Abstract
Previous reports have indicated that the survival rate of total mastectomy (TM) is higher than that of breast-conserving surgery (BCS). This study established survival prediction models for T1-stage locally advanced breast cancer (LABC) comparing TM and BCS, aiming to identify risk factors for overall survival (OS) associated with different surgical approaches and provide a basis for individualized treatment by clinicians. Cases of pathologically confirmed T1 LABC between 2010 and 2015 were retrieved from the Surveillance Epidemiology and End Results (SEER) database. COX regression analysis was used to analyze the relationship between LABC TM, BCS and various factors. Hazard ratio (HR) and 95% confidence interval (95%CI) were calculated to determine the possible influencing factors. Significant factors from multivariate COX regression were included into the models construct nomograms. Receiver operating characteristic curves (ROC), area under the curve of ROC (AUC), calibration curves, and the Hosmer-Lemeshow goodness-of-fit test for the calibration curves were generated. Model validation was conducted in a separate validation group. The results of COX regression analysis on survival rates for T1 LABC patients undergoing TM and BCS showed that the 5-year overall survival (OS) and breast cancer-specific survival (BCSS) were higher in the BCS group compared to the TM group. Age, race, histological grade, N stage, molecular subtype, chemotherapy, and radiation therapy (RT) were associated with 5-year OS of BCS. Similarly, age, race, pathological type, histological grade, human epidermal growth factor receptor 2 (HER2) status, N stage, molecular subtype, chemotherapy, and RT were correlated with 5-year OS of TM. Prediction nomograms were established using the aforementioned predictors, resulting in AUCs of 0.743 (for 5-year OS of BCS) and 0.718 (for 5-year OS of TM) in the modeling group. Both models were well-validated in the validation group. This study found that the survival rate of the BCS group was higher than that of the TM group, indicating that tumor size determines the survival rate of BCS to some extent. Lymph node status cannot be considered a contraindication for BCS surgery, suggesting that BCS can be considered for LABC patients with smaller tumors and more lymph node metastases. However, patients with primary tumors in N3 stage, triple-negative, and inner upper quadrant have a higher risk of death after BCS compared to other groups, so BCS should be carefully considered for these patients.
Similar content being viewed by others
Introduction
In 2022, the World Health Organization (WHO) reported that 2.3 million women worldwide were diagnosed with breast cancer (BC)1. The 8th edition of the American Joint Committee on Cancer (AJCC) defines BC in clinical stages IIIA-C as locally advanced breast cancer (LABC)2. With increasing public awareness of BC and advancements in medical technology, the mortality rate of BC has declined. However, for patients diagnosed as LABC, the mortality rate remains high, making their subsequent treatment critically important.
The treatment of LABC is a multidisciplinary and comprehensive treatment based on surgery, supplemented by radiotherapy (RT), chemotherapy, endocrine therapy, immunotherapy, and targeted therapy. Breast-conserving surgery (BCS) and total mastectomy (TM) represent the two mainstream surgical treatments for BC. According to literature, BCS can effectively improve patients’ quality of life compared to TM3. A meta-analysis revealed that BCS offers a higher overall survival (OS) rate than TM4. Xiang W compared the survival rates of stage I-III BC patients who received BCS + RT and TM + RT, and found that stage II BC patients who received BCS + RT had a higher survival rate than those who received TM + RT. Meanwhile, after identifying good predictors of BCSS for stage II BC, a relevant model was established to provide good guidance for the subsequent treatment of stage II BC5. However, the models did not perform well in terms of OS, especially those related to LABC. Therefore, it is important to set up a convenient, economical and effective clinical model for predicting BCS and TM survival related to T1 LABC .
On the one hand, this study aims to clarify the survival difference between BCS and TM, so as to provide guidance for clinicians to formulate more effective and individualized treatment plans for LABC. On the other hand, by establishing relevant models, it provides certain reference value for predicting the survival rate of LABC after BCS or TM, so as to provide clinicians with a general understanding of the survival rate after the choice of surgical methods. It is helpful to provide the optimal treatment plan for patients.
Materials and methods
Data source and patient population
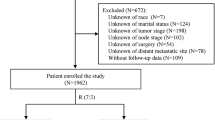
Analysis was performed on data from the National Cancer Institute (NCI)’s Surveillance, Epidemiology, and End Results (SEER). SEER is an open database, and regularly collects data on patient demographics, primary tumor location, tumor morphology and stage of diagnosis, and first course of treatment, and vital status follow-up data are regularly collected. This study searched the 17-SEER database submitted in November 2021. The inclusion criteria encompassed patients with a pathological diagnosis of BC between 2010 and 2015, female, aged ≥ 18 years, T1N2-3M0, who underwent BCS and TM. The exclusion criteria were: (1) cases with unknown or missing information; (2) cases where the surgical procedure involved no surgery, extended radical mastectomy, breast reconstruction, prophylactic contralateral mastectomy, or other surgical methods. Based on these inclusion and exclusion criteria, a total of 3,880 patients were finally included. The flowchart of patient inclusion is shown in Fig. 1. This study obtained permission to access the SEER database, with additional data on surgical procedures, chemotherapy, and RT.
Statistical methods
RStudio 4.3.1 software (R Foundation for Statistical Computing, Vienna, Austria) was used to analyze the data. The measurement data were expressed as mean ± standard (M ± SD), and the percentage of cases was used to express the count. The basic information of T1 LABC patients and the clinical characteristics of tumors were analyzed by t-test and Chi-square test. The “dplyr”, “survminer”, “survival”, “ggpubr”, and “ggplot2” functions of RStudio version 4.3.1 were used for survival analysis and data visualization, and the “coxph” function was used for univariate and multivariate COX analysis. Variables that were statistically significant in univariate analysis were included in multivariate COX regression analysis to identify factors that might affect BCS and TM survival in T1 LABC. The “rms” and “Hmisc” software package and “cph” functions of RStudio were used to fit the COX regression prediction model, and the significant factors in the multivariate COX regression were included in the model and the “plot” function was used to draw a Nomogram. For internal validation of the model, this study used the sample() function to divide the data into training set (70%) and test set (30%) by simple random sampling (Table 1). The Receiver operating characteristic curve (ROC) of the nomogram model for the training set and the test set were drawn respectively, and the area under ROC curve (AUC) and calibration curve were calculated. The Bootstrap repeated self-sampling method was used to draw the calibration curve of the model, and the Hosmer-Lemeshow goodness-of-fit test of the calibration curve was performed by the R software package “ResourceSelection”.
The software SPSS 25.0 (IBM Corp., Armonk, NY, USA) was used to clean the data, and P < 0.05 was considered statistically significant.
Ethics declarations
This study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). The SEER program is the authoritative source of information on cancer incidence and survival in the United States, and the SEER repository does not require institutional review board permission because it is an open, definitive database.
Results
Basic information about the patient and tumor characteristics
Among 3,880 female patients with T1 LABC included in the study, 1,902 patients (49.02%) were in the BCS group and 1,978 patients (50.98%) were in the TM group. In the modeling group, there were 1,348 patients (49.63%) in the BCS subgroup with an average age of 59.85 ± 12.20 years, and 1,368 patients (50.37%) in the TM subgroup with an average age of 59.89 ± 13.31 years. The follow-up period ended on December 31, 2020, with an average follow-up time of 84.41 months. T-test and chi-square test were used to analyze the differences between groups (Table 2). The results showed that in terms of adjuvant therapy, the proportion of BCS patients receiving RT was higher than that of TM patients (BCS vs. TM = 66.8% vs. 58.8%), but there was no significant difference in the distribution of chemotherapy (P = 0.598). At the same time, this study found that BCS and TM were significantly associated with race, T stage, N stage, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER-2) status, primary tumor location and RT distribution were statistically different (P < 0.05).
To analyze the long-term survival outcomes of BCS versus TM
Kaplan-Meier curves for 5-year OS and BCSS were plotted separately for BCS and TM. The results revealed that 1,902 patients opted for BCS, while 1,978 patients chose TM. The 5-year OS and BCSS rates for BCS were 80.73% and 85.94%, respectively. For TM, the 5-year OS and BCSS rates were 75.78% and 82.83%, respectively. Notably, the survival rates for BCS were higher to those for TM (OS: P < 0.001; BCSS: P = 0.001) (Fig. 2a, b).
Univariate and multivariate COX analysis results of BCS and TM in the modeling group
Variables identified as significant in the univariate COX analysis were included in a multivariate COX regression analysis. The results showed that age, race, histological grade, N stage, molecular type, chemotherapy and RT were associated with 5-year OS of BCS. And those showed that age, race, pathological type, histological grade, HER2 status, N stage, molecular type, chemotherapy and RT were associated with 5-year OS for TM. The study found that the total mortality risk of patients with N3 BCS was 0.503 times higher than that of patients with N2 BCS, and the overall risk of death was 0.365 times higher among blacks compared with whites. In terms of molecular subtypes, the overall risk of death of triple-negative LABC was 2.256 times higher than that of HER2-positive LABC. The risk of death after BCS for primary tumors in the upper inner quadrant was higher than that in the other groups. See Table 3 for details.
Nomogram for 5-year OS of BCS and TM in T1 LABC and validation
The significant variables in the multivariate COX analysis were included in the prediction model and the Nomogram was plotted. The individual scores of each influencing factor were obtained by the score scale, and then the scores of each factor were added to obtain the total score. And the corresponding 5-year OS probabilities of BCS and TM were obtained by comparing the scores of the total scale below the Nomogram. It was found that race and molecular typing had the greatest impact on 5-year OS in T1 LABC BCS, and the survival probability of the predicted model ranged from 0.10 to 0.95 (Fig. 3a). Race and pathological type had the greatest impact on OS at 5 years for T1 LABC TM, and the predictive model had survival probabilities ranging from 0.10 to 0.95 (Fig. 3b). RStudio version 4.3.1 was used to draw the ROC, and the AUC of the modeling group was 0.743 (5-year OS of T1 LABC BCS) and 0.718 (5-year OS of T1 LABC TM), respectively (Fig. 3c). The AUC of the validation group was 0.753 (5-year OS for T1 LABC BCS) and 0.685 (5-year OS for T1 LABC TM), respectively (Fig. 3d). Internal validation of the nomogram model was conducted by employing the bootstrap repeated resampling method. The calibration curve obtained through 1000 repetitions of bootstrap self-sampling revealed that the simulated curve and the actual curve in the modeling group were consistent in direction and approximately close to the ideal curve, indicating a favorable calibration effect of this model (Fig. 3e and f). Similarly, the simulated curve and the actual curve in the validation group were consistent in direction and approximately close to the ideal curve, suggesting a good calibration effect of the model (Fig. 3g and h).
Cox survival model, roc, and calibration curves. (a, b) In the nomogram of T1 LABC COX survival prediction model, the line corresponding to each variable is marked with a scale, representing the value range of the variable, and the length of the line segment reflects the contribution of the factor to clinical outcome events. Points: Represents the corresponding single-item scores for each variable at different values. Total points: Represents the total value of the sum of the corresponding single item scores after the values of all variables. (c) The ROC curves of BCS and TM in the modeling group were calculated. (d) The ROC curves of BCS and TM in the validation group were calculated. (e–h) Calibration curve: Apparent: the probability of direct prediction according to the model, that is, the original prediction probability of the model output; Ideal: a perfect prediction in which the predicted probability is exactly the same as the observed probability; Bias-corrected: predicted probability corrected by bootstrap calibration method. The Bootstrap repeated self-sampling method was used to internally verify the nomogram model. The calibration curve obtained by repeated Bootstrap self-sampling for 1000 times showed that the trend trajectories of the simulated curve and the actual curve were basically the same, which had a strong consistency, indicating that the calibration effect of the model was good. (e) OS at 5 years of BCS in the modeling group. (f) OS at 5 years of TM in the modeling group. (g) OS at 5 years of BCS in the validation group. (h) OS at 5 years of TM in the validation group.
Discussions
Advances in modern medicine have, to some extent, reduced the mortality rate of BC. However, the increasing incidence of BC over the years has heightened people’s attention to the disease, especially LABC, which has unique characteristics. This study starts with the common surgical methods for T1 stage LABC. By establishing a COX regression prediction model, it provides a reference for predicting the 5-year OS and BCSS survival probabilities of BCS and TM. This gives clinicians a general understanding of survival rates after surgical method selection, which aids in providing optimal treatment plans for patients.
Compared to the BCS group, patients with N3 disease who received TM had a higher incidence rate (31.7% vs. 25.7%). This suggests that clinicians have traditionally considered the number of lymph node metastases as an important indicator for selecting surgical methods. This may partially explain the lower OS observed for TM compared to BCS in the Kaplan-Meier curve. However, it’s important to note that the study results are influenced by various factors. Whether removing the factor of lymph node metastasis count would significantly impact the study findings remains unknown, and further research is needed to confirm this.
The comparison of survival differences between BCS and TM has been a hot topic. Previous studies have confirmed that the survival rate of BCS is higher than that of TM6,7. It is worth noting that this study compares the survival differences between pure BCS and TM, while previous studies tended to focus on BC + RT and TM. Rosenberg SM’s team studied the postoperative quality of life and psychosocial aspects of BCS and TM patients, concluding that BCS patients have better long-term body image, sexual health, and lower anxiety levels compared to post-TM patients8. In recent years, there has been a growing speculation that BCS may offer superior survival benefits compared to TM. On one hand, the application of neoadjuvant therapy has broadened the indications for BCS, which has facilitated clinicians’ increased selectivity of patients to some extent. On the other hand, cancer cells can interact with surrounding stromal cells and inflammatory cells to form an inflammatory tumor microenvironment (TME). Cells within the TME exhibit high plasticity and can influence tumor progression by altering their phenotypic and functional characteristics9. Based on two special types of cells, disseminated tumour cells (DTCs) and circulating tumour cells (CTCs), the breast tumor homing hypothesis has been proposed. This hypothesis suggests that during the development of BC, cancer cells not only have the ability to spread throughout the body via the bloodstream or lymphatic system but also possess the capability to return to the primary tumor site10. After treatment, DTCs and CTCs persist in peripheral blood or bone marrow in a dormant state11. The epithelial-mesenchymal transition (EMT) process, induced by the TME providing favorable conditions, can lead to the exit of DTCs and CTCs from dormancy12. Once activated, these tumor cells can proliferate and grow under the regulation of various cells and genes in the organism. Compared to the “new environment” of TM, the TME present in the primary lesion of BCS provides a more suitable environment for the rapid proliferation of DTCs and CTCs, laying the foundation for local recurrence. This explains why patients undergoing BCS are more prone to local recurrence at the primary tumor site compared to those undergoing TM. The majority of deaths in BC patients are caused by tumor metastasis. In patients undergoing TM, DTCs and CTCs make various organs throughout the body suitable containers for tumor cells, providing natural nutrients for distant metastasis and recurrence of the tumor, ultimately leading to the death of the organism. Therefore, the breast tumor homing hypothesis partly explains why the survival rate of TM is inferior to that of BCS13.
Age has always occupied an irreplaceable position in BC. Previous studies have shown that the choice of LABC surgical methods was related to age. With the rise of the trend of young BC, more and more young patients were opting for TM and contralateral prophylactic mastectomy14. Several studies had pointed out that young BC patients had a higher risk of recurrence and more aggressive disease compared with older patients with similar disease characteristics15,16. This study found that age is an independent influencing factor for both BCS and TM, and there is a negative correlation between age and OS in BCS and TM. This finding differs from the results of previous studies. We hypothesize that prior research may not have fully considered the unique characteristics of elderly patients when comparing the correlation between age and prognosis. Such patients may have received inadequate or excessive treatment due to factors such as incomplete management systems and the lack of evidence-based data to guide treatment, ultimately leading to delayed disease treatment17. In addition to the above reasons, the underlying diseases that may exist in elderly patients themselves can also affect OS. For example, Diabetes mellitus (DM) will not only increase the incidence of autoimmune diseases, but also damage the innate and adaptive immune systems18. As an important cause of death or disability in the world, hypertension has always occupied an irreplaceable position in medicine. The activation of innate and adaptive immune cells leads to hypertension. In the continuous state of hypertension, it will induce stroke, heart failure, renal failure and other diseases, and the risk of damage cannot be reduced even if the blood pressure is reduced to normal19. The irreversible damage caused by chronic diseases can lower the tolerance and immunity of elderly patients, whether it is the initial surgical treatment during the treatment period, or the subsequent chemotherapy and maintenance treatment. In addition, some chemotherapy drugs have different side effects, some of which can cause irreversible damage to the body. And this may lead to reduced selectivity of treatment options or ineligibility for existing treatment options. Although these results provide some speculation for the reasons why younger patients have better OS than elderly patients, more prospective studies are needed to explore and confirm the specific reasons and mechanisms.
The combination of BCS + RT for the treatment of BC has become an internationally recognized therapeutic approach. This study found that compared to the TM group, a higher proportion of patients in the BCS group received RT (BCS vs. TM = 66.8% vs. 58.8%). The first case of BC treated with RT was initiated by Emil Grubbé in 189620. Since then, the application of RT in BC has been favored by medical researchers, evolving from initial postoperative RT to intraoperative RT, and more recently to neoadjuvant radiotherapy (NART). The increasing use of RT in BC is reflected not only in the sequence of treatment with surgery, but also in the dosage and target areas21. Cláudia Sousa discovered that NART can effectively reduce the tumor size of inoperable LABC, transforming it into operable LABC22. Jasmina Mladenovic and colleagues included 134 patients with LABC in their study and observed the clinical tumor response after concurrent NART + TM. The results showed that patients who achieved pathological complete response (pCR) with NART demonstrated a trend of longer disease-free survival (DFS)23. For patients with positive sentinel lymph node (SLN) biopsy results, the traditional treatment approach has been routine axillary lymph node dissection (ALND). However, the AMAROS and ACOSOG Z011 trials have suggested that axillary radiotherapy (ART) can be used as an alternative to ALND for patients with cT1-2N1M0 BC24,25. Unfortunately, there are currently no relevant trials demonstrating the applicability of this treatment regimen to N2-3 BC patients. In recent years, it has been proposed that LABC patients who respond well to neoadjuvant therapy could consider BCS followed by postoperative RT as a substitute for TM26,27. Although this provides a promising approach for LABC, it still requires confirmation through prospective studies.
Interestingly, this study found that T1 LABC located in the upper inner quadrant had worse OS when receiving BCS compared to TM. In clinical practice, we have observed that tumor location plays a significant role in the prognosis of BC. Previous study has demonstrated that tumors in the medial quadrant have lower survival rates compared to those in the lateral quadrant28. Additionally, it has been suggested that this may be related to the difficulty of detecting these quadrants early through mammography and the higher likelihood of internal mammary lymph node metastasis29. We hypothesize that BC located in the medial quadrant, due to their elusiveness in early stages and proneness to intra-mammary lymph node metastasis, may result in some BC patients already experiencing intra-mammary lymph node metastasis when clinical intervention becomes necessary. The introduction of the concept of “lymphatic reflux” provides a compelling explanation for the findings of this study. When cancer cells metastasize to the internal mammary lymph nodes, they impede the normal lymphatic drainage of the internal mammary chain (IMC), causing subsequently produced lymphatic fluid to be diverted either to the axilla via lateral lymph nodes or to the liver via deep lymph nodes. For BC patients who have not undergone lymph node dissection, there is a high risk of metastasis to organs throughout the body. This may be one of the reasons why patients with tumors in the superomedial quadrant have lower survival rates after BCS compared to TM.
The primary strength of this study lies in its population-based characteristics, including the stratification of patients’ baseline and clinicopathological features. However, we also acknowledge several limitations of this research. Firstly, as a retrospective study, it signifies that we cannot completely eliminate confounding factors and potential selection biases. Nevertheless, due to the absence of a randomized controlled trial comparing outcomes between BCS and TM in LABC patients, our study reports results based on real-world data. Therefore, we believe that given the routine treatment practices of BC surgery, these findings can be considered by clinicians. Secondly, the SEER dataset lacks detailed information about chemotherapy regimens, endocrine therapy, anti-HER2 targeted therapy, or sequential surgery and chemotherapy data, as well as disease recurrence patterns and treatment histories after tumor relapse. Additionally, it excludes specifics on RT techniques, target volumes, and RT dosages. Thirdly, this study did not include patients receiving neoadjuvant therapy, and those who underwent axillary surgery were not separately grouped based on the type of surgery. Lastly, the SEER database does not capture the genetic information of tumors.
The advantages and disadvantages of BCS and TM have always been a hot topic for medical workers to discuss. And various guidelines and relevant expert consensus also have certain indications for the selection of BCS and TM procedures. Medicine is a discipline developing in a spiral of constant affirmation, denial and affirmation. Precision medicine is the stage goal of this discipline. In order to achieve this goal, it is particularly important to provide effective treatment plans. The emergence of clinical prediction model is an important node of medical progress. It integrates, analyzes and verifies different types of data, and then summarizes the probability value of an event in a specific scenario. Finally, it is presented in the way of data visualization to provide help for doctors to diagnose or predict the prognosis of diseases, so as to provide reference for clinical decision-making. The aim of this study was to compare the 5-year OS between BCS and TM in patients with T1 LABC, and to found out the risk factors of OS in different surgical procedures, so as to provide a basis for clinicians to individualized treatment.
Conclusions
This retrospective study found that the survival rate of the BCS group was higher than that of the TM group, indicating that tumor size determines the survival rate of BCS to a certain extent, while lymph node status cannot be considered a contraindication for BCS. The data from this study can inform clinicians that LABC with smaller tumor masses can be considered for BCS, and BCS should not be abandoned due to multiple lymph node metastases. This study also found that compared to stage N2, the overall risk of death for patients with stage N3 BCS increased by 0.503 times. The overall risk of death for triple-negative LABC was 2.256 times higher than that for HER2-positive LABC. The risk of death after BCS for primary tumors in the upper inner quadrant was higher than that for other groups. Therefore, caution should be exercised when considering BCS for patients with N3, triple-negative, or upper inner quadrant tumors.
Data availability
The data for this study can be reasonably obtained from the first author. And you can also submit a request to the SEER database to reasonably obtain data related to this study. https://seer.cancer.gov/.
References
World health organization. Breast cancer. (2024). www.who.int/zh/news-room/fact-sheets/detail/breast-cancer
Giuliano, A. E. et al. Breast Cancer-Major changes in the American joint committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 67, 290–303. https://doi.org/10.3322/caac.21393 (2017).
Zehra, S., Doyle, F., Barry, M., Walsh, S. & Kell, M. R. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer. 27, 534–566. https://doi.org/10.1007/s12282-020-01076-1 (2020).
De et al. Does Breast-Conserving surgery with radiotherapy have a better survival than mastectomy?? A Meta-Analysis of more than 1,500,000 patients. Ann. Surg. Oncol. 29, 6163–6188. https://doi.org/10.1245/s10434-022-12133-8 (2022).
Xiang, W. et al. Survival comparisons between breast conservation surgery and mastectomy followed by postoperative radiotherapy in stage I-III breast Cancer patients: analysis of the surveillance, epidemiology, and end results (Seer) program database. Curr. Oncol. 29, 5731–5747. https://doi.org/10.3390/curroncol29080452 (2022).
Rajan, K. K. et al. Overall survival after mastectomy versus breast-conserving surgery with adjuvant radiotherapy for early-stage breast cancer: meta-analysis. BJS Open. 8, zrae040. https://doi.org/10.1093/bjsopen/zrae040 (2024).
Van, Maaren, M. C. et al. 10 Year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 17, 1158–1170. https://doi.org/10.1016/S1470-2045(16)30067-5 (2016).
Rosenberg, S. M. et al. Association of breast Cancer surgery with quality of life and psychosocial Well-being in young breast Cancer survivors. JAMA Surg. 155, 1035–1042. https://doi.org/10.1001/jamasurg.2020.3325 (2020).
Denk, D. & Greten, F. R. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 8, 901–914. https://doi.org/10.1016/j.trecan.2022.07.002 (2022).
Kim, M. Y. et al. Tumor self-seeding by Circulating cancer cells. Cell 139, 1315–1326. https://doi.org/10.1016/j.cell.2009.11.025 (2009).
Goss, P. E. & Chambers, A. F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer. 10, 871–877. https://doi.org/10.1038/nrc2933 (2010).
Erin, N., Grahovac, J., Brozovic, A. & Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updat. 53, 100715. https://doi.org/10.1016/j.drup.2020.100715 (2020).
Mokbel, K. Unlocking the power of the homing phenomenon: why breast conserving surgery outshines mastectomy in overall survival. Clin. Breast Cancer. 24, 85–92. https://doi.org/10.1016/j.clbc.2023.10.003 (2023).
Nash, R. et al. State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral Early-Stage breast Cancer in the united States, 2004–2012. JAMA Surg. 152, 648–657. https://doi.org/10.1001/jamasurg.2017.0115 (2017).
Cancello, G. et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (< 35 years) with operable breast cancer. Ann. Oncol. 21, 1974–1981. https://doi.org/10.1093/annonc/mdq072 (2010).
Azim, H. A. Jr. et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin. Cancer Res. 18, 1341–1351. https://doi.org/10.1158/1078-0432 (2012).
Glaser, R., Marinopoulos, S. & Dimitrakakis, C. Breast cancer treatment in women over the age of 80: A tailored approach. Maturitas 110, 29–32. https://doi.org/10.1016/j.maturitas.2018.01.014 (2020).
Zhang, P. et al. Diabetes mellitus exacerbates experimental autoimmune myasthenia Gravis via modulating both adaptive and innate immunity. J. Neuroinflammation. 18 https://doi.org/10.1186/s12974-021-02298-6 (2021).
Madhur, M. S. et al. Hypertension: do inflammation and immunity hold the key to solving this epidemic?? Circ. Res. 128, 908–933. https://doi.org/10.1161/CIRCRESAHA.121.318052 (2021).
Grubbé, E. H. Priority in the therapeutic use of X-rays. Radiology 21, 156–162. https://doi.org/10.5694/mja16.01020 (1993).
Shah, C. et al. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol. 112, 9–16. https://doi.org/10.1016/j.radonc.2014.04.009 (2014).
Sousa, C. et al. Neoadjuvant radiotherapy in the approach of locally advanced breast cancer. ESMO Open. 4, e000640. https://doi.org/10.1136/esmoopen-2019-000640 (2020).
Mladenovic, J. et al. Tumor response and patient outcome after preoperative radiotherapy in locally advanced non-inflammatory breast cancer patients. J. BUON. 22, 325–333 (2017). PMID: 28534352.
Rutgers, E. J. et al. Abstract GS4-01: radiotherapy or surgery of the axilla after a positive Sentinel node in breast cancer patients: 10 year follow up results of the EORTC AMAROS trial (EORTC 10981/22023). Cancer Res. 79 https://doi.org/10.1158/1538-7445.SABCS18-GS4-01 (2019). GS4-01.
Bartels, S. A. L. et al. Radiotherapy or surgery of the axilla after a positive Sentinel node in breast cancer: 10-Year results of the randomized controlled EORTC 10981–22023 AMAROS trial. J. Clin. Oncol. 41, 2159–2165. https://doi.org/10.1200/JCO.22.01565 (2023).
Sun, Y., Liao, M., He, L. & Zhu, C. Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after good response to neoadjuvant chemotherapy: A PRISMA-compliant systematic review and meta-analysis. Med. (Baltim). 96, e8367. https://doi.org/10.1097/MD.0000000000008367 (2017).
Parmar, V. et al. Breast conservation treatment in women with locally advanced breast cancer - experience from a single centre. Int. J. Surg. 4, 106–140. https://doi.org/10.1016/j.ijsu.2006.01.004 (2006).
Colleoni, M. et al. Site of primary tumor has a prognostic role in operable breast cancer: the international breast cancer study group experience. J. Clin. Oncol. 23, 1390–1400. https://doi.org/10.1200/JCO.2005.06.052 (2005).
Han, Y. et al. Do breast quadrants explain Racial disparities in breast cancer outcomes? Cancer Causes Control. 30, 1171–1182. https://doi.org/10.1007/s10552-019-01222-x (2019).
Acknowledgements
We are deeply grateful to the Key Project of Jie ping Wu Medical Foundation Clinical Research Special Funding (No. 320.6750.2023-11-27) which believed in us and in our project, supporting economically our research group. We would like to thank all the members of our research team for their hard work.
Author information
Authors and Affiliations
Contributions
Fang Qian and Haoyuan Shen designed the study, Fang Qian, Dongtao Liu, Wei Chen collected the data, Fang Qian performed the statistical analysis, and Fang Qian and Haoyuan Shen jointly conceived the manuscript. Chenghao Liu provided important comments on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qian, F., Shen, H., Liu, C. et al. Establishment and validation survival prediction models for T1 locally advanced breast cancer after breast conservation surgery versus mastectomy. Sci Rep 15, 12189 (2025). https://doi.org/10.1038/s41598-025-91205-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91205-7