothed curve fitting revealed a positive relationship between WWI and the prevalence of CAD (Fig. 2A). As WWI increased, the prevalence of CAD exhibited an overall upward trend. Furthermore, the association between BMI and CAD demonstrated an inverted U-shaped pattern (Fig. 2B).
Subgroup analysis for the association of WWI and CAD prevalence
Subgroup analysis revealed differential associations between WWI and CAD across age groups. For participants ≤ 60 years old, the odds ratio (OR) was 1.71 (95% CI: 1.48, 1.98), while for those > 60 years old, the OR was 1.20 (95% CI: 1.09, 1.33) (Table 3). A significant interaction effect between age and WWI in relation to CAD risk was observed (P for interaction < 0.001). No statistically significant interactions were detected for other examined factors (all P for interaction > 0.05).
Comparative predictive performance of WWI and BMI for CAD prevalence
The ROC curve analysis showed that the AUC of WWI was 0.694(95% CI: 0.690, 0.698), and the AUC of BMI was 0.547(95% CI: 0.542, 0.551) (Fig. 3). WWI has moderate predictive ability for CAD, and its predictive ability was superior to BMI (De-long test P < 0.001). The best threshold of WWI was 11.23, and the sensitivity and specificity were 0.671 and 0.618, respectively.
Discussion
This study investigated the relationship between WWI and CAD prevalence using data of 49,240 participants from the NHANES. The main findings were: ①There was a significant positive association between WWI and CAD prevalence, which persisted after adjusting for confounding factors. ②Subgroup analysis indicated a stronger association between WWI and CAD in individuals aged ≤ 60 years compared to those > 60 years. ③ROC analysis showed that WWI had superior predictive performance for CAD compared to BMI. These results suggested that WWI could serve as a valuable tool for assessing CAD risk in the general population, potentially offering improved risk stratification compared to traditional anthropometric measures.
Obesity has long been recognized as a significant risk factor for cardiovascular disease11,12,13. Traditionally, BMI and WC have been the primary metrics used to assess obesity and its associated health risks14. Labounty TM et al. conducted a prospective study of 13,874 patients, revealing that obesity, as measured by BMI, was associated with an increased risk of CAD (OR 1.25 per + 5 kg/m2, 95% CI 1.20–1.30, P < 0.001)15. However, BMI has limitations in distinguishing between fat mass and lean mass. Consequently, researchers have explored alternative measures of adiposity. De Koning et al. performed a meta-analysis of 15 prospective studies (n = 258,114) and found that WC(RR 1.63, 95% CI 1.31–2.04) and waist-to-hip ratio (WHR) (RR 1.95, 95% CI 1.55–2.44)were more strongly associated with CAD risk than BMI16. More recently, the WWI has emerged as a novel anthropometric measure that combines elements of both WC and body weight7,17. WWI exhibits a positive correlation with the total abdominal fat area, visceral fat area, and the percentage of total tissue fat. Conversely, it shows a negative correlation with the appendicular skeletal muscle mass6. This characteristic of WWI may serve as an effective complement to the limitation of the BMI in reflecting central obesity. Previous studies have explored the relationship between WWI and cardiovascular outcomes. Fang et al. conducted a cross-sectional study of 21,040 US adults using NHANES data from 2009 to 2016, finding that each unit increase in WWI was associated with a 48% increased risk of cardiovascular disease (OR: 1.48, 95% CI: 1.25–1.74) after adjusting for covariates8. Han et al. examined 26,882 NHANES participants from 2005 to 2014 with a mean follow-up of 68.95 months, reporting that individuals with WWI ≥ 11.33 had a 95% higher risk of cardiovascular mortality (HR = 1.95, 95% CI 1.30–2.93) compared to those with lower WWI9. Qin et al. analyzed 3,082 adults aged ≥ 40 years from NHANES 2013–2014, demonstrating a positive association between WWI and abdominal aortic calcification scores (β = 0.34, 95% CI 0.05–0.63)10. These studies consistently showed that higher WWI levels were associated with increased cardiovascular risk. Several studies have indicated that other cardiometabolic indices, such as the triglyceride-glucose (TyG) index18, the stress hyperglycemia ratio (SHR)19, and the homeostasis model assessment of insulin resistance (HOMA-IR)20, were also related to cardiovascular risk, which typically rely on the combination of 2–3 laboratory test values. In contrast, the weight-adjusted waist index (WWI) utilizes anthropometric data, allowing for immediate risk assessment without the need for additional laboratory tests. This enhances the convenience of risk prediction by simplifying the parameters involved in the evaluation process.
This study provided compelling evidence for the association between WWI and CAD prevalence in a large, representative sample of US adults. Our study’s larger sample size (n = 49,240) and longer time frame (1999–2020) offered more robust and generalizable results. Our findings revealed a monotonic increasing relationship between WWI and CAD prevalence. However, the association between BMI and CAD did not exhibit a consistent pattern, displaying a non-monotonic relationship that complicates its use as a reliable predictor in cardiovascular risk assessment (Fig. 2B). DeLong’s test showed a superior predictive performance of WWI for CAD compared to BMI (P < 0.001). ROC analysis identified an optimal WWI threshold of 11.23 for CAD risk prediction, which could potentially be integrated with traditional risk factors and CKD metrics to enhance risk stratification frameworks. These findings underscored the limitations of BMI that were previously discussed, particularly its inability to accurately reflect central adiposity and its associated risks. In contrast, WWI emerged as a more effective predictor of CAD within our study cohort, offering a more consistent and robust association across varying levels of central adiposity. WWI was readily calculated using easily obtainable measurements (WC and weight), requiring no specialized equipment or invasive procedures. This simplicity rendered WWI an attractive candidate for large-scale screening programs or resource-constrained settings. The subgroup analysis revealed a more robust association between WWI and CAD in individuals aged ≤ 60 years, a finding of particular significance. This observation could be attributed to the cumulative effect of multiple risk factors in older adults, potentially attenuating the relative impact of WWI. Alternatively, it might have indicated that WWI was especially valuable for early risk stratification in younger cohorts. Notably, our subgroup analyses demonstrated consistent predictive performance of WWI across different sex and racial/ethnic groups, with no significant interaction effects (P for interaction > 0.05), suggesting its broad applicability across diverse populations. The enhanced predictive value of WWI in younger individuals aligns with emerging concepts in cardiovascular-kidney-metabolic (CKM) syndrome, where dysfunctional adipose tissue is recognized as a crucial early driver of CKM progression21. By enhancing early identification of at-risk individuals, particularly in younger demographics, WWI could potentially facilitate more efficient allocation of healthcare resources and mitigate the long-term economic burden of CAD. Given WWI’s stronger correlation with visceral adiposity, its implementation in screening protocols could potentially aid in early CKM risk stratification and intervention, particularly in younger populations.
The pathophysiological mechanisms underlying the association between increased WWI and CAD risk can be explained through several molecular and cellular pathways. Adipose tissue, particularly visceral fat, is metabolically active and secretes various adipokines and pro-inflammatory cytokines22. In an animal study, De Taeye et al. demonstrated that diet-induced obesity in mice led to increased expression of pro-inflammatory genes in adipose tissue, including TNF-α and IL-6, which contribute to systemic inflammation and insulin resistance23. At the cellular level, Mussbacher M et al. showed that adipocytes from obese individuals exhibit increased activation of the NF-κB pathway, promoting the release of inflammatory mediators that can directly impact vascular endothelial function24. Furthermore, excessive visceral adiposity is associated with altered lipid metabolism. Elevated free fatty acids induce oxidative stress and endoplasmic reticulum stress in endothelial cells, leading to endothelial dysfunction and accelerated atherosclerosis25,26. Additionally, obesity-induced leptin resistance could lead to sympathetic nervous system activation and increased blood pressure, further contributing to CAD risk27. A study demonstrated that a low eicosapentaenoic acid to arachidonic acid (EPA/AA) ratio is associated with increased vulnerability of coronary plaques, as assessed by optical coherence tomography (OCT), indicating a potential mechanistic link between altered lipid metabolism and plaque instability28. These mechanisms collectively elucidated how increased adiposity, as reflected by higher WWI, may contribute to the development and progression of CAD through inflammation, endothelial dysfunction, insulin resistance, and altered lipid metabolism.
Strengths and limitations
Our study has several notable strengths. First, we utilized two decades of nationally representative data from NHANES, with standardized measurements ensuring high data quality and reliability. Second, our study demonstrates the superior predictive performance of WWI compared to BMI through rigorous statistical analyses, including ROC curves and DeLong’s test. Third, we performed extensive subgroup analyses that validated WWI’s consistent performance across different demographic groups. Finally, our findings provide practical implications for clinical practice by identifying an optimal WWI threshold for CAD risk prediction.
This study had several limitations that should be considered when interpreting the results. Firstly, the cross-sectional design limited our ability to establish causal relationships between WWI and CAD prevalence. Secondly, the reliance on self-reported CAD diagnosis might introduce recall bias and potentially underestimate the true prevalence. Thirdly, the NHANES dataset, while representative of the US population, might not be generalizable to other populations with different ethnic compositions or lifestyle factors. Validation studies in other regions are essential, particularly in Asian populations where different patterns of central adiposity might necessitate population-specific WWI thresholds29. Fourthly, despite comprehensive adjustment for major confounders, residual confounding from unmeasured variables such as socioeconomic status and physical activity levels may still influence these associations, which needs to be considered in clinical application. Future research should address these limitations by conducting prospective studies with longer follow-up periods, incorporating more diverse populations, and including a wider range of potential confounding factors.
Conclusion
This study provided compelling evidence that the WWI is significantly associated with CAD prevalence in a large, diverse cohort from the NHANES dataset (1999–2020). Compared with BMI, WWI demonstrated superior predictive performance for CAD, particularly in younger individuals (aged ≤ 60 years). This finding highlighted the potential of WWI as a more sensitive indicator of cardiovascular health, which may enhance risk stratification and early intervention strategies. Given its simplicity, WWI can be easily incorporated into routine clinical assessments and large-scale public health initiatives, especially in resource-limited settings. Because of the cross-sectional nature of this study inherently limits causal inference, future prospective longitudinal studies are particularly needed to establish temporal relationships and potential causality between WWI and CAD development.
Data availability
Data are accessible in a public, open access repository. Open access data can be found on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
References
Roth, G. A. et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76 (25), 2982–3021 (2020).
Ralapanawa, U. & Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: a narrative review. J. Epidemiol. Glob Health 11 (2), 169–177 (2021).
Albar, H. M. et al. Prevalence of coronary artery disease and its risk factors in Majmaah City, Kingdom of Saudi Arabia. Front. Cardiovasc. Med. 9, 943611 (2022).
Haidar, A., Horwich, T. & Obesity Cardiorespiratory Fitness, and Cardiovascular Disease. Curr. Cardiol. Rep. 25 (11), 1565–1571 (2023).
Coutinho, T. et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of normal weight central obesity. J. Am. Coll. Cardiol. 61 (5), 553–560 (2013).
Kim, N. H., Park, Y., Kim, N. H. & Kim, S. G. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing 50 (3), 780–786 (2020).
Park, Y., Kim, N. H., Kwon, T. Y. & Kim, S. G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep. 8 (1), 16753 (2018).
Fang, H., Xie, F., Li, K., Li, M. & Wu, Y. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: a cross-sectional study. BMC Cardiovasc. Disord. 23 (1), 435 (2023).
Han, Y. et al. The weight-adjusted-waist index predicts all-cause and cardiovascular mortality in general US adults. Clin. (Sao Paulo) 78, 100248 (2023).
Qin, Z. et al. The association between weight-adjusted-waist index and abdominal aortic calcification in adults aged >/= 40 years: results from NHANES 2013–2014. Sci. Rep. 12 (1), 20354 (2022).
Powell-Wiley, T. M. et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 143 (21), e984–e1010 (2021).
Piche, M. E., Tchernof, A. & Despres, J. P. Obesity phenotypes, diabetes, and Cardiovascular diseases. Circ. Res. 126 (11), 1477–1500 (2020).
Elagizi, A., Kachur, S., Carbone, S., Lavie, C. J. & Blair, S. N. A review of obesity, physical activity, and Cardiovascular Disease. Curr. Obes. Rep. 9 (4), 571–581 (2020).
Grober-Gratz, D. et al. Body mass index or waist circumference: which is the better predictor for hypertension and dyslipidemia in overweight/obese children and adolescents? Association of cardiovascular risk related to body mass index or waist circumference. Horm. Res. Paediatr. 80 (3), 170–178 (2013).
Labounty, T. M. et al. Body mass index and the prevalence, severity, and risk of coronary artery disease: an international multicentre study of 13,874 patients. Eur. Heart J. Cardiovasc. Imaging 14 (5), 456–463 (2013).
de Koning, L., Merchant, A. T., Pogue, J. & Anand, S. S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur. Heart J. 28 (7), 850–856 (2007).
Li, Q. et al. Association of weight-adjusted-waist index with incident hypertension: the rural Chinese cohort study. Nutr. Metab. Cardiovasc. Dis. 30 (10), 1732–1741 (2020).
Liang, S. et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc. Diabetol. 22 (1), 170 (2023).
Zhao, S. et al. Association of the stress hyperglycemia ratio with coronary artery disease complexity as assessed by the SYNTAX score in patients with acute coronary syndrome. Diabetol. Metab. Syndr. 16 (1), 139 (2024).
Lee, J-H., Jeon, S., Joung, B., Lee, H. S. & Kwon, Y-J. Associations of Homeostatic Model Assessment for insulin resistance trajectories with Cardiovascular Disease incidence and mortality. Arterioscler. Thromb. Vasc. Biol. 43 (9), 1719–1728 (2023).
Ndumele, C. E. et al. A synopsis of the evidence for the Science and Clinical Management of Cardiovascular-kidney-metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 148 (20), 1636–1664 (2023).
Choi, S. H., Hong, E. S. & Lim, S. Clinical implications of adipocytokines and newly emerging metabolic factors with relation to insulin resistance and cardiovascular health. Front. Endocrinol. (Lausanne) 4, 97 (2013).
De Taeye, B. M. et al. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 293 (3), E713–E725 (2007).
Mussbacher, M. et al. Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front. Immunol. 10, 85 (2019).
Tabas, I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 107 (7), 839–850 (2010).
Khoi, C. S., Xiao, C. Q., Hung, K. Y., Lin, T. Y. & Chiang, C. K. Oxidative Stress-Induced Growth Inhibitor (OSGIN1), a Target of X-Box-Binding Protein 1, Protects Palmitic Acid-Induced Vascular Lipotoxicity through Maintaining Autophagy. Biomedicines 10(5), (2022).
Russo, B., Menduni, M., Borboni, P., Picconi, F. & Frontoni, S. Autonomic nervous system in obesity and insulin-resistance—the Complex interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 22 (10), 5187 (2021).
Hasegawa, T. et al. Serum n-3 to n-6 polyunsaturated fatty acids ratio correlates with coronary plaque vulnerability: an optical coherence tomography study. Heart Vessels 29 (5), 596–602 (2014).
Bajaj, S. S., Zhong, A., Zhang, A. L. & Stanford, F. C. Body Mass Index Thresholds for Asians: A race Correction in need of Correction? 1127–1129. (American College of Physicians, 2024).
Author information
Authors and Affiliations
Contributions
W.B. was responsible for conceptualization, methodology, data curation, conducted data analysis and writing the original draft. S.X. contributed to writing by reviewing and editing and supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, B., Sun, X. Weight-adjusted waist index shows superior detection of coronary artery disease than body mass index in NHANES 1999–2020. Sci Rep 15, 7077 (2025). https://doi.org/10.1038/s41598-025-90877-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90877-5
Keywords
This article is cited by
-
Exploring the association between relative fat mass and psoriasis risk: insights from the NHANES data
Lipids in Health and Disease (2025)