Abstract
To observe the current situation of fertility preservation among female breast cancer patients ≤ 40 years old and analyze the related factors which influence the utilization of fertility preservation. A single-center retrospective questionnaire was conducted investigating patients diagnosed with breast cancer attending The First Affiliated Hospital of Xian JiaoTong University between January 2016 and December 2019. The questionnaire was redesigned based on previous similar research and the questions needed in this study. Rates of utilization of preservation services were compared based on patients’ demographic and economic-social information, disease characteristic information and fertility related information. Univariate and multivariate logistic regression analysis was used to assess the relationship between utilization of fertility preservation and sociodemographic factors, previous reproductive-related problems. 313 patients were successfully interviewed. 60/313patients (19.2%) had utilized fertility preservation. Younger patients (< 30 years of age), patients with 1 or no child, and patients had higher education level were more likely to pursue fertility preservation than their matched counterparts. Age, parity, and education level of breast cancer patients may impact rates of fertility preservation among reproductive age women diagnosed with breast cancer. Thus, further attention to age difference and patient’s desire for future fertility could help to improve gaps in fertility preservation. These findings have guidance for counseling young breast cancer patients.
Similar content being viewed by others
Introduction
Since 1980, the incidence of breast cancer (BC) has been continuously increasing, with an annual growth of about 2-3%; it is expected that by 2030, the global annual number of new cases of breast cancer will increase to approximately 3.2 million1,2. Nowadays, breast cancer is the most common malignancy diagnosed among women, with an incidence rate surpassing that of lung cancer in women globally, accounting for one quarter of cancer cases2. Although the incidence of BC increases with age, approximately 30% of women with breast cancer are in their reproductive years3. Even young BC patients have a worse prognosis, with the continuous improvement of comprehensive treatment and early diagnosis of breast cancer, their 5-year survival rate has gradually increased. The overall 5-year survival rate for breast cancer patients under 40 in the United States has risen from 74.0% in the 1970s to 88.5% today, and even the 5-year survival rate for early-stage breast cancer and breast cancer has reached 99%4.
Along with the development and progress of society and the change of perspectives on life, the age of women’s first childbirth is gradually being postponed. Almost 25% of women in the United States give birth for the first time between the ages of 30–405. Therefore, many breast cancer patients don’t have a baby at the time of diagnosis and they may want to reproduce after successful cancer treatment. In J JU’s clinical investigation, 26.3% of breast cancer patients younger than 40 years still have fertility expectations after diagnosis6. Furthermore, about 75% of young cancer survivors without children at diagnosis hope to have children after treatment7.
However, some treatments for breast cancer may damage the fertility of young breast cancer patients permanently. Generally speaking, descriptions of cancer treatment often bring to mind more details about hair loss, decreased appetite, illness, and vomiting. Young women with breast cancer do not consider that infertility is related to the outcome of chemotherapy or radiation therapy. Young cancer patients have a 40–60% lower chance of successfully giving birth after treatment than the general population8. Females whose fertility ability is affected by cancer treatment are more likely to experience severe negative emotions during long-term survival compared to females who have successfully given birth. Females who become infertile after treatment have a higher level of depression symptoms9. It is essential to take certain measures to protect fertility before undergoing treatments. There are relatively many measures for protecting fertility, such as oocyte/embryo cryopreservation, ovarian tissue cryopreservation, temporary ovarian suppression with gonadotrophin-releasing hormone Agonists (GnRHa) during Chemotherapy and so on10.
However, to our knowledge, little information is available about the economic-social and doctor-patient factors that affect young BC patients’ adoption of fertility protection after diagnosis of breast cancer. The aim of this research is to ascertain the patients’ awareness of fertility-related information and their utilization of fertility preservation measures, while also analyzing variables potentially associated with the adoption of such measures and attempt to guide healthcare practitioners - including medical oncologists, radiation oncologists, gynecologic oncologists, surgeons, and others - to offer more tailored and personalized fertility protection counseling to patients.
Materials and methods
Participant selection
This was a single-center retrospective questionnaire study on reproductive aged women (18–40 years old at the time of diagnosis) who had been diagnosed with breast cancer. In this retrospective questionnaire study, we used hospital records of The First Affiliated Hospital of Xian JiaoTong University to identify all women, between January 2016 and December 2019. The ICD-10 diagnostic code C50 (malignancy of the breast) was used to identify female cancer patients from the electronic records. Hospital records were reviewed by a researcher to collect information about tumor stage, estrogen and progesterone receptor (ER/PR) expression, human epidermal growth factor receptor 2 (HER2) overexpression, treatments received and demographic information.
A patient was entitled to this study if she met the following criteria:
-
1.
Age younger than 40 years.
-
2.
The patient was diagnosed with primary breast cancer Stage I-III by pathologic diagnosis.
-
3.
The medical records were complete: including general data, disease treatment data and pathological data.
-
4.
The participant was voluntary and able to complete a telephone follow-up survey.
Women who with other types of malignancies or were unable to have child due to other diseases were excluded from the study.
Sample size
Based on the requirement of the Logistic regression model, the sample size required is usually determined according to the number of tests or independent variables. In order to ensure the stability of the model estimated based on samples, it is generally believed that the sample size is at least 10–20 times the number of independent variables. According to the results of previous relevant studies and the variable indicators of the questionnaire design, 16 potential independent variables are to be introduced, so it is estimated that 160–320 samples are required for this study. Considering for incomplete return of questionnaires in 20–30%, the sample size before survey is finally determined to be 430.
Survey instruments and distribution
The questionnaire was adapted from prior publication on similar topics on fertility preservation11,12. The questionnaire consisted of 17 questions in three sections: (1) background demographic and economic-social information, (2) disease characteristic information and (3) fertility related information. The survey questions were prepared by fertility specialists with input from clinical oncologists and breast cancer surgeons. The questionnaire was available in Chinese. The Validity of questionnaires is mainly judged by subjective experience. Researchers or experts are often used to evaluate the content validity of questionnaires. One statistical expert and two clinical experts were invited to evaluate the content validity of the questionnaire. After two revisions, it was agreed that the language expression was accurate and the content validity was good. Before finalization, the questionnaire was distributed to a trial group of 10 participants to evaluate its content, clarity, and length. Three weeks later, the 10 participants were followed up by telephone twice. The consistency of the general socio-demographic information and childbearing information of this questionnaire was 1 and 0.95, respectively. Minor changes in wordings and corrections in typos have been made.
Before the start of the investigation, we have trained investigators and started the formal investigation after the training was qualified. Every question in the questionnaire was checked again immediately after receiving an answer. After verbal consent, participants were asked to complete a telephone survey initiated by surveyors. The survey required approximately 8 min to complete. Completed paper questionnaires were collected by the researcher at the end of the consultation, who entered the data into the computer.
Statistical analysis
Results are expressed as mean ± standard deviation (SD), component ratio and rate. For descriptive data, the difference in categorical variables between cancer patients undergoing versus not undergoing fertility preservation (FP) was analyzed using the Chi-square. Univariate and multivariate logistic regression analysis was used to assess the relationship between concern on fertility preservation and sociodemographic factors, previous reproductive-related problems. All statistical analysis was conducted using SPSS version 26 (IIBM Corporation, Armonk, NY). A difference was considered statistically significant when the P < 0.05.
Ethics.
This study protocol, including the use of medical record data without patient consent, was reviewed and approved by the Institutional Review Board of The First Affiliated Hospital of Xian JiaoTong University, Xian, China. Separate approval from the Ethics Committee was not needed in this retrospective study, as no subjects or caregivers were contacted. The study has obtained the informed consent of all participants and/or their legal guardians and all experiments were performed in accordance with relevant guidelines and regulations.
Results
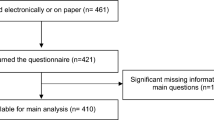
Ultimately, 515 patients were involved in our study. Those who did not meet the inclusion criteria were excluded in the research. In total, 313 women under age 40 at diagnosis consented on telephone and finished the interview. 92 patients refused the study or were missing. The flow of participant selection is shown in Fig. 1. Of these 313 patients, the median age was 36 years. This cohort included 50 women aged ≤ 30, 76 women aged 30–34, and 187 woman aged 35–40 at diagnosis. And the youngest woman of the cohort was 20 years old at diagnosis. Most women were married (92.3%). The demographic and economic-social information of the participants are shown in Table 1.
A total of 77 (24.6%) patients were diagnosed with breast cancer at Stage I and 236 (75.4%) were diagnosed at Stage II-III. There were 270 (86.3%) patients who were diagnosed with hormone receptor and/or HER2positive breast cancer and 43 (13.7%) were diagnosed with triple negative breast cancer. Most women had children (87.2%) and received mastectomy (76.0%). The clinical and fertility information of patients are shown in Table 2.
Knowledge of patients towards fertility preservation
In this follow-up population, only 167 (53.4%) women were aware of the impact of comprehensive breast cancer treatment on fertility, of which 133 (42.5%) explicitly stated that their doctors had told them that comprehensive treatment would affect their future fertility before starting treatment. In total, 77 women had heard of GnRHa-based fertility protection, 33 women had heard of egg or embryo cryopreservation, and only 13 women had heard of ovarian tissue cryopreservation. The awareness on the different modes of fertility preservation is shown in Fig. 2.
The main way to know that comprehensive treatment affects fertility was to be informed by doctors (79.6%), and a small number of people get this information through other ways, such as searching on the Internet (31.1%), communicating with patients (11.4%), television or other media (14.4%). The way to know fertility preservation was almost only to be informed by doctors (Table 3). To sum up, the way for patients to understand fertility preservation was lacking, and they cannot obtain satisfactory fertility related information.
Utilization of fertility preservation
60/313 (19.2%) of the participants had taken steps to protect their fertility and lower the chance that they would become infertile with cancer comprehensive treatment. Of these 60 women, all of them had utilized a gonadotropin-releasing hormone (GnRH) agonis and this was the only type of fertility preservation used.
253/313 (80.8%) women did not take fertility preservation, of which 17 people had been informed of the impact of comprehensive cancer treatment on fertility by doctors. The reasons for not taking fertility preservation are shown in Table 4. As of the follow-up date, one of the patients who had taken fertility preservation tried to get pregnant 30 months after treatment, but labor was finally induced due to multiple fetal malformations; One woman became pregnant 27 months after the end of treatment, and the pregnancy outcome was unknown. Both of them were hormone receptor-positive, and the endocrine therapy was interrupted due to pregnancy, and no recurrence or metastasis occurred.
Factors associated with utilization of fertility preservation
There were 60 patients in the FPT (Fertility Preservation Treatment) group and 253 patients in the non-FPT group. The age at diagnosis, marital status, census register, educational level, household income, occupation, stage of diagnosis, surgical method, and pregnancy or births at diagnosis were significant differences between the two groups (P < 0.05), as shown in Table 5. In univariate Logistic regression analysis, we tested a number of factors associated with utilization of fertility preservation. Then the factors with P ≤ 0.15 in the univariate analysis were included in the multivariate regression analysis. Multivariate binary logistic regression showed that age, parity and education level were significantly associated with utilization of fertility preservation. Younger age (<30 vs. 30–40, OR = 4.55, 95% CI: 1.76–11.75) and higher education level (Bachelor’s degree or above vs. vocational education or below, OR = 4.04, 95% CI: 1.79–9.16) was associated with using fertility preservation, whereas having children was associated with a lower likelihood of having a fertility preservation treatment (OR = 0.06, 95% CI: 0.02–0.18) in the final multivariate model (Table 4). Other demographic, economic-social, clinical and productive characteristics were not significant in multivariate models (Table 6).
Discussion
In this study, we investigated the fertility preservation measures undertaken by young women following a breast cancer diagnosis and examined the influencing factors. Using a retrospective questionnaire survey, we collected data on young women diagnosed and treated for non-advanced breast cancer at the First Affiliated Hospital of Xi’an Jiaotong University between January 2016 and December 2019. Ultimately, we gathered demographic information, disease details, and fertility-related information for 313 patients.
In this retrospective study, approximately 19% of the 313 female breast cancer patients adopted active fertility preservation measures. Women who were 30 years old or younger, had not yet given birth and had higher education levels were more proactive in undertaking fertility preservation measures before the commencement of breast cancer treatment, particularly gonadotoxic therapy compared to the older, already given birth and less educated women. Consistent with prior research, women diagnosed with cancer over the age of 35 are less likely to receive fertility counseling13,14. Studies from Sweden and the Memorial Sloan Kettering Cancer Center further indicated that women diagnosed with breast cancer who have undergone fertility preservation tend to be younger, have lower parity, or are nulliparous15,16.
Many factors may influence whether women adopt fertility preservation treatments. Firstly, the fertility status at the time of cancer diagnosis plays a crucial role. For nulliparous patients, the desire for future fertility was significant. Therefore, it is evident that nulliparous patients are more inclined to pursue fertility preservation treatments upon diagnosis17,18. In contrast, for parous patients, fertility may seem less important compared to cancer treatment, and they may be reluctant to risk delaying treatment for fertility preservation. In our study, 77.1% women expressed that they did not want fertility preservation before cancer treatment because they have already given birth to children and did not have wish to deliver child in the future. Influenced by traditional Chinese culture, women are burdened with high expectations of bearing offspring. While falling ill is unfortunate, when the disease is manageable, the potential conflicts arising from not being able to conceive or choosing not to can become increasingly pronounced, severely impacting women’s mental health, quality of life, and even leading to marital discord. Secondly, the older the patient, the less likely they are to opt for fertility preservation treatments. Older patients are more likely to have completed their family planning at the time of diagnosis, with potentially lower future fertility needs compared to nulliparous women. Additionally, as age advances, even in healthy women, ovarian function deteriorates, and post-treatment, patients should wait at least two years before considering pregnancy to avoid the high recurrence period of breast cancer after cancer treatment19. The individual’s level of education also influenced their decision to undergo fertility preservation treatments. Patients with a college education or higher were more likely to opt for fertility preservation, possibly because educated women tend to delay childbirth compared to those without higher education. At the time of breast cancer diagnosis, their need for fertility preservation is greater. Furthermore, their understanding of the impact of breast cancer treatment on fertility and the information regarding fertility preservation is more comprehensive, leading to a more thorough decision-making process that considers their true desires, resulting in optimal choices.
When discussing the adoption of fertility preservation treatment (FPT), one crucial aspect that cannot be overlooked is the genuine reproductive needs of patients. In consideration of reducing recall biases, the process of designing the questionnaire did not directly inquire about the patients’ reproductive desires at the time of diagnosis. Instead, we gained insight into their reproductive needs indirectly by asking about their awareness of chemotherapy-induced infertility, whether they opted for FPT, and the reasons for not choosing FPT. In this study, approximately 19% of patients ultimately opted for FPT, revealing that a substantial portion of this group had fertility needs following a breast cancer diagnosis. The American Helping Ourselves Helping Others study, encompassing a prospective cohort of 620 women with breast cancer, found that 51% of the women expressed concerns about their fertility12. In the European Helping Ourselves Helping Others study, 64% of the 297 women expressed fears of infertility post-cancer treatment, with 15% declining gonadotoxic tumor therapy due to potential fertility risks. Among women who had not completed their desired pregnancies, as high as 71% expressed a desire to continue family planning after comprehensive cancer treatment20. What requires particular attention is that patients’ desire for childbearing may change over time21, especially for those who have not yet had children at the time of cancer diagnosis. However, breast cancer patients are unaware of the adverse effects of breast cancer treatment on fertility and ovarian function22, and are also very unfamiliar with fertility preservation methods23. In this study, only 53.4% of patients were aware that comprehensive breast cancer treatment would affect their fertility. And our practice is still imperfect, with less than half of the women recalling that their doctors had told them that comprehensive treatment would affect their future fertility since cancer diagnosis. While the using of gonadotropin-releasing hormone agonists during chemotherapy was more well-known, established techniques such as oocyte cryopreservation or embryo cryopreservation were rarely known by cancer women. Most patients receive relevant information from their doctors, and other avenues for obtaining fertility preservation information are lacking. Adequate social support and the widespread dissemination of professional medical knowledge can potentially enable women with fertility needs to achieve their desires.
In our study, GnRH agonist administration was the sole methods of fertility preservation used in breast cancer women when it was commonly combined with a gold-standard technique. This may be due to limitations in the development of assisted reproductive centers in our study hospital, making it challenging to perform oocyte cryopreservation, embryo cryopreservation, and ovarian cryopreservation. In addition, the low cost of GnRH drugs may also play a role. Although the medical insurance for urban residents or rural cooperative medical insurance in China has basically reached 100%, its coverage does not involve fertility protection. Basically, patients have to bear all the costs. The cost of cryopreservation of oocytes, embryos or ovarian tissue in fertility protection measures is extremely high. But GnRHa drugs are much cheaper. The fertility protection measures adopted in this study were all GnRH drugs used before and during chemotherapy. The reason why personal annual income was significant in the univariate analysis but no longer statistically significant after adjusting for fertility status may be explained in this way, which also reflects from the side that GnRHa drugs are easy to operate and cheap. It may be easier for patients to accept and use. A more important reason may be doctors’ lack of knowledge about fertility preservation. According to an ESMO study published in 2018, 37% of 273 physicians surveyed across multiple disciplines (not just oncology) worldwide said they had never reviewed existing authoritative fertility protection guidelines24. Another online survey of reproductive specialists in Shanghai conducted by Fudan University in Shanghai, China25 showed that most (71%) reproductive specialists had an average or low level of knowledge about tumor-related fertility. The 2018 ASCO Clinical Practice Guideline update mentioned the potential utility of GnRH agonists in fertility preservation26. GnRH agonists can help preserve fertility by suppressing ovarian function, with a lower incidence of primary ovarian insufficiency during chemotherapy in patients receiving GnRH agonist therapy and a higher pregnancy rate post-treatment27. However, ASCO does not recommend using GnRH agonists as a substitute for more established fertility preservation strategies. In future clinical practice, oncologists or breast cancer specialists should consider patients’ diseases, financial capabilities, fertility desires and other factors, and take standard fertility preservation measures to ensure patients’ safety while enhancing the success rate of fertility preservation.
Our study found that patients’ access to information about fertility was lacking. In our study, patients learned about fertility preservation measures mainly by being told by their doctors, and a small number learned about them by searching the Internet on their own. However, our study found that doctors’ information to patients was very inadequate, although several guidelines clearly recommended that doctors should fully inform every female patient of childbearing age that chemotherapy would affect fertility before starting treatment, and patients interested in fertility should be promptly referred to reproductive experts for appropriate fertility preservation26. At present, several regions have established birth reservation decision support websites28,29 specifically for women of childbearing age with cancer. These websites provide detailed and professional information on the impact of cancer on fertility, the specific implementation process, advantages and disadvantages, and scope of application of different birth reservation measures, and consult different individuals online about their fertility concerns and needs. Give advice in real time to help patients make better decisions, reduce the degree of regret for subsequent decisions, and accurately guide patients to carry out fertility protection. Perhaps the Health economics department can organize oncologists, reproductive scientists, breast specialists, computer professionals, health economists, etc., to set up our own website to assist oncology clinicians in solving fertility related problems.
Being a retrospective study, it inevitably suffers from a certain degree of recall bias. And we excluded data from incomplete records tests, patients with other types of cancer, and patients with severe organic lesions, which may pose a risk of patient selection bias. The methodology employed in this study was based on questionnaire surveys, which may introduce a level of subjectivity into the results. Respondents might tend towards giving positive answers, leading to a potential overestimation of certain outcomes. The study population is drawn solely from a single large teaching hospital, which imposes limitations on the representativeness of the sample, thereby restricting the generalizability and external validity of the study’s conclusions. But our sample size is sufficient, the conclusions drawn from our research were credible. Additionally, the follow-up observation period in this study was relatively short, failing to capture the effectiveness of fertility preservation measures for patients and the impact of fertility preservation on patient prognosis, and longer follow-up in the future will help us understand these problems. Besides, decisions about fertility preservation were made before the data was collected, so we can only observed correlations between age, education level, etc., and the use of fertility preservation.
Despite the limitations of our study, the data analyzed in our research was sourced from a large-scale hospital with reliable and mandatory records. Conducted as a retrospective study, our research examined the influence of several potential factors on patients’ decisions to undergo fertility preservation measures, ultimately identifying three factors as significant influencers. These findings can serve as a reference for future prospective studies. The issues discovered during the research process can offer guidance for clinical work in the future, enhancing healthcare professionals’ awareness and attention to this matter.
Conclusion
Young breast cancer patients had a low level of awareness regarding fertility-related information, and they lack adequate access to such information. Young breast cancer patients who had not child or had one child, were younger, and had a higher level of education were more likely to proactively take fertility preservation treatment. Thus, further attention to age difference and a patient’s desire for future fertility could help to improve gaps in fertility preservation. These findings have guidance for counseling young breast cancer patients.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
da Costa Vieira, R. A., Biller, G., Uemura, G., Ruiz, C. A. & Curado, M. P. Breast cancer screening in developing countries. Clin. (Sao Paulo). 72 (4), 244–253. https://doi.org/10.6061/clinics/2017(04)09 (2017). PMID: 28492725; PMCID: PMC5401614.
Sung, H. et al. : GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338. (2020).
Heer, E. et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. ;8(8):e1027-e1037. (2020). https://doi.org/10.1016/S2214-109X(20)30215-1. PMID: 32710860.
Chen, L., Linden, H. M., Anderson, B. O. & Li, C. I. Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res. Treat. 147 (3), 609–616. https://doi.org/10.1007/s10549-014-3112-6 (2014). Epub 2014 Aug 28. PMID: 25164974; PMCID: PMC4174984.
Matthews, T. J. & Hamilton, B. E. First births to older women continue to rise. NCHS Data Brief. ;(152):1–8. (2014). PMID: 24813228.
Ju, J. et al. An investigation of the fertility needs of young patients with breast cancer. Zhonghua Zhong Liu Za Zhi. ;42(5):408–412. Chinese. (2020). https://doi.org/10.3760/cma.j.cn112152-112152-20191017-00672. PMID: 32482031.
Letourneau, J. M., Melisko, M. E., Cedars, M. I. & Rosen, M. P. A changing perspective: improving access to fertility preservation. Nat. Rev. Clin. Oncol. 8 (1), 56–60. https://doi.org/10.1038/nrclinonc.2010.133 (2011). Epub 2010 Aug 24. PMID: 20736926; PMCID: PMC3226819.
Lambertini, M. et al. Pregnancy after breast cancer: A systematic review and Meta-Analysis. J. Clin. Oncol. 39 (29), 3293–3305. https://doi.org/10.1200/JCO.21.00535 (2021). Epub 2021 Jul 1. PMID: 34197218.
Gonçalves, V. & Quinn, G. P. Review of fertility preservation issues for young women with breast cancer. Hum. Fertil. (Camb). 19 (3), 152–165. https://doi.org/10.1080/14647273.2016.1193228 (2016). Epub 2016 Aug 26. PMID: 27561757.
Christian, N. & Gemignani, M. L. Issues with Fertility in Young Women with Breast Cancer. Curr Oncol Rep. ;21(7):58. (2019). https://doi.org/10.1007/s11912-019-0812-4. PMID: 31098718.
Lambertini, M. et al. Prospective study to optimize care and improve knowledge on ovarian function and/or fertility preservation in young breast cancer patients: results of the pilot phase of the pregnancy and fertility (PREFER) study. Breast 41, 51–56 (2018). Epub 2018 Jun 22. PMID: 30007268.
Ruddy, K. J. et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J. Clin. Oncol. 32 (11), 1151–1156. https://doi.org/10.1200/JCO.2013.52.8877 (2014). Epub 2014 Feb 24. PMID: 24567428; PMCID: PMC4164759.
Goodman, L. R., Balthazar, U., Kim, J. & Mersereau, J. E. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Hum. Reprod. 27 (7), 2076–2081. https://doi.org/10.1093/humrep/des133 (2012). Epub 2012 May 2. PMID: 22552688; PMCID: PMC6457079.
Howlader, N., Cronin, K. A., Kurian, A. W. & Andridge, R. Differences in breast Cancer survival by molecular subtypes in the united States. Cancer Epidemiol. Biomarkers Prev. 27 (6), 619–626. https://doi.org/10.1158/1055-9965.EPI-17-0627 (2018). Epub 2018 Mar 28. PMID: 29593010.
Marklund, A. et al. Reproductive outcomes after breast Cancer in women with vs without fertility preservation. JAMA Oncol. 7 (1), 86–91. https://doi.org/10.1001/jamaoncol.2020.5957 (2021). PMID: 33211089; PMCID: PMC7677871.
Crown, A. et al. Fertility preservation in young women with breast cancer: impact on treatment and outcomes. Ann. Surg. Oncol. 29 (9), 5786–5796. https://doi.org/10.1245/s10434-022-11910-9 (2022). Epub 2022 Jun 7. PMID: 35672625; PMCID: PMC10118746.
Moravek, M. B. et al. Predictors and outcomes in breast cancer patients who did or did not pursue fertility preservation. Breast Cancer Res Treat. ;186(2):429–437. (2021). https://doi.org/10.1007/s10549-020-06031-4. Epub 2021 Jan 4. PMID: 33392838.
Crown, A. et al. Does use of neoadjuvant chemotherapy affect the decision to pursue fertility preservation options in young women with breast cancer?? Ann. Surg. Oncol. 27 (12), 4740–4749. https://doi.org/10.1245/s10434-020-08883-y (2020). Epub 2020 Aug 7. PMID: 32767225; PMCID: PMC7554118.
Hunan Expert Collaboration Group on Fertility Preservation for Breast Cancer Patients. Hunan expert consensus on implementation plan of fertility preservation for young breast cancer patients. Chin. J. Gen. Surg. 27 (11), 1361–1369. https://doi.org/10.7659/j.issn.1005-6947.2018.11.001 (2018).
Ruggeri, M. et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European centers. Breast 47, 85–92 (2019). Epub 2019 Jul 10. PMID: 31362134.
Armuand, G. M., Wettergren, L., Rodriguez-Wallberg, K. A. & Lampic, C. Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer. 22 (10), 2805–2812. https://doi.org/10.1007/s00520-014-2279-z (2014). Epub 2014 May 11. PMID: 24817617; PMCID: PMC4153973.
Urech, C. et al. Knowledge about and attitude towards fertility preservation in young female cancer patients: a cross-sectional online survey. Hum. Fertil. (Camb). 21 (1), 45–51 (2018). Epub 2017 Sep 22. PMID: 28934899.
Nahata, L., Sivaraman, V. & Quinn, G. P. Fertility counseling and preservation practices in youth with lupus and vasculitis undergoing gonadotoxic therapy. Fertil. Steril. 106 (6), 1470–1474. https://doi.org/10.1016/j.fertnstert.2016.07.1102 (2016). Epub 2016 Aug 10. PMID: 27521770.
Lambertini, M. et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast 42, 41–49 (2018). Epub 2018 Aug 22. PMID: 30170202.
Wang, Y. et al. Female oncofertility attitude and knowledge: a survey of reproductive health professionals in Shanghai, China. Future Oncol. ;15(4):371–379. doi: 10.2217/fon-2018-0428. Epub 2019 Jan 8. PMID: 30620219; PMCID: PMC6462868. (2019).
Oktay, K. et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. ;36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914. Epub 2018 Apr 5. PMID: 29620997. (2018).
Lambertini, M. et al. Gonadotropin-Releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: A systematic review and Meta-Analysis of individual Patient-Level data. J. Clin. Oncol. 36 (19), 1981–1990 (2018). Epub 2018 May 2. PMID: 29718793; PMCID: PMC6804855.
Tseng, L. M. et al. Developing a Web-Based shared Decision-Making tool for fertility preservation among Reproductive-Age women with breast cancer: an action research approach. J. Med. Internet Res. 23 (3), e24926. https://doi.org/10.2196/24926 (2021). PMID: 33729164; PMCID: PMC8074988.
Speller, B. et al. The begin exploring fertility options, risks and expectations (BEFORE) decision aid: development and alpha testing of a fertility tool for premenopausal breast cancer patients. BMC Med. Inf. Decis. Mak. 19 (1), 203 (2019).
Acknowledgements
We would like to thank the patients who participated in this study and the Precision Medicine Centerof the First Affliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, 710061, P.R. China.
Funding
This manuscript is supported by the National Natural Science Foundation of China (Juan Ren, 82473252, 81772793); the Scientific and Technological Research Foundation of Shaan’xi Province, Key Research and Development Project, General project (Juan Ren, 2023-YBSF-666); the Shaanxi Provincial Key Research and Development Plan Project (Key support projects Category A) (Juan Ren, 2021A011); the Basic and Clinical Integration Innovation Project of Xi’ an Jiao tong University (Juan Ren, YXJLRH2022006; Ying Gao, YXJLRH 2022004); and the Nanjing Tianqing Scientific Research Fund of First Affliated Hospital of Xi’an Jiaotong University (Juan Ren, TQ202205).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Min Chen, Xuan Wang. The first draft of the manuscript was written by Min Chen. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, M., Wang, X., Lan, N. et al. Prevalence and impact of fertility preservation among young women with breast cancer. Sci Rep 15, 7549 (2025). https://doi.org/10.1038/s41598-025-91197-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91197-4
Keywords
This article is cited by
-
Cancer and IVF: assessing fertility preservation options and associated cancer risks
Bulletin of the National Research Centre (2025)