Abstract
The bio-electro-fenton (BEF) system is a novel technology that can be utilized to degrade both emerging and persistent pollutants while producing clean, green, and sustainable energy. Various catalysts that have a high active surface area are employed in these systems to enhance the oxygen reduction reaction (ORR) efficiency. In this study, the Nickel/Cobalt metal–organic framework (Ni/Co BTC-MOF) as heterogeneous catalyst was synthesized and deposited by the cathodic electrochemical deposition method on the carbon felt (CF) and graphite plate (GP) electrodes. The results of FT-IR, Field Emission Scanning Electron Microscopy (FE-SEM), X-ray Diffraction (XRD), and Energy Dispersive X-ray spectroscopy (EDS) analysis proved that the synthesis of Ni/Co-BTC MOF successfully carried out. The performance and positive effect of the modified electrodes in ORR were investigated and compared in electrical energy generation. Finally, bio-electro-degradation of bisphenol-S (BPS) as one of the endocrine-disrupting compounds (EDCs) was studied by the optimal modified electrode. According to the results of electrochemical experiments, the highest maximum power density is equal to 133.6 mW/m2, which is related to Ni/Co-BTC@CF, and the highest production voltage is related to Ni/Co-BTC@CF, Ni/Co-BTC@GP, CF, and GP, respectively. The removal efficiency levels of bisphenol S in this system at different concentrations of 1.0, 5.0, and 10.0 mg/l after 24 h were 98.0%, 84.0%, and 41.0%, respectively. Based on the obtained results, the improved BEF system with Ni/Co-BTC@CF catalyst can be a suitable technology to achieve more electricity flow and at the same time have a positive effect on the decomposition of bisphenol S pollutant.
Similar content being viewed by others
Introduction
Bio-electrochemical systems (BES) represent an innovative and environmentally friendly approach to recovering energy from biomass and treating wastewater, which has garnered considerable attention from researchers1,2,3. In the BESs, energy production occurs through oxidation and reduction reactions. Microorganisms play a crucial biocatalytic role by oxidizing organic carbon materials in the anode chamber, resulting in the production of electrons. These electrons can then be utilized to generate energy or to reduce and recycle metals4,5. All BESs rely on the electrochemical activity of microorganisms, enabling the decomposition of organic materials and the production of energy using bacteria6. Recent studies have shown that to significantly improve the efficiency of BESs in removing emerging contaminants, pharmaceutical compounds, and other persistent organic pollutants, these systems should be combined with advanced treatment processes. One effective approach for this combination is the use of advanced oxidation processes. Advanced oxidation processes, which come in various types, are highly effective at removing resistant and pharmaceutical organic compounds by generating highly reactive chemical oxidants, such as hydroxyl free radicals7,8,9. Because it produces homogeneous hydroxyl radicals, the electro-Fenton reaction is one of the most popular and extensively used advanced electrochemical oxidation techniques. Notwithstanding the many benefits of electro-Fenton processes, some drawbacks have led to a number of studies intended to address these issues7,10,11. Compared to conventional Fenton processes, research shows that the electro-Fenton process combined with BESs can greatly increase the efficacy of pollutant degradation. Known as bio-electro-Fenton (BEF) systems, these integrated systems use both biological and electrochemical processes to more effectively destroy persistent pollutants.
BEF systems also offer greater energy efficiency, particularly when renewable energy sources are incorporated. These systems are currently under research and development and have great potential to improve broad applications in the field of sustainable energy production. Additionally, they are more cost-effective and environmentally friendly than other Fenton-based methods, as they require fewer chemical reagents and produce hydrogen peroxide internally. Furthermore, BEF systems are easier to control and can achieve more precise treatment of pollutants8,12,13. In these systems, chemical energy found in wastewater can be transformed into electrical energy, hydrogen, and valuable compounds through the oxidation-reduction processes14,15. Equations 1–2 shows the reactions in the anode chamber16. After the decomposition of organic matter and the production of electrons, Next, the generated protons and electrons pass through the proton exchange membrane (PEM) and external circuit, respectively, to enter the cathode chamber17,18,19. Ultimately, H2O2 is continuously produced in the cathode chamber through the reduction of two oxygen electrons. The generated H2O2 is utilized to create homogeneous highly oxidative hydroxyl radicals (.OH) by establishing the Electro-Fenton process11,20. These radicals have a high destructive power and can degrade persistent organic pollutants (POPs)21. Persistent organic pollutants are broken down in BEF systems during reactions 4–67,22. These systems have been used to break down tetracycline, phenol, erthromycin, and other substances7,23,24.
Anodic reaction:
Cathodic reactions:
It is noteworthy that the selection of material type for modification of electrodes in the BEF is one of the important concepts. This point can affect the ORR efficiency with emphasis on the chemical and physical stability, porosity, surface area, and catalysis features of the selected material. Using cathodic catalysts improves electricity production, reduces energy loss, and gets rid of pollutants that are resistant to removal25,26,27,28,29. Various catalysts are employed in these systems to enhance the oxygen reduction reaction (ORR) efficiency. There is an increasing growth in the use of metal–organic frameworks (MOFs), as a novel class of nanoporous materials, to develop stable catalysts for BES. MOFs consist of hierarchically crystalline networks of electron-donor and electron-acceptor agents30,31,32 and have been utilized for various applications, including their use as adsorbents and catalysts in the removal and degradation of pollutants33,34. The use of MOFs as heterogeneous electrocatalyst has been facilitated by their features, which include high surface area, crystalline open framework, adjustable pore size, high conductivity, and thermal stability35,36,37,38. According to a study conducted in 2022 by Yang et al., the TiO2@ZIF-67/ZIF-8 MOF generated a higher maximum power density than ZIF-67/ZIF-8 when employed as a cathodic catalyst. As demonstrated by this study, MOFs serve as the structural backbone of composite materials, possessing integrity, stability, and high electrochemical activity. On the other hand, titanium dioxide provides the surface and centers required for catalytic reactions39.
It should be mentioned that despite the positive characteristics of these materials, instability in the aquatic environment is one of their defects40,41. One of the MOFs that has been introduced for a variety of applications is Ni/Co-BTC MOF. This MOF can be more stable in wet conditions because of the presence of two metallic cations, which increase its wet stability, specific surface area, catalytic points, and bond strength42,43,44,45. The Ni/Co-BTC MOF’s performance has been investigated in a number of applications. Benzene, toluene, ethylbenzene, and xylene isomers were extracted and analyzed from urine samples using the Ni/Co-BTC MOF as a solid adsorbent in a dynamic headspace-needle trap device in a previous study. The findings showed that this MOF provides a quick, easy, and accurate way to track unmetabolized BTEX compounds in urine biologically40. Co-MOF and Ni/Co-MOF catalysts were used in 2024 to improve antibiotic photocatalytic degradation. Cefoperazone was used as a model pollutant to test the efficacy of these synthesized catalysts when exposed to visible light. According to the study’s findings, 88.9% of the original cefoperazone was broken down under ideal circumstances. The Ni/Co-MOF catalyst’s photostability and reusability were also noteworthy; although its photodegradation efficiency was high, it decreased slightly from 98–72%46. The benefits of using this MOF in conjunction with Ni/MOF to improve electrocatalysis and supercapacitor performance were recently highlighted in a study. Because of its higher specific capacity, the results show that the Ni/Co-MOF outperforms the Ni/MOF and ZIF-67 in terms of supercapacitors. Furthermore, it significantly enhances the ORR’s electrocatalytic performance44.
Different techniques have been reported for synthesis and deposition of MOFs31,32,47. In this study, Ni/Co-BTC MOF was fabricated by cathodic electrodeposition (CED) technique in green conditions such as aqueous solution, room temperature, and atmospheric pressure30,31,47,48.
Eliminating persistent organic and emerging contaminants is the primary objective of employing BEF systems in conjunction with different catalysts23,24. Bisphenol S (BPS) is one prominent emerging contaminant. Emerging contaminants, or pollutants that are hard to biodegrade, have become more prevalent in recent years due to a combination of factors like population growth, lifestyle changes, and industrial expansion9,49. Drugs, personal care items, and endocrine-disrupting substances are the three general categories into which emerging contaminants fall. Endocrine disrupting chemicals are synthetic or natural chemical substances discovered in consumer goods or the environment that interfere with the body’s endocrine system by imitating or blocking endocrine hormones and harming living things50,51.
BPS is frequently used as an alternative to bisphenol A (BPA) in thermal paper products, personal care products, and other applications. Chemically speaking, BPS is an organic compound with the formula C12H10O4S that is made up of a sulfonyl group and two phenol functional groups. Because their benzene rings contain hydroxy groups, BPS and BPA are both categorized as endocrine disruptors52,53. The characteristics of BPS that contribute to its persistence in aquatic environments include a longer half-life, high skin permeability, and significant chemical inertness. BPS concentrations in a variety of water sources, such as raw water and wastewater effluents, have been estimated to range from 10 ng/L to 300 µg/L54. According to toxicological research, BPS is linked to metabolic and reproductive problems, a higher risk of cancer, and the potential to induce gene mutations. The breakdown of BPS using a variety of approaches, such as advanced oxidation, BES, activated carbon filtration, and biological degradation techniques, has been the subject of research in recent years55.
Even though there are many efficient techniques for getting rid of new pollutants, each one has drawbacks. As a result, numerous studies have sought to find and investigate novel methods that are more efficient in eliminating newly discovered pollutants while also being economical and energy-efficient. The objective is to reduce the risks that dangerous pollutants pose to the environment and human health. This study focuses on using BEF systems for the destruction of the pollutant BPS because of their advantages over conventional Fenton-based methods, including their high efficiency in removing pollutants and their capacity to use microorganisms to transform wastewater’s chemical energy into electrical energy.
According to the best of our knowledge, there is not any reported study employing the Ni/Co-BTC MOF film as a heterogeneous cathodic material in the BEF system for simultaneous removal of BPS and electrical energy generation. So, in this work, the Ni/Co-BTC MOF-modified electrodes have been fabricated by the CED technique in green conditions, and employed as a heterogeneous cathodic catalyst in the BEF system for bio-electro-degradation of BPS and in-situ electrical energy production.
Materials and methods
Chemical and materials
Bisphenol-S was provided from Sigma-Aldrich. Different chemicals required in the experiments, such as Benzene-1,3,5-tricarboxylic acid (H3BTC; 95%), Potassium hydrate (KOH; 99%), Sodium chloride (NaCl; 99%), Ethanol (C2H5OH; 99%), Perchloric acid (HClO4), Ammonium chloride (NH4Cl), Potassium dihydrogen phosphate (KH2PO4), Dipotassium hydrogen phosphate (K2HPO4), Sulfuric acid (H2SO4 95.0–97.0%), Glucose (C6H12O6, H2O), Magnesium sulfate (MgSO4), Potassium chloride (KCl), Calcium chloride (CaCl2), Sodium hydroxide (NaOH), Hydrochloric acid (HCl 37.0%), peptone, Heptahydrated ferrous sulfate (FeSO4·7H2O), Anhydrous sodium sulfate (Na2SO4), Acetonitrile (HPLC grade) were purchased from Merck company. Also, Nafion 117, Cobalt nitrate hexahydrate (Co (NO3)2·6H2O; 99%), and Nickel (II) nitrate hexahydrate (Ni (NO3)2⋅6H2O; 99%) were prepared from Aldrich Chemical Company. All of the electrosynthesis and electrodeposition procedures were done by distilled water (Millipore Milli-Q water purification instrument) at room temperature and atmospheric pressure. All of the analytical grade materials were used without extra purification.
Electrochemical synthesis and deposition of Ni/Co-BTC MOF
Modification of carbon felt (CF) and graphite plate (GP) electrodes with Ni/Co-BTC MOF films were performed in a three electrode home-made reactor by CED technique30,31,42,47. The designed three electrode home-made reactor was fabricated based on a beaker equipped with CF or GP (5.0 mm× 0.2 mm× 5.0 mm) as working electrode, two stainless steel plates as auxiliary electrodes, and Ag/AgCl as reference electrode. Electrochemical synthesis and deposition of Ni/Co-BTC MOF films were accomplished at green conditions (room temperature and atmospheric pressure).
For electrosynthesis and deposition of Co/Ni-BTC MOF films, 0.46 g Ni (NO3)2·6H2O, 0.46 g Co (NO3)2⋅6H2O as cation source, and 0.127 g KNO3 as supporting electrolyte were dissolved to 15.0 mL distilled water (as solution A). at the other hands, 0.42 g Trimesic acid (H3BTC) as ligand source was added in 15.0 mL ethanol (as solution B). At the next step, solution A was added to the solution B under vigorous stirring for 30 min before the electrochemical procedure. Modification of the CF and GP electrodes with the Ni/Co-BTC MOF film was performed by applying a suitable current density for a period of time (1.0 mA cm2−, 30 min) at the steady-state condition. By applying the suitable current density and starting the electrosynthesis and electrodeposition process, the activated form of ligands will be produced by in-situ electrogenerated hydroxide ions due to the electroreduction of water molecules on the cathodic electrode. Crystallization of Ni/Co-BTC-MOF on the cathodic electrode will be done by coordination of the activated ligands with the accessible cationic ions (Ni2+ and Co2+). At the end of process, the Ni/Co-BTC MOF film was synthesized and deposited onto the GP (Fig. 1) and CF (Fig. 2) electrodes30,31,47.
The final Ni/Co-BTC MOF modified electrodes were washed lightly with distilled water and ethanol to extraction of the unreacted raw materials from the porosities and cavities. The Ni/Co-BTC@CF and Ni/Co-BTC@GP modified electrodes were aged overnight at 100 ◦C for evaporation of trapped solvents and activation of bi-MMOF film40,41,42,43.
Setup and operation of reactor
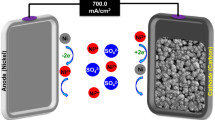
The BEF experiments were performed in a homemade reactor. Plexiglas sheets were used to build a two-chamber reactor including an anaerobic anodic and an aerobic cathodic chamber with a useful volume of 450.0 mL. A Nafion 117 membrane was used to separate the two chambers and pass the proton ions. Anodic and cathodic electrodes with sizes of (30.0 × 40.0 × 2.0) mm were connected to the data logger by a copper wire with a diameter of 0.5 mm and an external resistance of 1000 Ω4. To form the biofilm layer on the anodic electrode, the anaerobic sludge of Urmia sewage treatment plant was used to supply enriched anaerobic microorganisms. Before running, the modified electrodes were first soaked in 1.0 M HCl solution for 24 h, then in 1.0 M NaOH solution for 24 h, and finally washed twice with distilled water to remove the impurities. Also, to the creation of anaerobic and aerobic conditions, N2 gas and air pump were injected into the anodic and cathodic chambers, respectively14,56,57,58,59.
To set up the system, the anodic chamber was filled with synthetic wastewater made with glucose, NH4Cl, KCl, NaCl, CaCl2, MgSO4, K2HPO4, KH2PO4, 1.0 ml of trace elements, and the cathodic chamber was filled with phosphate buffer solution60. Phosphate buffer was consistently present during the investigation. The water matrix remained stable when phosphate buffer was present, regardless of whether the system was set up for electrode comparison or the BEF process for BPS degradation. The system with chemical oxygen demand (COD) equal to 2000.0 mg/L was set up with bared CF, Ni/Co-BTC@CF, bared GP, and Ni/Co-BTC@GP cathodes to compare and obtain the optimal electrode and catalytic effect of Ni/Co-BTC MOF by conducting electrochemical tests. Finally, to establish the electro-fenton process, Na2SO4 as a supporting electrolyte with a concentration of 0.1 M and ferrous sulfate powder with a concentration of 5.0 mg/L were added to the chamber7,61. As a diagnostic test, BPS decomposition analysis was done in different concentrations in the cathodic chamber. Figure 3 shows a schematic view of a BEF reactor.
Instrumentation of characterization
Investigating the morphological and surface characteristics of bared GP, Ni/Co MOF@GP, bared CF, Ni/Co MOF@CF electrodes as cathodic electrodes and the anodic electrode before and after biofilm formation were performed using a field emission scanning electron microscope (FE-SEM) (TESCAN MIRA3) with 15 kW accelerator voltage was investigated. A Perkin Elmer FT-IRGX spectrometer was employed for recoding FT-IR spectra of deposited MOF films. X-ray diffraction (XRD) analysis was performed to investigate the crystal structure of Ni/Co MOF@GP and Ni/Co MOF@CF electrodes. This analysis was done with Rigaku Ultima IV using copper element (λ = 0.154 nm). It was done at 40KW voltage and 40 mA current and the diffraction patterns were investigated at 2θ angle in the range of 5–80 degrees. Finally, the Energy Dispersive X-ray spectroscopy (EDS) detector (BRUKER XFLASH 6/10) was used to determine the elemental composition of Ni/Co MOF@GP and Ni/Co MOF@CF electrodes.
Electrochemical analysis
For the automatic recording of the voltage and power of the system with different electrodes, a data logger device manufactured by Danesh Gostar Hamgam Ba Sanat Company (Babol-Iran) was employed. The open circuit voltage (OCV) of the system was recorded and the time curve for the voltage to stabilize was obtained for all electrodes. The polarization and power density curves were determined by applying different external resistances (0.1–1000.0 Ω) for the system with different electrodes. Finally, the power density (P = RI2/A) and the current density (I = V/RA) were obtained by considering the surface area of the anodic electrode62,63.
Chemical analysis
All tested samples from the anodic and cathodic chambers were filtered with 0.45 μm filters before the desired analyses. Analysis of BPS was performed in concentrations of 1.0, 5.0, and 10.0 mg/L at different times using high-performance liquid chromatography (HPLC) (Agilent 1260 Infinity HPLC) with UV-Vis detector and C18 chromatography column during 254.0 nm wavelength. The HPLC mobile phase consisted of acetonitrile/water with a ratio of 40.0/60.0 at a temperature of 25 ◦C53,64. Finally, the removal efficiency of BPS was obtained using Eq. 4:
Where C0 and Ct are respectively the concentration of BPS before and after the BEF process.
The changes in the amount of COD were investigated simultaneously with the purification of BPS in different concentrations using a COD reactor in closed reflux conditions and colorimetry according to D5220 method of the standard book14.
Results and discussion
Characterization of modified electrodes
The Ni/Co-BTC MOF modified electrodes were characterized by FT-IR, XRD, FE-SEM, EDS, and mapping analysis techniques for investigating and obtaining further insight into the morphological properties and structural characteristics, before electrochemical investigations. Similar to earlier research, the discovered crystal structure sufficiently demonstrates the synthesis of Ni/Co MOF on CF and G65.
Figure 4 shows the FT-IR spectra of Trimesic acid ligand and as-synthesized Ni/Co-MOF films on the CF and GP surfaces to investigate the functional groups and bonding. The absence of the carboxylic acid group peaks (3260.0–2470.0 cm− 1) in the MOFs spectra, absence of characteristic free C = O functional groups in 1722.0 cm− 1, shifting of the characteristic couple bands from 1722.0 to 1608.0 cm− 1 and 1455.0-1404.0 cm− 1 in the ligand spectrum to 1671.0–1524.0 cm− 1 and 1473.0-1372.0 cm− 1 in the MOFs spectra, can be assigned to the contribution of these functional groups in the formation of the MOF structures. These results are similar to the scratched MOF films from CF and GP films and consistent with the previously reported studies30,31,40,42.
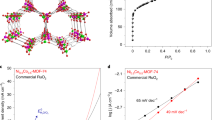
The XRD patterns of Ni/Co-MOF electrodeposited films onto the CF and GP electrodes were recorded for the evaluation of X-ray crystallography information (Fig. 5). The recorded XRD patterns of both Ni/Co-MOF deposited films onto CF and GP electrodes proved three distinguished peaks of Ni/Co-BTC MOF placed at the 2θ (17.6°, 18.7°, and 26.7°) which can be related to plan of lattice structures (220, 111, and 311). According to this patterns and in consistent with previously reported studies, Ni/Co-BTC MOF has been electrodeposited onto the CF and GP electrodes41,42,43.
The X-ray energy diffraction spectroscopy (EDS) analysis of modified electrodes was performed for the evolution of the elemental analysis and elemental mapping electrodeposited films. Figures 6 and 7 (up section) illustrated the characteristic peaks of Ni and Co elements in addition to the presence of O and C elements that can be assigned to the uniform deposition of Ni/Co MOF film onto the CF and GP electrodes, respectively. The superior purity of the implanted Ni/Co-BTC MOF is guaranteed by the lack of additional energy peaks in the EDS spectrum. Furthermore, Ni and Co elements are distributed uniformly and appropriately in carbon and oxygen clouds, as demonstrated by elemental mapping images onto the CF and GP surfaces, respectively (Figs. 6 and 7-down section).
The morphology of the synthesized and deposited MOF thin films are the effective parameters on the electrochemical experiments that can be followed by FE-SEM images. Figure 8 illustrates recorded FE-SEM images of bared and modified electrodes with Ni/Co-BTC MOF film in different magnifications. Compared with bared electrodes (Fig. 8a and d), modified electrodes show full coverage of the under layer substrate by the hierarchical microstructure of the MOF crystals. Furthermore, compared with the CF (Fig. 8b and c) and GP (Fig. 8d and f) modified electrodes, growth of needle- and cubic-shaped 3D hexagonal cylindrical microcrystals with nearly uniform configuration can be seen which are confirmed by the results of earlier investigations40,41,42. It should be noted that the needle shape and smaller size of the deposited crystals onto the CF compared to cubic shape and larger size of the GF electrode can be assigned to the applied electrochemical technique (galvanostatic conditions)30,31 and lager surface area of the CF electrode.
MOFs as heterogeneous electrocatalyst has eye-catching features such as high surface area, crystalline open framework, adjustable pore size, high conductivity, and thermal stability. According to the previous reports, low conductivity and poor stability of the MOFs can be a negative point of them for using in the electrochemical wet experiments40,41,43. This concern will be addressed by utilizing of two metallic cations MOF modified electrodes with suitable structural stability and conductivity32,42. In the simultaneous synthesis and deposition of Ni/Co-BTC MOF film via the CED technique, Upon the applying constant current electrolysis, the increased local pH at the cathodic surface electrode due to the gradual in-situ electrogeneration of hydroxide ions (OH−) by electroreduction of water, make regular deprotonation of ligands (H3btc to btc3−). The activated ligands (btc3−) in the vicinity of cathodic surface electrode could be coordinated to the available cations (Ni2+ and Co2+) to starting crystallinity of Ni/Co-BTC-MOF. Eventually, nucleation and growth of Ni/Co-BTC-MOF are managed on the cathodic surface electrode without the need for any ex-situ base/probes at the aqueous solution with the dual task as a green solvent and hydroxide source30,47.
Performance of Ni/Co-BTC MOF in electricity production
An investigation was conducted on Ni/Co-BTC MOF catalytic efficiency in the BES system using bared CF, Ni/Co-BTC@CF, bared GP, and Ni/Co-BTC@GP as cathodic electrodes under constant conditions. The generated voltage by each cathode and the stability of modified electrodes with Ni/Co-BTC MOF were monitored using the OCV curve over four-time intervals (Fig. 9). Figure 9a indicates an advantageous impact of Ni/Co-BTC MOF film on the generated voltage during each time. So, the maximum output voltage for Ni/Co-BTC@CF; Ni/Co-BTC@GP; CF, and GP were 580.0 mV, 490.0 mV, 380.0 mV, and 331.0 mV, respectively. The stability and reusability of the electrodes can also be examined by OCV testing66. The stability of Ni/Co-BTC MOF on CF and GP electrodes can be seen using Fig. 9a. The results show that the maximum voltage generated by the BES system with the Ni/Co-BTC@GP cathode decreases with time and subsequent system startup. In fact, on the fourth day, it decreased by 92.82 mV compared to the first day, indicating the gradual loss of the catalyst. While the maximum voltage of the system with the Ni/Co-BTC@CF cathode was constant in all four periods. These results indicate that the Ni/Co-BTC MOF catalyst on CF is stable and the Ni/Co-BTC@CF electrode is reusable. In another study, the maximum production voltage and maximum power density could be observed to benefit from modified carbon when utilizing various catalysts, as demonstrated by the OCV of BEF systems. For instance, in Xiang et al. study, MFC-FeVO4/CF’s OCV was equal to 980.0 mV, 75.0% higher than MFC-CF7,67.
By adjusting external resistances, the polarization curve and power density-current density were examined to further insight into the catalytic function of Ni/Co-BTC MOF catalyst. It is noteworthy that there was a direct correlation between the system resistance and current density, where higher resistance is equal to the lower current density. As shown in Fig. 9b, the maximum power density of Ni/Co-BTC@CF was 133.6 mW/m2, which is 1.78 times bared CF. Furthermore, the maximum power density of Ni/Co-BTC@GP was 90.44 mW/m2 which is 1.62 times bared GP. These results can be seen from two viewpoints. First, the key role of supported material onto the bared electrodes, and second, the important role of the underlying substrate. According to the obtained results, the maximum power density of the modified electrodes is better than the bared electrodes. Therefore, Ni/Co-BTC MOF can have a positive impact on BES due to the enhanced wet stability, porosity, surface area, and electrical conductivity. This point can be assigned to the simultaneous presence of the two metallic cations in the MOF structure consistent with the previously reported documents. The maximum power obtained from the microbial fuel cell with Ni-Co alloy was 1.27 times that of carbon cloth in the study by Powell et al., which was conducted to investigate Co-Ni catalyst effect in the microbial fuel cell68. A selection of MOF-based catalysts utilized in BES is displayed in Table 1.
On the other hand, according to the above-mentioned data, the maximum power density of the modified CF electrode is better than the modified GP electrode. So, the modified CF can have a positive impact on BES due to the enhanced mechanical stability, porosity, and significant active surface area. Das et al. confirmed suitable efficiency of Zr based MOF in bio-electrochemical system by modifying carbon electrode70. CF electrodes and electrodes modified with catalysts, whose base is CF, usually have high surface area and porosity, which makes them more compatible and stable. In various studies, researchers have used CF cathode electrodes modified with different catalysts to increase hydrogen peroxide production. Xu et al., in a study aimed at treating coal wastewater, operated a BEF system with a modified cathode, in which the cathode was modified with a FeVO₄ film on the CF electrode. The results of this study showed an increase in power density and current production following the electrode modification18. In a different study, Mi et al. employed RCW nanosheets as catalysts to remove ciprofloxacin from a CF cathode electrode. The results of the SEM analysis verified that RCW was completely deposited on the rough and porous CF surface. in order to raise the process’s power density and ultimately achieve 100% removal of the targeted antibiotic after an hour76.The close-up and distant views of the Ni/Co-BTC@CF electrode (8b, 8c) show how well the needle-shaped microcrystals of Ni/Co-BTC MOF fit into the coil-like space and pores of CF. Ni/Co-BTC MOF is a good choice for reactions involving oxygen reduction (ORR) in BES because of its accessible crystal morphology, adjustable pores, wet stability, and high surface area. Therefore, Ni/Co-BTC@CF can have additional potential for oxygen diffusion and electron acceptor due to the electrochemical analysis results and electrode morphological features. Along with the FE-SEM results, the obtained data from the polarization curves and power-current wells (Table 2) demonstrate the improved efficiency of Ni/Co-BTC@CF over Ni/Co-BTC@GP electrode.
Performance of Ni/Co-BTC@CF in BEF degradation of bisphenol-S
A BEF system was designed to degrade BPS contaminants using a Ni/Co-BTC@CF electrode based on the morphological analyses of various cathodic electrodes and obtained data from electrical analyses. The impact of initial concentration and reaction time on the efficiency of BPS removal is depicted in Fig. 10. The BEF system efficiency increases with time, as demonstrated by comparing BPS removal efficiency in 4.0, 12.0, and 24.0 h at different concentrations. This steep slope can be explained by the gradual rise in hydrogen peroxide production, which in turn led to an increase in hydroxyl radical production. Given that the majority of emerging pollutants are resistant to breakdown, aging and prolongation of the decomposition process improve the system ability to break down and degrade the targeted pollutant, The impact of the initial pollutant concentration on the BPS removal rate revealed that the BPS removal efficiency was 98.0%, 84.0%, and 41.0%, respectively, at concentrations of 1.0, 5.0, and 10.0 mg/L.
Figure 10d demonstrates that cathode COD removal efficiency increased by decreasing BPS concentrations. So that, the cathode COD removal efficiency at a concentration of 1.0 mg/L BPS was 1.14 times greater than that of the cathode at a concentration of 10.0 mg/L BPS.
The degradation of bisphenol S over a 48-h operating period was also examined in this study. Nevertheless, the findings demonstrated that the degradation efficiency did not significantly alter after a day and at the optimal operating time, satisfactory results of bisphenol S degradation were observed.
In the researcher’s previous study, we looked into and measured the amount of H2O2 produced in the BEF system7. The quantity of H2O2 generated in the system has been continuously tracked throughout the course of this investigation. The BEF system’s electrochemical H2O2 production typically occurs in three stages: the descending stage, the steady-state stage, and the ascending production stage. The H2O2 reagent is gradually produced in the cathode chamber in the first stage following the activation of the BEF system. The H2O2 equivalent then stays constant at its maximum concentration based on the rate of production and breakdown in the steady-state stage. Lastly, because of its consumption in the Fenton reaction, the H2O2 concentration progressively drops during the descending stage7.
In this study, the degradation of the BPS pollutant in the BEF system at neutral pH was investigated. The majority of research indicates that Fenton-based processes and pollutant degradation are more effective at acidic pH values between 2 and 3. At pH values higher than 4, the formation of ferrous/ferric hydroxide complexes results in catalyst deactivation, which lowers the amount of .OH. However, the performance of pollutant degradation is also inhibited when the pH is below 213. Nonetheless, some research shows that BEF systems can function at neutral pH67. Investigating this process at neutral pH is justified by the following factors: increased treatment costs and sludge production due to acidic conditions. Actually, changing the pH both before and after the process raises operating expenses, which is a significant drawback in and of itself11. In a study aimed at using a BEF equipped with an anode and cathode modified with PPy/AQDS for the degradation of azo dyes under neutral conditions in the cathode chamber, it was carried out because the mineralization of organic pollutants is more likely to occur at neutral pH. The azo dye mineralization in this study was also positively impacted by the use of PPy/AQDS modified anode and cathode77. The impact of acidic cathode conditions on the biological component of the system is an important consideration. Since the BEF system depends on the presence and activity of microorganisms, acidic cathode chamber conditions may affect proton transfer and interfere with anode chamber microorganism activity. Additionally, a study at neutral pH may be helpful for the practical application of these systems in industries11,77. Therefore, the effectiveness of the BEF system equipped with a cathode modified with Ni/Co-BTC MOF for the destruction of bisphenol S pollutant in neutral conditions has been investigated in the current study, taking into account the operating costs, the impact of pH on the activity of microorganisms, and wastewater conditions on real scales.
Compared to the outcomes of the BPS biological experiment, the current study findings indicated that combining the biological process with electro-fenton may be an appropriate option for removing this pollutant. In the anode compartment of the BES system, Shi et al. also reported the breakdown of BPS at a concentration of 5.0 mg/L using a carbon anode electrode modified with NZVI-HA, NZVI, and HA as 92.2%, 78.4%, and 62.2%, respectively52.
Promising results are reported by electro-fenton analysis of other emerging pollutants and dyes in BESs (Table 3). In this system, tetracycline at a concentration of 5.0 mg/L was destroyed between 91% and 99.04% in 24 h7. Using a catalyst modified with powdered activated carbon and iron oxide, sodium didecyl sulfate was destroyed in the BEF system in 120 min with an approximate 78.0% reduction in activity78.
According to Table 3, the type of electrodes and membranes used as well as the pollutant removal efficiency at various operating times determine how effective these systems are. Among these studies, the BEF system for phenol degradation has the highest removal rate and power generation with a removal efficiency of 100% and a maximum power output of 1746 mW/m279. The current study aimed at the degradation of BPS in the BEF system with a modified cathode, shows a degradation efficiency of 98% at a concentration of 1.0 mg/L and a power density of 88 mW/m2 in an operating time of 24 h.
Figure 11 displays the power density and polarization curve of the BEF system at various BPS concentrations. The maximum power density was 88.0–60.47 and 42.7 mW/m2, respectively, in concentrations of 1.0, 5.0, and 10.0 mg/L BPS. The findings indicate that when the concentration of pollutants in the system rises, the energy generated within the system is utilized for additional pollutant breakdown, resulting in a decrease in the maximum power density.
Characterization of bio-anode
Anaerobic sludge was utilized in the anode chamber and inoculated with electrogenic microorganisms in order to create a biofilm layer on the anode. And it became evident as time went on that the biofilm layer was forming. Figure 12 displays FE-SEM images of the anode electrode prior to and following the formation of the biofilm. Because of the porous and pore-filled surface of CF and the fact that enough time was allowed for the formation of a biofilm layer on the anode electrode at the start of the project and prior to the main experiments, biofilm colonies of target bacteria can be seen on the CF filaments in Fig. 12b and c, which is in line with previously published documents14. The system’s ability to carry out electrochemical and BES reactions was shown by the FESEM test and the observation of the biofilm layer on the anode electrode. Furthermore, as BES was implemented, the system’s production power increased over time, confirming that the required amount of biofilm had formed. Prior research has demonstrated the growth of bacteria with a 1.0 mm length and bacillary shape. Schwannella, Geobacter, Arcobacter, Cucamonas, Dichloromonas, Sphingobacterium, Desulfobulbacterium, Firmicutes, and Clostridium are the bacteria that primarily reside in biofilms4,7,16. These electrogenic bacteria use two mechanisms to transfer electrons to the electrode: direct and indirect electron transfer. In direct transfer, oxidation-active proteins like cytochromes C-type or nanowires carry electrons to the electrode. Actually, conductive nanowires and cytochromes are features of microorganisms that aid in the movement of electrons from the interior of the cell to the electrode surface. This method is highly efficient due to the direct contact of microorganisms with the electrode. Some bacteria also have a pilus protein that acts like nanowires and plays the role of transferring electrons to the electrode. The second method, which is indirect, is that microorganisms transfer electrons to the electrode through electron mediators such as flavin, quinone, etc., which is called mediated transfer16.
Conclusion
In this study, the BEF system assisted by Ni/Co BTC-MOF modified electrode as a novel technology utilized in terms of energy production and pollutant degradation of bisphenol S. The Ni/Co BTC@CF was synthesized by the cathodic electrochemical deposition method and carefully analyzed and optimized as a cathodic electrode in the BEF system. The Ni/Co-BTC MOF catalyst’s organic framework gives it a large and porous surface area, as demonstrated by FESEM tests. More active sites are available for the adsorption of oxygen molecules to the catalyst due to its large surface area. Conversely, the presence of two metal ions Ni and Co as metallic catalyst breaks the oxygen molecule’s double bond and creates active oxygen ions. The ORR happens more quickly as a result of these two processes. These two metals’ presence also enhances electron transfer and lowers the energy needed to complete the reaction. High selectivity for ORR is also caused by the selectivity feature of MOFs, such as Ni/Co-BTC MOF.
OCV, polarization, and power-current density were among the electrochemical analyses used to examine the Ni/Co-BTC MOF catalyst’s performance. The catalyst’s electrical potential and stability are revealed by the OCV analysis. The Ni/Co-BTC MOF-modified electrodes generated a higher maximum voltage in the system than the CF and GP electrodes, according to the OCV results. Additionally, the catalyst’s stability on the CF electrode was ascertained. The polarization and power-current density curves aid in assessing the catalyst’s performance and offer details on how the catalyst behaves in the system. so that it was feasible to compare the electrodes’ current and power production through these analyses. These analyses’ findings demonstrated that adding the Ni/Co-BTC MOF catalyst to the electrodes enhanced the system’s power. With power densities of 133.6 and 90.44 mW/m2, respectively, the Ni/Co MOF@CF and Ni/Co MOF@GP electrodes outperformed the CF and GP electrodes among the four electrodes.
The degradation of pollutants in the BEF system may be directly impacted by the Ni/Co-BTC MOF catalyst’s beneficial effect on ORR. because the electro-Fenton reactions in the cathode chamber may be impacted by ORR. The Ni/Co-BTC MOF catalyst improves ORR, which in turn improves H2O2 production and, eventually, hydroxyl radical production. One can anticipate an improvement in the degradation of pollutants in the BEF system given the damaging effect of hydroxyl radicals. The current study’s findings demonstrated that bisphenol S, at a concentration of 1 mg/L, had a 98% removal efficiency in a 24-hour period in the BEF system fitted with the Ni/Co-BTC MOF catalyst.
Based on the obtained results; the improved BEF system with Ni/Co-BTC@CF catalyst can be a suitable technology to achieve more electricity flow and at the same time have a positive effect on the decomposition of bisphenol S pollutant.
Data availability
The authors declare that the all data generated or analyzed during this study are included in this published article.
References
Wang, H. & Ren, Z. J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 31, 1796–1807 (2013).
Rozendal, R. A., Hamelers, H. V. M., Rabaey, K., Keller, J. & Buisman, C. J. N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 26, 450–459 (2008).
Rashadi, F., Navidjouy, N., Aghapour, A. A. & Rahimnejad, M. Application of Dual Chamber Microbial Fuel Cell with Aeration Cathode for Bioelectricity Generation and Simultaneous Industrial Wastewater Treatment (2021).
Rahmani, A. R. et al. Application of the eco-friendly bio-anode for ammonium removal and power generation from wastewater in bio-electrochemical systems. J. Clean. Prod. 243, 118589 (2020).
Rahimnejad, M., Bakeri, G., Najafpour, G., Ghasemi, M. & Oh, S. E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 1, 7–15 (2014).
Wang, A. J., Liang, B., Li, Z. L. & Cheng, H. Y. Bioelectrochemistry Stimulated Environmental Remediation (Springer, 2019).
Soltani, F., Navidjouy, N., Khorsandi, H., Rahimnejad, M. & Alizadeh, S. A novel bio-electro-Fenton system with dual application for the catalytic degradation of Tetracycline antibiotic in wastewater and bioelectricity generation. RSC Adv. 11, 27160–27173 (2021).
Soltani, F., Navidjouy, N. & Rahimnejad, M. A review on bio-electro-Fenton systems as environmentally friendly methods for degradation of environmental organic pollutants in wastewater. RSC Adv. 12, 5184–5213 (2022).
Esfandiaribayat, M. et al. Tetracycline removal from wastewater via g-C3N4 loaded RSM-CCD-optimised hybrid photocatalytic membrane reactor. Sci. Rep. 14, 1163 (2024).
Pignatello, J. J., Oliveros, E. & MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 36, 1–84 (2006).
Feng, C. H., Li, F. B., Mai, H. J. & Li, X. Z. Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment. Environ. Sci. Technol. 44, 1875–1880 (2010).
Olvera-Vargas, H., Trellu, C., Oturan, N. & Oturan, M. A. Electro-Fenton Process: New Trends and Scale-Up 29–56 (eds Zhou, M.) (Springer, 2018).
Li, X., Chen, S., Angelidaki, I. & Zhang, Y. Bio-electro-Fenton processes for wastewater treatment: advances and prospects. Chem. Eng. J. 354, 492–506 (2018).
Rahmani, A. R. et al. Effect of different concentrations of substrate in microbial fuel cells toward bioenergy recovery and simultaneous wastewater treatment. Environ. Technol. 43, 1–9 (2022).
Li, S., Hua, T., Li, F. & Zhou, Q. Bio-electro‐Fenton systems for sustainable wastewater treatment: mechanisms, novel configurations, recent advances, LCA and challenges. An updated review. J. Chem. Technol. Biotechnol. 95, 2083–2097 (2020).
Navidjouy, N., Soltani, F. & Rahimnejad, M. Biological Fuel Cells 301–320 (Elsevier, 2023).
Birjandi, N., Younesi, H., Ghoreyshi, A. A. & Rahimnejad, M. Enhanced medicinal herbs wastewater treatment in continuous flow bio-electro-fenton operations along with power generation. Renew. Energy 155, 1079–1090 (2020).
Xu, P., Xu, H. & Shi, Z. A novel bio-electro-fenton process with FeVO4/CF cathode on advanced treatment of coal gasification wastewater. Sep. Purif. Technol. 194, 457–461 (2018).
Ebrahimzadeh, P., Navidjouy, N., Khorsandi, H. & Rahimnejad, M. Application of bioelectrochemical system in nitrogen removal via simultaneous autotrophic nitrification and denitrification from wastewater. ChemElectroChem 11, e202400432 (2024).
Ling, T., Huang, B., Zhao, M., Yan, Q. & Shen, W. Repeated oxidative degradation of Methyl orange through bio-electro-fenton in bioelectrochemical system (BES). Bioresour. Technol. 203, 89–95 (2016).
Raj, R., Sathe, S. M., Das, S. & Ghangrekar, M. M. Nickel–iron-driven heterogenous bio-electro-fenton process for the degradation of methylparaben. Chemosphere 341, 139989 (2023).
Zhao, Q., An, J., Wang, X. & Li, N. In-situ hydrogen peroxide synthesis with environmental applications in bioelectrochemical systems: a state-of-the-art review. Int. J. Hydrog. Energy 46, 3204–3219 (2021).
Li, B. et al. Enhanced bio-electro-fenton degradation of phenolic compounds based on a novel Fe–Mn/graphite felt composite cathode. Chemosphere 234, 260–268 (2019).
Li, S. et al. Microbial electro-Fenton: A promising system for antibiotics resistance genes degradation and energy generation. Sci. Total Environ. 699, 134160 (2020).
Li, M., Zhou, S. & Xu, M. Graphene oxide supported magnesium oxide as an efficient cathode catalyst for power generation and wastewater treatment in single chamber microbial fuel cells. Chem. Eng. J. 328, 106–116 (2017).
Satar, I. et al. Performance of titanium–nickel (Ti/Ni) and graphite felt-nickel (GF/Ni) electrodeposited by Ni as alternative cathodes for microbial fuel cells. J. Taiwan Inst. Chem. Eng. 89, 67–76 (2018).
Valipour, A., Ayyaru, S. & Ahn, Y. Application of graphene-based nanomaterials as novel cathode catalysts for improving power generation in single chamber microbial fuel cells. J. Power Sources 327, 548–556 (2016).
Shan, Y., Cui, J., Liu, Y. & Zhao, W. TiO2 anchored on MoS2 nanosheets based on molybdenite exfoliation as an efficient cathode for enhanced cr (VI) reduction in microbial fuel cell. Environ. Res. 190, 110010 (2020).
Mashkour, M., Rahimnejad, M., Raouf, F. & Navidjouy, N. A review on the application of nanomaterials in improving microbial fuel cells. Biofuel Res. J. 8, 1400–1416 (2021).
Alizadeh, S. & Nematollahi, D. Electrochemically assisted self-assembly technique for the fabrication of mesoporous metal–organic framework thin films: composition of 3D hexagonally packed crystals with 2D honeycomb-like mesopores. J. Am. Chem. Soc. 139, 4753–4761 (2017).
Alizadeh, S. & Nematollahi, D. Convergent and divergent paired electrodeposition of metal-organic framework thin films. Sci. Rep. 9, 14325 (2019).
Rahimpoor, R., Soleymani-Ghoozhdi, D., Firoozichahak, A. & Alizadeh, S. Needle trap device technique: from fabrication to sampling. Talanta 1, 126255 (2024).
Kalashgrani, M. Y. et al. Synthesis of isoreticular metal organic framework-3 (IRMOF-3) porous nanostructure and its effect on naphthalene adsorption: optimized by response surface methodology. Separations 10, 261 (2023).
Zhang, L. et al. A novel metal organic framework-derived carbon-based catalyst for oxygen reduction reaction in a microbial fuel cell. J. Power Sources 384, 98–106 (2018).
Zhao, X. et al. Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water. Sci. Rep. 5, 11849 (2015).
Gangu, K. K., Maddila, S., Mukkamala, S. B. & Jonnalagadda, S. B. A review on contemporary metal–organic framework materials. Inorg. Chim. Acta 446, 61–74 (2016).
Tajik, S. et al. Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A 9, 8195–8220 (2021).
Shah, S. S. A. et al. Recent trends in wastewater treatment by using metal-organic frameworks (MOFs) and their composites: A critical view-point. Chemosphere 349, 140729 (2024).
Yang, J. et al. Improving oxygen reduction reaction of microbial fuel cell by titanium dioxide attaching to dual metal organic frameworks as cathode. Bioresour. Technol. 349, 126851 (2022).
Rahimpoor, R. et al. Bio-monitoring of non-metabolized BTEX compounds in urine by dynamic headspace-needle trap device packed with 3D Ni/Co-BTC bimetallic metal–organic framework as an efficient absorbent. Microchem. J. 166, 106229 (2021).
Ghamari, F., Raoufi, D., Alizadeh, S., Arjomandi, J. & Nematollahi, D. Construction of highly efficient new binder-free bimetallic metal–organic framework symmetric supercapacitors: considering surface statistical and morphological analyses. J. Mater. Chem. A 9, 15381–15393 (2021).
Souri, Z., Mazloum-Ardakani, M., Alizadeh, S. & Nematollahi, D. Template-free electrodeposition of sponge-like porous polymer interwoven with the bi-metallic metal–organic framework and reduced graphene oxide and application in energy storage device. J. Energy Storage 55, 105381 (2022).
Rahimpoor, R. et al. Determination of halogenated hydrocarbons in urine samples using a needle trap device packed with Ni/Zn–BTC bi-MMOF via the dynamic headspace method. RSC Adv. 11, 21537–21547 (2021).
Xia, H. et al. 2D MOF nanoflake-assembled spherical microstructures for enhanced supercapacitor and electrocatalysis performances. Nano-Micro Lett. 9, 1–11 (2017).
Chen, C. et al. Formation of bimetallic metal–organic framework nanosheets and their derived porous nickel–cobalt sulfides for supercapacitors. Dalton Trans. 47, 5639–5645 (2018).
El Salam, H. M. A. & El-Fawal, E. M. Optimized photocatalytic degradation of antibiotics with modified Co-MOF and NiCo-MOF catalysts. Environ. Processes 11, 40 (2024).
Naseri, A. M. et al. Synthesis and application of [Zr-UiO-66-PDC-SO3H] Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods. Sci. Rep. 11, 16817 (2021).
Pourghobadi, R., Nematollahi, D., Baezzat, M. R., Alizadeh, S. & Goljani, H. Electropolymerization of catechol on wireless graphite electrode. Unusual cathodic polycatechol formation. J. Electroanal. Chem. 866, 114180 (2020).
Ahmad, A., Priyadarshani, M., Das, S. & Ghangrekar, M. M. Role of bioelectrochemical systems for the remediation of emerging contaminants from wastewater: A review. J. Basic Microbiol. 62, 201–222 (2022).
Deblonde, T., Cossu-Leguille, C. & Hartemann, P. Emerging pollutants in wastewater: a review of the literature. Int. J. Hyg. Environ. Health 214, 442–448 (2011).
Grelska, A. & Noszczyńska, M. White rot fungi can be a promising tool for removal of bisphenol A, bisphenol S, and nonylphenol from wastewater. Environ. Sci. Pollut. Res. 27, 39958–39976 (2020).
Shi, C. et al. Enhanced bisphenol S anaerobic degradation using an NZVI–HA-modified anode in bioelectrochemical systems. J. Hazard. Mater. 403, 124053 (2021).
Mehrabani-Zeinabad, M., Achari, G. & Langford, C. H. Advanced oxidative degradation of bisphenol A and bisphenol S. J. Environ. Eng. Sci. 10, 92–102 (2016).
Zhang, S., Jiang, J. Q. & Petri, M. Preliminarily comparative performance of removing bisphenol-S by ferrate oxidation and ozonation. NPJ Clean Water 4, 1 (2021).
Sun, J. et al. Highly efficient transformation of bisphenol S by anaerobic cometabolism in the bioelectrochemical system. J. Environ. Eng. 145, 04019079 (2019).
Safari, M., Rezaee, A., Ayati, B. & Jonidi-Jafari, A. Bio-electrochemical reduction of nitrate utilizing MWCNT supported on carbon base electrodes: A comparison study. J. Taiwan Inst. Chem. Eng. 45, 2212–2216 (2014).
Mahmoodzadeh, F., Navidjouy, N., Alizadeh, S. & Rahimnejad, M. Investigation of microbial fuel cell performance based on the nickel thin film modified electrodes. Sci. Rep. 13, 20755 (2023).
Soltani, F., Navidjouy, N., Khorsandi, H., Alizadeh, S. & Rahimnejad, M. Tetracycline removal from wastewater and electricity generation in microbial electro-Fenton system in different electrical circuit conditions. J. Mazandaran Univ. Med. Sci. 32, 123–134 (2022).
Mahmoodzadeh, F., Navidjouy, N., Alizadeh, S. & Rahimnejad, M. Application of electrodeposition for nickel nanoparticle-modified electrodes in biochemical systems. MethodsX 1, 103115 (2024).
Khorsandi, H., Movahedyan, H., Bina, B. & Farrokhzadeh, H. Innovative anaerobic/upflow sludge blanket filtration bioreactor for phosphorus removal from wastewater. Environ. Technol. 32, 499–506 (2011).
Wang, Y., Feng, C., Li, Y., Gao, J. & Yu, C. P. Enhancement of emerging contaminants removal using Fenton reaction driven by H2O2-producing microbial fuel cells. Chem. Eng. J. 307, 679–686 (2017).
Izadi, P., Rahimnejad, M. & Ghoreyshi, A. Power production and wastewater treatment simultaneously by dual-chamber microbial fuel cell technique. Biotechnol. Appl. Chem. 62, 483–488 (2015).
Li, N., Liu, L. & Yang, F. Power generation enhanced by a polyaniline–phytic acid modified filter electrode integrating microbial fuel cell with membrane bioreactor. Sep. Purif. Technol. 132, 213–217 (2014).
Rahimzadeh, H., Rahmani, A., Samadi, M. T., Farmany, A. & Asgari, G. Sono-photo‐assisted heterogeneous activation of peroxymonosulfate by Fe/CMK‐3 catalyst for the degradation of bisphenol A, optimization with response surface methodology. Water Environ. Res. 92, 189–201 (2020).
Hong, J., Park, S. J. & Kim, S. Synthesis and electrochemical characterization of nanostructured Ni-Co-MOF/graphene oxide composites as capacitor electrodes. Electrochim. Acta 311, 62–71 (2019).
Mahmoodzadeh, F., Navidjouy, N., Alizadeh, S. & Rahimnejad, M. Comprehensive and Comparative Survey of Microbial Fuel Cell Performance in the Presence of Graphite and Carbon Felt Electrodes Modified with Nano Bio-Nickel Thin Film (2023).
Wang, X. Q., Liu, C. P., Yuan, Y. & Li, F. -b. Arsenite oxidation and removal driven by a bio-electro-Fenton process under neutral pH conditions. J. Hazard. Mater. 275, 200–209 (2014).
Włodarczyk, P. P. & Włodarczyk, B. Preparation and analysis of Ni–Co catalyst use for electricity production and COD reduction in microbial fuel cells. Catalysts 9, 1042 (2019).
Tian, P. et al. Porous metal-organic framework Cu3(BTC)2 as catalyst used in air-cathode for high performance of microbial fuel cell. Bioresour. Technol. 244, 206–212 (2017).
Das, I., Noori, M. T., Shaikh, M., Ghangrekar, M. M. & Ananthakrishnan, R. Synthesis and application of zirconium metal–organic framework in microbial fuel cells as a cost-effective oxygen reduction catalyst with competitive performance. ACS Appl. Energy Mater. 3, 3512–3520 (2020).
Zhong, K. et al. Cobalt/nitrogen-Co-doped nanoscale hierarchically porous composites derived from octahedral metal-organic framework for efficient oxygen reduction in microbial fuel cells. Int. J. Hydrog. Energy 44, 30127–30140 (2019).
Tang, H. et al. Metal–organic-framework‐derived dual metal‐and nitrogen‐doped carbon as efficient and robust oxygen reduction reaction catalysts for microbial fuel cells. Adv. Sci. 3, 1500265 (2016).
Yan, Y. et al. Bimetallic organic framework-derived, oxygen-defect-rich FexCo3-xS4/FeyCo9-yS8 heterostructure microsphere as a highly efficient and robust cathodic catalyst in the microbial fuel cell. J. Power Sources 472, 228582 (2020).
Zhang, Y., Tian, P., Li, K., Liu, Y. & Zhang, Z. C3N4 coordinated metal-organic-framework-derived network as air-cathode for high performance of microbial fuel cell. J. Power Sources 408, 74–81 (2018).
Wang, H. et al. Enhanced bioelectrochemical performance caused by porous metal-organic framework MIL-53 (Fe) as the catalyst in microbial fuel cells. Process Biochem. 99, 147–153 (2020).
Mi, X. et al. Enhanced catalytic degradation by using RGO-Ce/WO3 nanosheets modified CF as electro-Fenton cathode: influence factors, reaction mechanism and pathways. J. Hazard. Mater. 367, 365–374 (2019).
Feng, C., Li, F., Liu, H., Lang, X. & Fan, S. A dual-chamber microbial fuel cell with conductive film-modified anode and cathode and its application for the neutral electro-Fenton process. Electrochim. Acta 55, 2048–2054 (2010).
Sathe, S. M. et al. A novel bio-electro-Fenton process for eliminating sodium Dodecyl sulphate from wastewater using dual chamber microbial fuel cell. Bioresour. Technol. 341, 125850 (2021).
Zhu, X. & Logan, B. E. Using single-chamber microbial fuel cells as renewable power sources of electro-Fenton reactors for organic pollutant treatment. J. Hazard. Mater. 252, 198–203 (2013).
Yong, X. Y. et al. Bio-Electron-Fenton (BEF) process driven by microbial fuel cells for Triphenyltin chloride (TPTC) degradation. J. Hazard. Mater. 324, 178–183 (2017).
Tao, H. C., Wei, X. Y., Zhang, L. J., Lei, T. & Xu, N. Degradation of p-nitrophenol in a BES-Fenton system based on limonite. J. Hazard. Mater. 254–255, 236–241 (2013).
Zhuang, L., Zhou, S., Yuan, Y., Liu, M. & Wang, Y. A novel bioelectro-Fenton system for coupling anodic COD removal with cathodic dye degradation. Chem. Eng. J. 163, 160–163 (2010).
Acknowledgements
Urmia University of Medical Sciences provided funding for this study in 2023 under the Ethical Identifier IR.UMSU.REC.1401.335. The Faculty of Health’s post-graduate laboratory is where the article analysis was completed.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: N Navidjouy, M. Alinasab, S. Alizadeh, M. Rahimnejad; data collection: M.A Author, N.N Author, and S.A Author; Analysis and interpretation of results: M.A Author, N.N Author and S.A. Author; draft manuscript preparation: M.A Author, N.N Author, S.A Author and M.R. Author. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alinasab, M., Navidjouy, N., Alizadeh, S. et al. Bio-electro-fenton system assisted with metal–organic framework for degradation of bis-phenol S in wastewater as an emerging contaminant. Sci Rep 15, 6475 (2025). https://doi.org/10.1038/s41598-025-90969-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90969-2