Abstract
The relationship between niacin and osteoarthritis (OA) is not clear. Using a retrospective cohort study from the National Health and Nutrition Examination Survey (NHANES), this study aimed to investigate the association between niacin intake and osteoarthritis. This study conducted a cross-sectional analysis using data from the National Health and Nutrition Examination Survey 1999–2018 to investigate the association between niacin intake and osteoarthritis. The association between niacin and osteoarthritis was assessed using univariate and multivariate weighted logistic regression models and restricted cubic spline curves (RCS). Nonlinear correlation is analyzed by fitting smooth curve. In this study, 30,620 participants were examined, with 1,864 individuals diagnosed with osteoarthritis, resulting in a prevalence of 5.74%. Utilizing multivariate weighted logistic regression, a consistent inverse relationship between Niacin and osteoarthritis was observed (OR = 0.99, 95% CI: 0.98–0.99, P = 0.003). When Niacin was treated as a categorical variable, the highest Niacin quartile (Q4) exhibited a 33% reduced risk of osteoarthritis compared to the lowest quartile (Q1) (OR = 0.67, 95% CI: 0.53–0.83, P = 0.0004). The restricted cubic spline analysis revealed a non-linear association between Niacin and osteoarthritis risk (non-linear P = 0.022), with 33.53 as the inflection point. Subgroup analyses further highlighted a stronger inverse relationship between Niacin and osteoarthritis in Non − Hispanic Black and other Race patients. The results showed a negative linear relationship between niacin intake and OA risk. By increasing the intake of niacin-rich foods, the risk of osteoarthritis can be reduced, providing ideas for the prevention and treatment of osteoarthritis. Further future studies are recommended to validate our findings.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a degenerative joint disease that primarily affects articular cartilage, subchondral bone, and synovial membranes. It is characterized by joint pain, stiffness, and functional limitations, leading to reduced quality of life. OA is one of the most common musculoskeletal disorders globally, affecting millions of adults, particularly those over the age of 601. As the global population ages and obesity rates continue to rise, the incidence of OA is expected to gradually increase2. The etiology of OA is multifactorial, involving genetic predisposition, mechanical stress, and systemic inflammation3. In addition to these factors, recent studies have highlighted the role of endocrine disorders, such as elevated plasma aldosterone concentration, in the development of bone and joint diseases, including osteoporosis and gout4,5,6. Moreover, the use of certain antihypertensive medications has been shown to impact bone health, further complicating the pathophysiology of OA7. Despite its prevalence and impact, effective preventive and therapeutic strategies for OA remain an ongoing challenge.
Niacin, also known as vitamin B3, plays essential roles in cellular metabolism. It is a precursor for the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), which participate in redox reactions and energy production8. It is known that niacin has a positive role in maintaining skin homeostasis9. Beyond its classical role in preventing pellagra, niacin has gained attention for its potential effects on musculoskeletal health. The study by Sahin K et al.10 found that oral supplementation with nicotinamide and undenatured type II collagen (UCII) alleviated the inflammatory response of most OA symptoms in rats. Recent studies suggest that niacin may modulate inflammation, oxidative stress, and mitochondrial function, all of which could influence OA pathogenesis11,12. A longitudinal cohort study analyzed dietary questionnaires from 2,375 OA patients and found a significant association between specific dietary intake and reduced knee pain, as well as improved quality of life13. However, the relationship between dietary niacin intake and OA risk remains incompletely understood.
In this study, we aim to investigate the association between dietary niacin intake and the prevalence of OA in U.S. adults. By analyzing data from the National Health and Nutrition Examination Survey (NHANES) spanning 1999 to 2018 to explore the relationship between niacin intake and OA risk.
Materials and methods
Study population and design options
NHANES is a series of cross-sectional surveys conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC)14.The study is designed to collect data on the health, nutritional status, and behavior of noninstitutionalized adults and children in the U.S. NHANES is a cross-sectional survey study that collects data through personal interviews, physical examinations, and laboratory assessments on demographics, dietary habits. For more information about the survey and related research data, visit the official NHANES website (https://www.cdc.gov/nchs/nhanes/). NHANES is widely used for the analysis of various diseases.
Exclusion criteria
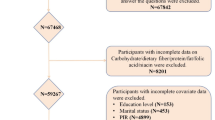
A total of 135,310 participants were enrolled in this study, and 10 NHANES data cycles (NHANES 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, 2017–2018 cycles) in which the survey was completed. Of these, we excluded 67,842 participants who lacked OA, 8,201 who lacked niacin, 153 for whom educational attainment was unavailable, 435 for whom marital status was unavailable, 4,899 for whom poverty-to-income ratios were unavailable, 585 for whom body mass index (BMI) information was unavailable, 10 for whom diabetes mellitus (DM) history was not missing at this time, 10 for whom blood pressure information, 17 were unable to obtain smoking status, and 7191 were unable to obtain alcohol consumption status. Therefore, a total of 32,484 participants were ultimately included in this study. A flowchart detailing the study design can be seen in Fig. 1. The NHANES data were approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent15.
Data collection
Exposure variable
We exposed data using dietary niacin intake, and this data source was collected primarily through two recall interviews. Participants were asked to recall what they had eaten in the past 24 h, with the first interview taking place at a mobile examination center (MEC) and the second follow-up visit taking place by telephone 3 to 10 days later16. Detailed information on all foods and beverages consumed by participants in the past 24 h was collected through the dietary recall method. To obtain detailed nutritional content of each diet, the researchers used the USDA’s Food and Nutrition Dietary Studies database (FNDDS)17. This database summarizes individual nutrient intakes, and niacin intake was assessed according to the NHANES guidelines, with the final niacin intake being the average of the two dietary interviews that were calculated.
Outcome variable
To assess whether a patient had OA, all participants were asked two questions about osteoarthritis. Firstly, if they answered “yes” to the question “Your doctor said you have arthritis” then they were considered to have arthritis (MCQ160A). However, there are many types of arthritis, and to further differentiate between the types of arthritis suffered, participants who answered “yes” to the first question were asked, “What type of arthritis” (MCQ195). Response options included “rheumatoid arthritis”, “osteoarthritis”, “psoriatic arthritis”, “other ”, “refused” and “don’t know”, and only those who answered “osteoarthritis” were considered to have OA.
Covariates
Covariates were selected based on prior literature and OA risk assessment and included age, gender, race, marital status, education level, body mass index (BMI), smoking and alcohol use, PIR, and baseline medical history status (hypertension and diabetes). We categorized race into four total: Mexican American, non-Hispanic white, non-Hispanic black, and other. Marital status was categorized into two categories, widowed/divorced/separated/never married or married/living with partner. Educational level was categorized using high school as the dividing line into less than a high school degree, a high school degree, and more than a high school degree (which includes college graduates or higher).BMI was measured offline by height and weight measurements at the first interview, and was categorized as normal weight (BMI < 25 kg/m2 ), overweight (25 kg/m2 ≤ BMI < 30 kg/m2 ), and obese (BMI ≥ 30 kg/m2 ) based on BMI. Smoking status was categorized into three types, never smoked/previously smoked/currently smoked. Alcohol consumption was defined as at least 12 drinks per year.
Standardized diagnoses were used for both hypertension and diabetes. Higher than normal systolic and diastolic blood pressure or a self-reported diagnosis of hypertension or currently taking antihypertensive medication were considered to be hypertension. Diabetes mellitus was defined as a physician diagnosis of diabetes mellitus or higher than normal values of glycosylated hemoglobin, fasting glucose, random glucose, or being on glucose-lowering medications or insulin. Measurement details for these variables are available at https://www.cdc.gov/nchs/nhanes/.
Statistical analysis
To mitigate the effects associated with the intricate multi-stage sampling design employed by NHANES, we utilized the Day 1 dietary sample weight (WTDRD1) as delineated by the guidelines established by NHANES and performed weighted analyses to augment the precision of the data. Continuous variables are presented as weighted means with standard errors, and categorical variables are presented as counts with corresponding percentages. Subsequently, Subsequently, participants’ baseline features were assessed based on OA status using the Kruskal-Wallis and chi-square tests. To estimate the adjusted odds ratio (OR) and their 95% confidence interval (CI) for niacin quartiles, weighted logistic regression models were employed. The study constructed three weighted logistic regression models: Model 1 had no adjustments; Model 2 was adjusted for age, race, marital status, education level, and PIR; and Model 3 included further adjustments for BMI, hypertension, diabetes, smoking status, and alcohol consumption. Additionally, the study applied weighted restricted cubic splines (RCS) to clarify the dose-response relationship between Niacin and OA risk, adjusting for potential confounders. In order to investigate any potential differential connections between subgroups, we subsequently stratified the patients by age, race, BMI, smoking status, alcohol intake, hypertension, and diabetes and performed interaction analyses. Nonlinear correlation is analyzed by fitting smooth curve. Statistical analyses were performed using R software (version 4.4.1; R Foundation, Vienna, Austria; http://www.R-project.org), with statistical significance set at a two-sided P-value of less than 0.05.
Results
Baseline population characteristics
A total of 32,484 eligible participants, aged between 20 and 85 years, were included in the final analysis. As shown in Table 1, of these participants, 1,864 self-reported having osteoarthritis and 30,620 reported normal joint function, resulting in a prevalence of OA of 5.74%. In the OA group, approximately 56.17% of the subjects were ≥ 60 years of age, 7.66% had less than high school education, 30.29% had low household income, 46.45% had a body mass index ≥ 30 kg/m², 65.92% had a history of smoking, and 79.59% had a background of alcohol consumption. In addition, in the OA group, 17.02% of the subjects were diagnosed with diabetes and 62.03% with hypertension. Statistically significant differences were found between the two groups of subjects in terms of age, race, gender, marital status, PIR, education, BMI, smoking status, drinking status, and prevalence of hypertension, diabetes mellitus, and niacin (P < 0.05).
Association between niacin and OA
We used weighted multivariate logistic regression analysis to investigate the relationship between niacin levels and OA risk in different models. The results, as shown in Table 2, showed a negative association between niacin levels and OA risk. Both univariate and multivariate weighted logistic regression models showed a negative association between niacin and lower OA risk. In addition, we transformed niacin into a categorical variable expressed in quartiles to enhance analytic scrutiny. In Model 3, after adjusting for all possible covariates, participants in the highest quartile of niacin (Q4) had a 33% lower risk of developing OA compared with those in the lowest quartile (Q1) (Q4 vs. Q1, OR: 0.67; 95% CI: 0.53–0.83; P = 0.0004, trend P = 0.0001).
Dose-response curve analysis using the RCS showed a nonlinear relationship between niacin and OA risk, with an inflection point of 33.53. OA risk decreased with increasing niacin, with the rate of reduction varying before and after the inflection point (Total P < 0.001; nonlinear P = 0.022; Fig. 2). The weighted curve fit plot in Fig. 2 includes 100% of the available data from the NHANES study.
The dose-response relationship of the Niacin with the risk of OA. RCS curve adjustment factors: age, gender, race, education level, BMI, PIR, smoking status, drinking status, hypertension, and diabetes. The yellow line and green shading represent the OR and 95% confidence interval (CL) for the association between niacin and osteoarthritis (OA) risk. The two dashed lines represent the range of niacin intake around the median value (22.23). The inflection point is 33.53.
Subgroup analysis
We performed stratified analyses to assess whether the relationship between niacin and OA differed across subgroups (Fig. 3). Our findings indicated an interaction between race and niacin (P < 0.05 for interaction). Specifically, after adjusting for covariates, the effect of niacin on reducing the risk of OA was more pronounced in the Non-Hispanic Black races and Other race. However, the ORs for the relationship between niacin intake and OA were less than 1 in all races, and the sample sizes varied widely across the four races. The results may have been influenced by sample size, which interfered with the interaction results. In the other subgroups analyzed, the relationship between niacin and OA risk was not significantly different (P > 0.05 for interaction).
Discussion
Our study conducted a national cross-sectional analysis of the association between dietary niacin intake and risk of osteoarthritis (OA) using data from the NHANES surveys from 1999 to 2018. It was found that both univariate and multivariate models indicated an inverse relationship between niacin levels and OA risk, regardless of whether niacin was quantified as a continuous variable or in quartiles. These findings suggest that increased niacin intake plays a protective role in preventing OA.
Niacin, also known as vitamin B3, is an essential nutrient that is consumed through the diet, with major food sources including meat, fish, vegetables, and grains18. Before the advent of statins, niacin was commonly used to treat dyslipidemia19. In addition, niacin has been found to be associated with pellagra20, glaucoma retinal function21, and a variety of neurological disorders22, so the importance of maintaining proper niacin levels in the body cannot be overstated.
Increased niacin intake was found to have significant improvements in knee pain and quality of life in a longitudinal cohort study13. More previous studies on the relationship between diet and arthritis exist, and a number have found that specific dietary intake can slow arthritis symptoms23,24,25. However, there is a lack of analyzing the elements of the diet that work to get more precise access to beneficial foods. Most of the previous studies on the association of niacin with osteoarthritis are from cohort studies and have the disadvantage of small sample sizes13. There was also a double-blind, placebo-controlled study on niacin and OA, which included 72 patients with OA and had a small sample size26. In conclusion, studies on the effect of niacin intake on OA are still scarce and deserve further exploration. The present study, found that increased niacin intake was associated with a reduced risk of OA through a large sample. This provides ideas for clinicians in the treatment of OA, as well as prevention for patients with joint pain and OA. More studies between niacin and OA should be added in the future, which will also reduce the NHS burden of OA.
The exact mechanism by which niacin prevents osteoarthritis (OA) is not yet fully understood, but several potential pathways have been proposed. First, niacin has been shown to influence cholesterol metabolism in cartilage, which could help mitigate the progression of OA. Niacin, an ancient cholesterol-lowering drug, promotes cholesterol efflux from chondrocytes, which is critical in maintaining healthy cartilage function. Recent studies have demonstrated that increased cholesterol efflux from chondrocytes reduces lipid accumulation and inflammation in cartilage, slowing the progression of OA27. Another study suggests that niacin may reduce cholesterol deposition in joint tissues, thereby alleviating joint damage associated with OA. Additionally, another study found that niacin enhances cholesterol hydroxylase uptake and increases the production of oxysterol metabolites in OA chondrocytes. These metabolites, in turn, may alter cartilage metabolism, reducing the inflammation and oxidative stress that contribute to OA progression. Moreover, retinoic acid-associated orphan receptor α (RORα), which is involved in regulating cholesterol metabolism, has been implicated in OA progression. Studies in mice models have shown that RORα plays a key role in modulating cholesterol metabolism, suggesting that niacin’s impact on this receptor may also influence OA development28.
Secondly, niacin may modulate neuroinflammation and chronic pain, which are hallmark features of OA. Niacin activates niacin receptor 1 (NIACR1), also known as G protein-coupled receptor 109 A (GPR109A), an anti-inflammatory receptor that has been shown to play a crucial role in regulating neuroinflammation. Emerging research has demonstrated that activation of NIACR1 by niacin reduces microglia activation in the central nervous system, which is critical in managing chronic pain and neuroinflammation associated with OA. This anti-inflammatory effect could reduce the pain and stiffness experienced by OA patients, improving their overall quality of life. Furthermore, the ability of niacin to regulate neuroinflammation suggests that it may have broader implications for pain management in OA29. Thirdly, niacin exhibits significant antioxidant properties, which may contribute to its protective effects on OA and overall joint health. Oxidative stress is a key factor in the progression of OA, as it accelerates cartilage degradation and joint inflammation. Niacin, by increasing the production of NAD + and enhancing mitochondrial function, may reduce the accumulation of reactive oxygen species (ROS) in joint tissues. Several studies have demonstrated that niacin’s antioxidant effect reduces oxidative damage in joint tissues, thereby protecting cartilage and maintaining joint function. This ability to neutralize ROS may slow the degeneration of cartilage and alleviate the symptoms of OA9,30,31. Despite these promising mechanisms, further studies are needed to fully elucidate the molecular biological pathways by which niacin protects against OA. Animal studies, as well as well-designed clinical trials, are essential to confirm these mechanisms and establish a clear understanding of how niacin influences OA at the molecular level. Moreover, whether the intake of a specific niacin-targeted diet is better protective against OA and what the optimal dose is still needs to be verified in large-scale clinical trials.
The strength of this study is that it focuses on the relationship between niacin intake and OA and is supported by a large sample. There are then some limitations to the study. First, the diagnosis of OA that we used was self-reported from participants, and although this method allows for rapid data collection from a large population, there is a risk of lack of accuracy. And, because the study was retrospective in design, it was not possible to confirm a causal relationship between exposure and outcome. Second, although we adjusted for common confounders affecting OA, there may still be residual confounders, which could potentially affect the relationship between niacin and OA. Third, the population we studied was the US population, and further, larger-sample studies are needed to determine whether the benefits of niacin intake can be generalized to other populations.
Conclusions
In conclusion, this cross-sectional study based on the NHANES is cycle (1999–2018) found a negative association between niacin intake and OA in the US population after adjusting for potential confounders. This study provides a novel approach for exploring impactful dietary interventions to reduce the incidence of OA. The results of this study provide ideas for further research to confirm these relationships and determine the optimal dose for therapeutic effects. In the future, more randomized controlled trials are needed to confirm this finding and provide more precise and effective prevention and treatment for OA.
Data availability
All datasets used during the current study can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes).
References
Global and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. ;5(9):e508-e522. eng. Epub 2023/09/07. (2023). https://doi.org/10.1016/s2665-9913(23)00163-7. Cited in: Pubmed; PMID 37675071.
Hu, Y., Chen, X., Wang, S., Jing, Y. & Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. ;9(1):20. eng. Epub 2021/03/19. (2021). https://doi.org/10.1038/s41413-021-00147-z. Cited in: Pubmed; PMID 33731688.
Guan, S. Y. et al. Global burden and risk factors of musculoskeletal disorders among adolescents and young adults in 204 countries and territories, 1990–2019. Autoimmun Rev. ;22(8):103361. eng. Epub 2023/05/26. (2023). https://doi.org/10.1016/j.autrev.2023.103361. Cited in: Pubmed; PMID 37230312.
Song, S. et al. Plasma aldosterone concentrations elevation in hypertensive patients: the dual impact on hyperuricemia and gout. Front. Endocrinol. (Lausanne). 15 https://doi.org/10.3389/fendo.2024.1424207 (2024). :1424207. eng. Epub 2024/08/14.
Song, S. et al. Effectiveness of spironolactone in reducing osteoporosis and future fracture risk in Middle-Aged and elderly hypertensive patients. Drug Des. Devel Ther. 18, 2215–2225. https://doi.org/10.2147/dddt.S466904 (2024). eng. Epub 2024/06/17.
Song, S. et al. Correlation between plasma aldosterone concentration and bone mineral density in middle-aged and elderly hypertensive patients: potential impact on osteoporosis and future fracture risk. Front. Endocrinol. (Lausanne). 15 https://doi.org/10.3389/fendo.2024.1373862 (2024). :1373862. eng. Epub 2024/05/29.
Ma, H. et al. Association of systemic inflammatory response index with bone mineral density, osteoporosis, and future fracture risk in elderly hypertensive patients. Postgrad. Med. 136 (4), 406–416. https://doi.org/10.1080/00325481.2024.2354158 (2024). eng. Epub 2024/05/16.
Ferrell, M. et al. A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat. Med. 30 (2), 424–434. https://doi.org/10.1038/s41591-023-02793-8 (2024). eng. Epub 2024/02/20.
Boo, Y. C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants (Basel). ;10(8). eng. Epub 2021/08/28. (2021). https://doi.org/10.3390/antiox10081315. Cited in: Pubmed; PMID 34439563.
Sahin, K. et al. Niacinamide and undenatured type II collagen modulates the inflammatory response in rats with monoiodoacetate-induced osteoarthritis. Sci Rep. ;11(1):14724. eng. Epub 2021/07/21. (2021). https://doi.org/10.1038/s41598-021-94142-3. Cited in: Pubmed; PMID 34282229.
Godin, A. M. et al. Nicotinic acid induces antinociceptive and anti-inflammatory effects in different experimental models. Pharmacol Biochem Behav. ;101(3):493-8. eng. Epub 2012/03/01. (2012). https://doi.org/10.1016/j.pbb.2012.02.012. Cited in: Pubmed; PMID 22366213.
Freitas, C. S. et al. Anti-inflammatory and Anti-nociceptive Activity of Ruthenium Complexes with Isonicotinic and Nicotinic Acids (Niacin) as Ligands. J Med Chem. ;58(11):4439-48. eng. Epub 2015/05/15. (2015). https://doi.org/10.1021/acs.jmedchem.5b00133. Cited in: Pubmed; PMID 25973517.
Zheng, Z., Luo, H. & Xue, Q. Association between niacin intake and knee osteoarthritis pain and function: a longitudinal cohort study. Clin. Rheumatol. 43 (2), 753–764. https://doi.org/10.1007/s10067-023-06860-w (2024). eng. Epub 2024/01/05.
Bailey, R. L., Pac, S. G., Fulgoni, V. L. 3, Reidy, K. C. & Catalano, P. M. rd, Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw Open. ;2(6):e195967. eng. Epub 2019/06/22. (2019). https://doi.org/10.1001/jamanetworkopen.2019.5967. Cited in: Pubmed; PMID 31225890.
Fang Zhang, F. et al. Trends and Disparities in Diet Quality Among US Adults by Supplemental Nutrition Assistance Program Participation Status. JAMA Netw Open. ;1(2):e180237. eng. Epub 2018/12/01. (2018). https://doi.org/10.1001/jamanetworkopen.2018.0237. Cited in: Pubmed; PMID 30498812.
Li, T. et al. Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). Nutrients. ;14(19). eng. Epub 2022/10/15. (2022). https://doi.org/10.3390/nu14194038. Cited in: Pubmed; PMID 36235691.
Pan, J., Hu, Y., Pang, N. & Yang, L. Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018. Nutrients. ;15(19). eng. Epub 2023/10/14. (2023). https://doi.org/10.3390/nu15194128. Cited in: Pubmed; PMID 37836412.
Hrubša, M. et al. On Behalf Of The O. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B(1), B(2), B(3), and B(5). Nutrients. ;14(3). eng. Epub 2022/03/13. (2022). https://doi.org/10.3390/nu14030484. Cited in: Pubmed; PMID 35276844.
Boden, W. E., Sidhu, M. S. & Toth, P. P. The therapeutic role of niacin in dyslipidemia management. J. Cardiovasc. Pharmacol. Ther. 19 (2), 141–158. https://doi.org/10.1177/1074248413514481 (2014). eng. Epub 2013/12/24.
Prabhu, D., Dawe, R. S. & Mponda, K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced Pellagra. Photodermatol Photoimmunol Photomed. 37 (2), 99–104. https://doi.org/10.1111/phpp.12659 (2021). eng. Epub 2021/01/21.
Hui, F. et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 48 (7), 903–914. https://doi.org/10.1111/ceo.13818 (2020). eng. Epub 2020/07/29.
Wuerch, E., Urgoiti, G. R. & Yong, V. W. The promise of niacin in neurology. Neurotherapeutics 20 (4), 1037–1054. https://doi.org/10.1007/s13311-023-01376-2 (2023). eng. Epub 2023/04/21.
Morales-Ivorra, I., Romera-Baures, M., Roman-Viñas, B. & Serra-Majem, L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients. ;10(8). eng. Epub 2018/08/09. (2018). https://doi.org/10.3390/nu10081030. Cited in: Pubmed; PMID 30087302.
Messier, S. P. et al. Effect of diet and exercise on knee pain in patients with osteoarthritis and overweight or obesity: A randomized clinical trial. Jama 328 (22), 2242–2251. https://doi.org/10.1001/jama.2022.21893 (2022). eng. Epub 2022/12/14.
Lv, X. et al. Causal relationship between diet and knee osteoarthritis: A Mendelian randomization analysis. PLoS One. ;19(1):e0297269. eng. Epub 2024/01/31. (2024). https://doi.org/10.1371/journal.pone.0297269. Cited in: Pubmed; PMID 38295091.
Jonas, W. B., Rapoza, C. P. & Blair, W. F. The effect of niacinamide on osteoarthritis: a pilot study. Inflamm Res. ;45(7):330-4. eng. Epub 1996/07/01. (1996). https://doi.org/10.1007/bf02252945. Cited in: Pubmed; PMID 8841834.
Lee, G. et al. Enhancing intracellular cholesterol efflux in chondrocytes alleviates osteoarthritis progression. Arthritis Rheumatol. https://doi.org/10.1002/art.42984 (2024). Sep 11. eng. Epub 2024/09/12.
Choi, W. S. et al. The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature 566 (7743), 254–258. https://doi.org/10.1038/s41586-019-0920-1 (2019). eng. Epub 2019/02/08.
Taing, K., Chen, L. & Weng, H. R. Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain. Neural Regen Res. ;18(4):763–768. eng. Epub 2022/10/08. (2023). https://doi.org/10.4103/1673-5374.354514. Cited in: Pubmed; PMID 36204834.
Tian, S. et al. Dietary niacin intake in relation to depression among adults: a population-based study. BMC Psychiatry. 23 (1), 678. https://doi.org/10.1186/s12888-023-05188-8 (2023). eng. Epub 2023/09/19.
Montserrat-de la Paz, S. et al. Niacin and its metabolites as master regulators of macrophage activation. J. Nutr. Biochem. 39, 40–47. https://doi.org/10.1016/j.jnutbio.2016.09.008 (2017). eng. Epub 2016/10/25.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Study design, data collection and statistical analysis, XFL, XMD, RL, Writing-original draft, XFL, XMD, RL; Writing-review & editing, XFL, XMD, RL, SSL, RHW, ZHZ, YLL, XCD, YL; Supervision: YL.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and informed consent statement
NHANES is a publicly available, free database that has been approved by the NCHS Research Ethics Review Board and agreed to by all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, X., Deng, X., Lai, R. et al. Association between niacin intake and osteoarthritis in the US population based on NHANES 1999–2018. Sci Rep 15, 6470 (2025). https://doi.org/10.1038/s41598-025-91063-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91063-3