Abstract
The autonomic regulation of heart rate (HR) reactivity to acute hypoxia remains unclear. Parasympathetic cardioneuroablation (PCNA) may serve as a novel model for the analysis of physiological consequences of reduced vagal influence over sinus node in humans. We studied 11 adult patients scheduled for PCNA for the treatment of vasovagal syncope. HR reactivity to hypoxia was studied before and after PCNA with brief nitrogen gas administrations. Each test was followed by an atropine challenge to evaluate the contribution of parasympathetic tone to the resting HR. Additionally, we assessed changes in cardiac baroreflex sensitivity and HR variability following the procedure. PCNA led to partial parasympathetic denervation of sinus node at rest (67.0 ± 20.1%). This translated into a significant change in HR reactivity to hypoxia (0.58 ± 0.21 vs. 0.22 ± 0.13 beats min− 1%SpO2 − 1, p = 0.0001) which was proportional to the degree of cardiac vagal denervation (R = 0.76, p = 0.01). There was no change in peak HR on atropine following PCNA implying unchanged sympathetic input to sinus node. This suggests that HR reactivity to acute hypoxia is significantly influenced by parasympathetic system. Additionally, despite incomplete vagal denervation PCNA resulted in profoundly depressed HR variability and cardiac baroreflex sensitivity. The clinical meaning of the latter should be explored in further studies.
Similar content being viewed by others
Introduction
Tachycardia in response to acute hypoxia constitutes a basic, homeostatic mechanism common for all freely breathing mammals1. It originates from the activation of the glomus cells responding to reduced oxygen level. Those cells are found in two distinct anatomical locations: (1) at the bifurcation of the common carotid artery (carotid bodies – the predominant peripheral chemoreceptors), and (2) in the walls of the aortic arch (aortic bodies). Previously, we documented that bilateral carotid body resection did not disrupt hypoxic heart rate (HR) reactivity highlighting the role of the aortic bodies in this phenomenon in heart failure patients2. However, due to the possibility of a compensatory reaction from aortic bodies following carotid body resection the contribution of the former might be overestimated. On the other hand, direct activation of carotid bodies by local administration of adenosine in conscious humans leads rather to bradycardia than tachycardia3. Nevertheless, in a dog model Kato et al.4 elegantly demonstrated the role of the pulmonary stretch receptors (Hering-Brauer reflex)5,6 in tachycardic response to hypoxia due to hyperventilation mediated by intact carotid bodies. The influence of baroreceptors (unloading secondary to hypoxia-induced vasodilatation)7 in this response was also suggested; however, our recent study using controlled breathing in healthy subjects did not confirm those hypotheses8. Additionally, we are still lacking direct evidence from an invasive study linking aortic bodies activation to tachycardic response in humans. While our functional understanding of the afferent arm of the reflex is far from certain, the exact mechanism regarding the autonomic efferent arm also remains a matter of controversy.
The early works on the subject focused on the activation of the sympathetic system during hypoxic conditions. Cunningham et al. documented elevated plasma catecholamines9, and Hoon et al. noted raised urinary catecholamines10 at high altitude. Later, in several studies, an acute hypoxic challenge was found to produce an increase in the global sympathetic tone as measured with microneurography11,12,13. Those together with the documented link between the overall sympathetic activity and HR14 led to the notion of sympathetic system involvement in hypoxic tachycardia. The vagal withdrawal was also considered to play a role in the tachycardic response to hypoxia. In a dog model, Hamill et al. found that only simultaneous blockade of sympathetic (adrenalectomy combined with 6-hydroxydopamine) and parasympathetic systems (atropine) abolished HR reactivity to hypoxia15. Similar results were reported in humans where a combination of atropine and propranolol was necessary to prevent changes in HR during hypoxia16. Interestingly, in another study tachycardic effect of hypoxia during exercise persisted despite combined β-adrenergic and muscarinic receptor inhibition suggesting that α-adrenergic transmission might be involved in the HR response17. However, the contribution of non-autonomic mechanisms cannot be ruled out7. One such mechanism could be potentially related to the stretch-activation of cation nonselective ion channels within the sinoatrial node18 perpetuated by increased contractility19 due to hypoxic pulmonary vasoconstriction20.
Unfortunately, the papers cited above share one major drawback limiting the possibility of a direct assessment of the vagal influence over HR response to hypoxia. The blockade of the parasympathetic system with atropine (by abolition of tonic activity) results in a vast and consistent elevation of HR which obscures the potential HR changes occurring due to hypoxia. This is clearly seen in the paper by Koller et al. where complete autonomic blockade (atropine + propranolol) led to higher HR at normoxia compared to HR seen at maximal hypoxia in controls16. It suggests that atropine alone (i.e. in the setting of preserved sympathetic reactivity) would result in even greater baseline HR making unplausible the observation of potential HR changes due to sympathetically-mediated reflex responses to hypoxia. A similar problem is encountered in the work by Siebenmann et al. carried out again in the setting of chronic hypoxia. Here, the case for the dominant role of vagal tone in hypoxic HR response was made indirectly (i.e. by the exclusion of sympathetic influence) as adrenergic blockade did not affect HR reactivity to hypoxia21.
Hence, the model of selective parasympathetic cardioneuroablation (PCNA) recently introduced to cardiac electrophysiology for the treatment of vagally mediated syncope22,23 provides a unique and novel opportunity to study HR responses to hypoxia in human subjects. PCNA involves the percutaneous introduction of a radiofrequency catheter into the right and left atrium with subsequent thermoablation of ganglionated plexi located in epicardial adipose tissue (Fig. 1). PCNA rarely leads to complete vagal cardiac denervation24; however, the degree of denervation achieved with PCNA may be easily calculated using atropine challenges before and after the procedure. Those facts taken together allow for a direct assessment of relationships between the changes in cardiac parasympathetic tone and the changes in HR under hypoxic conditions.
Electroanatomical map depicting left atrium where red dots indicate ablation lesions applied to right superior ganglionated plexus supplying parasympathetic fibres to sinus node (A) and both atria where red dots indicate additional lesions applied to the same plexus from the right atrium (transparent). Green dots show the location of the phrenic nerve, which is spared during ablation (B).
Additionally, acute reduction in cardiac parasympathetic drive following PCNA may affect other important indices of HR reactivity. Those include heart rate variability (HRV) and cardiac baroreflex sensitivity (cBRS). Both are related to cardiovascular outcomes with low values seen in individuals with higher risk of cardiac events25,26. We are not aware of previous studies looking at those parameters together after PCNA and believe that potential changes in HRV and cBRS could not only shed new light on the role of the parasympathetic system in HR regulation in humans but may also carry important clinical meaning.
To address those issues we designed a study where HR response to hypoxia was assessed using previously validated methodology27 before and shortly after PCNA and where the magnitude of parasympathetic cardiac denervation was analysed with repeated atropine administrations. We also intended to report the changes in other indices of HR reactivity namely HRV and cBRS.
Methods
The study protocol was approved by the local Institutional Ethics Committee (Komisja Bioetyczna przy Uniwersytecie Medycznym we Wrocławiu; approval no. KB-331/2022) and conformed to the standards set by the Declaration of Helsinki. An informed consent has been obtained in writing from all study participants.
Inclusion and exclusion criteria
We enrolled patients hospitalized in our Institution for elective PCNA who had sinus rhythm on admission and were between 18 and 80 years old. We excluded pregnant women and individuals with previously documented atropine intolerance, glaucoma and those with structural heart disease or on chronic treatment with beta-blockers.
Study protocol
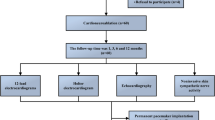
The study protocol (Fig. 2) consisted of: (1) Baseline non-invasive hemodynamic and electrocardiographic recording (lasting 15–20 min and preceded by 5 min of familiarization with study equipment); (2) Assessment of hemodynamic and ventilatory responses to acute hypoxia (approximately 30–40 min); (3) Atropine administration (10 min); (4) PCNA procedure. Items 1–3 were performed twice i.e. day before and the day after PCNA with approximately 48 h interval between the sets. All tests (items 1–3) were performed in a supine position in a quiet, light-attenuated room with a stable temperature of 22–24 °C. Subjects were asked to avoid caffeine intake for 24 h preceding testing.
Study protocol with examples of the tracings taken from the same study participant before (A) and after (B) parasympathetic cardioneuroablation (PCNA). An asterisk (*) indicates the familiarization period, while the arrow points to a high-dose atropine bolus. Note that, PCNA leads to the suppression of beat-to-beat cardiac parasympathetic activity while blood pressure variability remains unchanged.
Circulatory and ventilatory parameters
Hemodynamic measurements including HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), cardiac output (CO) and systemic vascular resistance (SVR) were taken noninvasively using Finapress monitor (FMS, Enschede, Netherlands). Ventilation was assessed with a one-way open breathing circuit (Hans Rudolph, Inc., Shawnee, United States). The inhalation arm of the circuit served to administer room air or nitrogen gas (100% N2) during trials of acute hypoxia. Exposure to N2 was silently controlled using a high-pressure electric valve. The exhale arm of the breathing circuit was connected to a flow head (MLT3000L; ADInstruments, Sydney, Australia) fitted with a differential pressure transducer (FE141 Spirometer; ADInstruments) which served to measure breathing rate (BR) and tidal volume (TV), and from this minute ventilation (MV) was calculated. A pulse oximeter (Radical-7; MasimoCorp., Irvine, CA, USA) with a lightweight ear clip was employed to evaluate blood oxygen saturation (SpO2). Measurement of end-tidal CO2 (ETCO2) was carried out with a capnograph (CapStar; CWE Inc., Ardmore, USA) at the expiratory arm of the breathing circuit.
For hemodynamic and ventilatory parameters (Table 1) we averaged the last 30 s of the recording directly preceding initiation of hypoxic testing. For the assessment of HR changes due to atropine, we averaged the last 30 s before atropine injection and the last 30 s from 5 min period after atropine administration.
Atropine challenge test
To achieve complete parasympathetic blockade we used high dose i.v. atropine (0.04 mg/kg)28. This manoeuvre was performed before and after PCNA to measure the total preprocedural parasympathetic tone and residual postoperative parasympathetic tone. A difference between those (shown as % of the total tone) represented the degree of parasympathetic cardiac denervation. In one case acute urinary retention following atropine challenge precluded its administration following PCNA.
Hemodynamic and ventilatory responses to hypoxia
To assess hemodynamic and ventilatory responses to hypoxia we used methodology based on repeated, acute, poikilocapnic (allowing for varying levels of ETCO2) hypoxia previously validated27 and used by our group2,29. Briefly, subjects were silently switched using pressure-controlled valve from breathing room air to breathing 100% N2 for the time period lasting 15 s. Participants were not aware of the type of breathing mixture they were receiving. This procedure was repeated 5–8 times (depending on tolerability) per testing session (over a total period of 30–40 min; Fig. 2). The lengths of the following N2 exposures were adjusted ad hoc based on the fall in SpO2 caused by the first 15 s N2 administration. The sequence of subsequent exposures (of different durations) was chosen randomly. After each N2 administration study participants were allowed to rest for approximately 5 min breathing room air, which was sufficient for studied parameters to return to the baseline. Minimal SpO2 achieved during hypoxic reactivity testing was comparable before and after PCNA (59.4 ± 9.1 vs. 63.8 ± 6.6%, p = 0.21). Previous studies documented the safety of this methodology related to very short periods of desaturation27,30,31.
In order to measure HR response to hypoxia we plotted SpO2 nadir (achieved with first N2 exposure) against the post-hypoxic peak value of HR providing Point (A) The same was done for baseline (averaged from 90 s prior to N2 administration) SpO2 and corresponding baseline HR providing Point (B) The slope of the regression line linking Point A and Point B was identified. This was repeated for every N2 exposure. The arithmetic average of the values of the slopes for all N2 administrations was interpreted as a measure of HR response to hypoxia (hHR slope). The same procedure was performed for SBP, DBP and MV to calculate respective hypoxic reactivities: hSBP slope, hDBP slope and hMV slope.
Assessment of heart rate and systolic blood pressure variability
For HRV analysis we used methods based on time-domain, spectral-domain and Poincaré plot32. From various time-domain indices we report the standard deviation of NN intervals (SDNN, ms), a measure validated in short-term recordings33 with a documented prognostic significance, and the proportion of NN50 (the number of times successive heartbeat intervals exceed 50ms divided by the total number of NN intervals, pNN50, %) – a marker of parasympathetic phasic activity under resting conditions34,35,36. SDNN was calculated using Nevrokard BPV version 9.0.0 (Nevrokard Kiauta, Izola, Slovenia; https://www.nevrokard.eu) and pNN50 with LabChart Pro (ADInstruments) from 5 min period of an acceptable quality taken from the baseline recording and from the recording after atropine administration. The same period and software were used to determine the absolute power for HRV (ms2) and systolic blood pressure variability (SBPV, mmHg2) in the low-frequency band (LF, 0.04–0.15 Hz) and high-frequency band (HF, 0.15–0.4 Hz). HRV and SBPV were separated into their components using Fast-Fourier Transformation and Hanning window. The Poincaré plot was constructed by plotting each R-R interval as a function of the previous one using Nevrokard BPV version 9.0.0 (Nevrokard Kiauta, Izola, Slovenia; https://www.nevrokard.eu). The dispersion of this cloud of points was characterized by two indices: the standard deviation along the identity line (SD2) and along its perpendicular (SD1). While SD1 represents beat-to-beat HRV and is a marker of parasympathetic influence37, SD2 reflects long-term HRV and gives insight into both sympathetic and parasympathetic activity38.
Assessment of cardiac baroreflex sensitivity
For the calculation of cBRS we employed two methods. (1) The sequence method is based on the identification of sequences consisting of three (or more) consecutive heartbeats where an increase (or decrease) in RR interval duration by at least 5.0 ms is accompanied by an increase (or decrease) in SBP by at least 0.5 mmHg as described by Di Rienzo et al.39 This spontaneous variability in SBP is related to the interaction between the sympathetic system, ventilation, nitric oxide release, renin-angiotensin-aldosterone system, behavioral and emotional factors40. An average slope of all regression lines linking RR intervals duration to SBP (an average slope of all sequences) was considered a measure of cBRS gain (cBRS-seq, ms mmHg− 1)39. We reported cBRS-seq only if at least 3 sequences were identified. We also presented the number of sequences as a valuable measure of baroreflex function41 – especially after PCNA (or atropine) when the low number of sequences occasionally precluded reliable analysis based on averaged regression slopes. (2) In the spectral method cBRS is expressed as a ratio of HRV and SBPV within two frequency bands: 0.04–0.15 Hz (cBRS-αLF, ms mmHg− 1) and 0.15–0.4 Hz (cBRS-αHF, ms mmHg− 1)42,43. The coherence criterion of > 0.5 was not required as in subjects with severely depressed baroreflex the coherence tends to zero44. For the cBRS assessment, we used the same 5 min periods as for HRV. The calculations were carried out with Nevrokard BRS version 6.3.0 (Nevrokard Kiauta, Izola, Slovenia; https://www.nevrokard.eu). One individual with frequent ectopic beats had to be removed from the analysis of HRV and cBRS.
Parasympathetic cardioneuroablation
After obtaining the vascular access, a transseptal puncture was performed. Then, the mapping / ablation catheter was introduced to the left atrium (Thermocool SmartTouch, Biosense Webster, Irvine, USA) to produce a three-dimensional map using an electroanatomical system (CARTO, Biosense Webster, Irvine, USA). Next, based on anatomical landmarks a thermoablation of parasympathetic ganglionated plexi was performed45,46,47. After ablating all regions of interest within the left atrium we moved to the right atrium where additional ablation lesions were applied with careful attention to sparing the phrenic nerve and sinus node (Fig. 1). It ought to be mentioned that PCNA results in the disruption of both efferent (from nucleus ambiguus ventrolateral to the heart) and afferent (from the heart to nucleus tractus solitarius) parasympathetic fibers. The functionality of both types is interwoven as the afferent fibers are involved in the regulation of the efferent parasympathetic and sympathetic activity48. No periprocedural complications were noted, and all patients were fully ambulant on the following day.
Statistical analysis
Spotfire Statistica version 13.3.721.0 (TIBCO Software Inc., Santa Clara, USA; https://docs.tibco.com/products/spotfire-statistica), LabChart Pro (ADInstruments) and MATLAB (MathWorks, Natick, USA) were used to analyse the data. Shapiro-Wilk test was used to assess the normality. For normally distributed variables we performed: a paired test for two-group comparisons, one-way analysis of variance (ANOVA) with repeated measures followed by a Bonferroni post-hoc test for > 2 groups comparisons, and Pearson coefficient for correlations. For variables with skewed distribution, we carried out: the Wilcoxon test for two-group comparisons, Friedman non-parametric ANOVA followed by post-hoc Wilcoxon test (with Bonferroni correction) for > 2 groups comparisons and Spearman coefficient for correlations. Variables within text and tables are shown as mean and standard deviation. A P value < 0.05 was considered statistically significant.
Results
Characteristics of the studied group
The study was carried out in a group of 11 subjects scheduled for PCNA due to symptomatic vasovagal syndrome. All experienced recurrent syncopes nonresponsive to other forms of conservative therapy. The studied group comprised 5 males and 6 females without additional cardiovascular diseases (apart from one individual diagnosed with mild hypertension). The mean age was 36.8 ± 14.1 years and the mean body mass index was 23.6 ± 3.4 kg m− 2. Laboratory results showed a haemoglobin level of 14.4 ± 1.6 g% and a creatinine serum concentration of 0.8 ± 0.1 mg%. Echocardiography revealed a left ventricle ejection fraction of 62 ± 2.5%, a left ventricle end-diastolic diameter of 48.4 ± 5.6 mm, and a left atrium diameter of 35.8 ± 6.2 mm. None of the included participants was on calcium channel blocker, digoxin, or any antiarrhythmic medication possibly affecting HR. One male participant with mild hypertension was treated with an angiotensin-converting-enzyme inhibitor only. None of the participants was diagnosed with any metabolic or endocrine disorder (such as diabetes, thyroid dysfunction etc.) which could influence HR reactivity.
Changes in baseline hemodynamic and ventilatory parameters
We found a significant increase in baseline HR and CO with a concomitant decrease in SVR following PCNA. However, there was no change in SBP and DBP. All measured ventilatory parameters remained stable. Detailed data describing changes after PCNA are given in Table 1.
Changes in hypoxic reactivity
Minimal SpO2 obtained at hypoxic reactivity testing was 59.4 ± 9.1% before PCNA and 63.8 ± 6.6% after PCNA (p = 0.21). PCNA led to attenuation of hHR slope (0.58 ± 0.21 vs. 0.22 ± 0.13 beats min − 1%SpO2 − 1, p = 0.0001) and hDBP slope (0.24 ± 0.14 vs. 0.17 ± 0.13 mmHg %SpO2 − 1, p = 0.03). hMV slope (0.27 ± 0.14 vs. 0.36 ± 0.16 l min − 1%SpO2 − 1, p = 0.08) and hSBP slope (0.38 ± 0.26 vs. 0.33 ± 0.21 mmHg %SpO2 − 1, p = 0.41) remained unchanged.
Effect of atropine on heart rate control
Intravenous atropine injection (0.04 mg/kg) resulted in HR increase both before (from 60.5 ± 10.3 to 102.4 ± 15.8 beats min− 1, p < 0.0001) and after PCNA (from 83.0 ± 9.0 to 96.7 ± 12.0 beats min− 1, p = 0.02). HR augmentation was significantly greater before PCNA when compared to post-PCNA state (Δ = 41.9 ± 20.1 vs. Δ = 13.7 ± 9.1 beats min− 1, p = 0.0006). Maximal HR following atropine challenge did not differ before and after PCNA (102.4 ± 15.8 vs. 96.7 ± 12.0 beats min− 1, p = 1.00). The mean degree of parasympathetic cardiac denervation achieved with PCNA procedure was 67.0 ± 20.1% (see Fig. 3 for details).
Changes in heart rate and blood pressure variability
PCNA decreased HRV indices both in the time-domain and spectral-domain. We found significant reductions in: SDNN, pNN50, HRV-LF power and HRV-HF power (p < 0.05 for all). Similarly, SD1 and SD2 derived from Poincaré analysis showed a decrease post-PCNA (p < 0.05 for both). However, the HRV-LF/HF ratio, SBPV-LF power, and SBPV-HF power were unaltered following PCNA (p = NS for all) – see Table 2 for details.
Changes in cardiac baroreflex sensitivity
Cardiac BRS was profoundly inhibited following PCNA. We noted a decrease in: the number of sequences, cBRS-seq (where a number of sequences was sufficient to calculate the gain), cBRS-αLF and cBRS-αHF. P-value was < 0.05 for all pairwise comparisons between pre-PCNA and post-PCNA – see Fig. 4 for details.
Effects of atropine on heart rate variability, blood pressure variability and cardiac baroreflex sensitivity
Atropine given pre-PCNA reduced all indices of HRV and cBRS (Table 2). The changes in HRV and cBRS after atropine administered pre-PCNA and the changes produced by PCNA itself were comparable and significantly greater to the changes after atropine given post-PCNA (Fig. 5) Atropine did not exert an additional effect on SDNN, pNN50, SD1, SD2, HRV-LF power and HRV-HF power, number of sequences, cBRS-αLF and cBRS-αHF beyond PCNA (Table 2). Atropine did not affect SBPV (in both power bands) either pre-PCNA or post-PCNA (Table 2).
Absolute changes in the indices of heart rate variability (left panels) and cardiac baroreflex sensitivity (right panels) as a consequence of parasympathetic neuroablation (PCNA), atropine administration before PCNA (atropine pre-PCNA) and atropine administration after PCNA (atropine post-PCNA). Data are presented as means ± SD.
Relationships between the degree of parasympathetic cardiac denervation and changes in measured parameters
We found a significant correlation between the degree of parasympathetic cardiac denervation and relative change in hHR slope (R = 0.76, p = 0.01, Fig. 6, one patient was excluded from this analysis due to previously unknown atropine intolerance). No such relationships were present for the degree of parasympathetic cardiac denervation and relative changes in: hMV slope, hSBP slope, hDBP slope, SDNN, pNN50, SD1, SD2, HRV-LF power, HRV-HF power, SBPV, number of sequences, cBRS-αLF, and cBRS-αHF (p = NS for all).
Discussion
The procedure of PCNA is believed to be highly specific regarding the parasympathetic arm of the autonomic innervation of the heart49. However, based on our data and on previous reports PCNA rarely leads to total parasympathetic cardiac denervation50,51 which does not preclude high clinical effectiveness regarding syncopal events52. The simple observation that resting HR achieved after PCNA is usually ~ 20–30 bpm lower than HR seen after preprocedural atropine administration goes in line with this notion. On the other hand, the possibility of a concomitant decline in the sympathetic tone following PCNA was previously raised24 – perhaps due to inadvertent damage to the adrenergic fibres supplying sinus node. However, this observation was based solely on the analysis of the LF/HF ratio which usefulness for the measurement of sympathetic input to the heart is rather controversial53. Additionally, in a study by Qin et al.51 changes in the LF/HF ratio showed the opposite direction pointing at the questionable reliability of this parameter.
In the current study degree of parasympathetic cardiac denervation was calculated using atropine. Based on the previous report we assumed that atropine given in a dose of 0.04 mg/kg leads to complete parasympathetic blockade, and thus reveals the magnitude of sympathetic influence over resting HR28. By eliminating parasympathetic input to sinus node the remaining determinants of HR are: (1) sympathetic input and (2) intrinsic activity of pacemaker cells. Because the change in intrinsic HR after PCNA can be excluded due to careful electrophysiological mapping of the sinus node location it is the varying sympathetic input that may drive any potential changes between preprocedural and postprocedural HR on atropine. In our study, we found no significant difference between those values (p = NS for HR on atropine pre-PCNA vs. HR on atropine post-PCNA, see Fig. 3) implying unaltered sympathetic innervation of the sinus node. This is further supported by unchanged SBPV which in a paper by Laitinen et al. was related to sympathetic control of HR54.
Variable parasympathetic cardiac denervation (67.0 ± 20.1%) seen in our study is most likely determined by two factors. First, the penetrance of radio-frequency energy delivered from the endocardial side could have been not sufficient for the complete destruction of ganglionated plexi embedded at various depths into the pericardial fat tissue55. Second, some of the ganglionated plexi56 supplying sinus node may not have been identified using our approach due to high anatomical variability57.
Bearing in mind the lack of significant change in sympathetic activity in our study at normoxia, the two-fold decrease in hHR slope reflecting diminished hypoxic reactivity of the sinus node to acute hypoxia must be seen in light of the attenuated parasympathetic input to the heart. Importantly, we found a significant relationship between changes in hHR slope and the degree of parasympathetic cardiac denervation (Fig. 6) which strengthens the case for the significant role of parasympathetic system in mediating tachycardic response to hypoxia. Due to non-uniform and usually not complete denervation, we could observe some HR responses to hypoxia which otherwise would be obscured after atropine16 making potential adrenergically-mediated HR reactivity impossible to assess.
Our observation of the leading role of the parasympathetic system in HR reactivity to hypoxia ought to be discussed in the context of the studied population. Apart from being diagnosed with vasovagal syndrome, we included healthy, young and not overweight individuals. Our results cannot be generalized to patients with sympathetically mediated diseases such as hypertension, heart failure or sleep apnea where sympathetic tone may play dominant role in HR control upon acute hypoxia27,58.
Furthermore, we assessed the responses to acute bursts of hypoxia (lasting for several seconds) which removes from the equation additional factors such as down-regulation of cardiac β-adrenergic receptors59, increased affinity and density of muscarinic receptors60, baroreceptor resetting due to increased arterial blood pressure61 or changes in pH / electrolyte concentration62 that could be happening with more prolonged exposure. Thus, our results might be difficult to compare to previous studies where HR responses to hypoxia were measured following several days of high altitude exposure21,63,64.
It has been shown that isolated electrical stimulation of vagal afferents (through oesophageal electrical stimulation) increases the parasympathetic tone of the heart. Thus, deafferentation related to PCNA may contribute to diminished cardiac parasympathetic outflow and further blunt HR reactivity resulting from the ablation of the efferent parasympathetic fibres. However, based on our data it is impossible to ascertain whether decreases in hHR slope, cBRS and HVR are attributable to afferent or efferent cardiac denervation.
Interestingly, apart from a decrease in hHR slope following PCNA we also found diminished hDBP slope. This could be related to the changes in some of the hemodynamic parameters seen after PCNA. An increase in HR possibly together with an increased stroke volume resulted in a proportional augmentation in CO. This in turn led to a compensatory, likely baroreflex-mediated, reduction in SVR to maintain arterial blood pressure (Table 1). Since variations in DBP (compared to SBP) better reflect the changes in muscle sympathetic nerve activity (MSNA)65, it can be hypothesized that diminished sympathetic constraint over peripheral vasculature (reflected by attenuated SVR) may have also caused the reduced DBP response to hypoxic stimulus. However, in our study we did not employ MSNA measurements therefore this notion remains a speculation.
The maintained hMV slope speaks for intact transduction of the hypoxic stimulus through peripheral chemoreceptors of the carotid bodies and its independence regarding the evident changes in baroreflex function which we report in our study. On the surface, this could be a surprising finding in the view of studies describing inhibitory interaction between arterial baroreflex and peripheral chemoreflex regarding response to hypoxia (but not to hypercapnia)66,67, which is likely related to the convergence of the afferents in the dorsomedial medulla68. It needs to be remembered though that in a current study we measured BRS indirectly i.e. through capturing HR responses to spontaneous changes in BP and therefore effectively analysing cardiac baroreflex slopes. Thus, we believe that reported changes in BRS are due to isolated changes in HR reactivity which do not reflect the BP regulating properties of baroreflex.
One of the major acute consequences of PCNA was a profound decrease in HRV. It held true for both time-domain, frequency-domain, and Poincaré plot-based indices (Table 2). These results are in line with the widely accepted concept that HF power and SDNN reflect alterations in cardiac vagal tone in response to respiration32. The physiological meaning of LF power is less clear69. Changes in LF power must be taken with caution as they may be produced by alterations in both the sympathetic and parasympathetic system and be related to BP regulation via baroreceptors33,70,71. The sympathetic system usually does not produce rhythms above 0.1 Hz, while the parasympathetic system may affect heart rhythms down to 0.05 Hz32. Indeed, Our findings suggest a dominant role of vagal tone (perhaps via altered baroreflex function) also in regards to the origin of LF power – at least in a short-term recording, and in a population of relatively young participants without significant cardiovascular or metabolic comorbidities.
Among various HRV indices, we report pNN50 – an established measure of extreme cardiac parasympathetic activity at rest. It is of particular importance in the studied group where subjects experienced recurrent syncopes related to vagal surge towards the heart under resting conditions. Interestingly, we found that the baseline mean pNN50 (23.2%) was comparable to elite athletes (24.2%)73 while none of the studied subjects reported more than regular physical activity. Reassuringly, PCNA led to the complete eradication of pNN50, being in line with the high clinical efficacy of the ablation procedure52.
Another important issue is related to the clinical meaning of reduced HRV after PCNA. It should be noted that some of the indices of HRV are used as markers of cardiovascular risk with the SDNN being a “gold standard” for risk stratification in clinical34,35 and general population36. The relation between low SDNN and higher all-cause mortality most likely reflects sympathetic/parasympathetic imbalance (loss of vagal activity and/or sympathetic hyperactivity) and as such should be seen rather as a biomarker (e.g. of ominous ventricular arrhythmias) and not as a causal factor for the malignant events.
On the other hand, respiratory sinus arrhythmia (RSA) – the major contributor to SDNN and HF power32, has been shown to significantly improve pulmonary gas exchange and circulatory efficiency in an elegant canine experiment73. The abolition of RSA in the cited experiment led to a two-fold increase in fractional intrapulmonary shunt indicating the physiological importance of this phenomenon observed among vertebrates throughout the evolution74. In fact re-instating the RSA utilizing a special pacing protocol, which could be incorporated into conventional implantable pacing systems, has been proposed as a treatment for chronic heart failure75. Therefore, further research is required to better delineate the risk-benefit ratio of PCNA procedures beyond commonly reported inadequate sinus tachycardia52.
Finally, we found that changes in all studied HRV indices observed after PCNA were almost identical to changes seen after parasympathetic blockade with the use of atropine (Fig. 5). Having in mind variable and often incomplete degree of parasympathetic cardiac denervation achieved with PCNA it speaks for the high vulnerability of HRV to even modest changes in cardiac parasympathetic tone. As previously suggested by Goldberger et al.76 the relation between vagal effect over heart and HRV might simply not be a linear one.
PCNA exerts a significant effect on cBRS. The predominant role of vagal tone in the spontaneous cardiac baroreflex function has been elegantly shown by Parlow et al.77 In their study atropine virtually eliminated the baroreflex slope and the subsequent addition of propranolol did not alter it further. As discussed above our methodology was focused solely on the cardiac efferent arm of baroreflex. Nonetheless, we consistently reported diminished HR response to spontaneous BP fluctuations using both sequential and spectral analysis. While not as prominent as changes in HRV (Table 2; Fig. 5), the disturbed HR reactivity to naturally occurring changes in BP is indicative of disrupted cardiac baroreflex with potential physiological and clinical consequences. First, the intact baroreflex provides compensatory tachycardia in the settings of suddenly decreasing arterial BP with the homeostatic aim of constant CO. Whether higher baseline HR after PCNA would in part counterbalance this untoward effect remains to be unravelled in further studies. Second, functional cardiac baroreflex also carries an important cardioprotective property. In a case of systemic catecholamine surge with concomitant vasoconstriction, it increases parasympathetic tone protecting the heart against arrhythmias78.
Interestingly, a possibility of parasympathetic renervation following PCNA has been reported79,80. On the one hand, over time it may reduce the clinical effectiveness of PCNA regarding syncopal events. On the other hand, it could diminish detrimental physiological alterations related to HRV, cBRS and hypoxic reactivity. Further studies are necessary to establish whether the clinically accepted balance between those two effects could be obtained with PCNA procedures.
Our study is not without limitations. We did not use beta-blocking agents (e.g. propranolol infusion) to further explore the relations between HR reactivity to hypoxia and autonomic tone. This was not performed due to safety issues related to the low baseline HR (pre-PCNA) in participants presenting with recurrent syncopes. Nonetheless, we believe that achieving complete vagal blockade with high-dose atropine together with an unchanged intrinsic sinus node activity following PCNA (due to electrophysiological mapping) allows for the assessment of changes in cardiac sympathetic constraint. Also, additional agents affecting the parasympathetic system other than atropine (e.g. clonidine, pirenzepine) were not employed. While clonidine has been found to be beneficial for the treatment of baroreflex failure81 it may have an opposite effect in vasovagal syncope82,83. The mixed results obtained with clonidine for the treatment of neurogenic syncope most likely reflect its complex mode of action. On the one hand, it does increase parasympathetic cardiac activity84, which would enhance the propensity towards vagally-mediated syncope. On the other hand, clonidine causes cardiac sympathetic inhibition85, which is known to be protective against vasovagal episodes86.
The incorporation of direct invasive analysis of sympathetic outflow with microneurography would allow for a more robust conclusion regarding global sympathetic tone following PCNA. The reported measurement of SVR should be taken with caution as it is derived from other noninvasively taken parameters. Higher than expected baseline SBP ought to be noted. It might have been related to the study protocol itself which incorporated quite a short period for the familiarization with rather elaborate study equipment. On the other hand, prolonging the familiarization part of the protocol could have resulted in agitation and tiredness at the end of the study when the atropine challenge was performed. Nonetheless, Finapres technology is not a tool validated for the measurement of absolute values of BP87, but rather for reliable tracing of changes in the hemodynamic parameters. Because SBP after PCNA was not statistically different compared to baseline values we do not expect that it significantly influenced the main study results. The post-hoc analysis performed after the exclusion of one individual with mild hypertension yielded results consistent with primary analysis regarding the statistical significance and direction of the reported changes.
To summarize, the novel model of selective cardiac parasympathetic denervation allowed us to demonstrate that vagal tone significantly contributes to HR reactivity to acute hypoxia in humans. Furthermore, intact parasympathetic activity is critical for the maintenance of HRV and cBRS whilst PCNA does significantly reduce those physiological mechanisms. The clinical relevance of the reported finding requires further investigations to warrant that the benefit of PCNA outweighs the potential harm.
Data availability
Data will be made available upon reasonable request. For that purpose please contact the corresponding author – Piotr Niewinski at piotr.niewinski@umw.edu.pl.
References
Joyce, W. & Wang, T. Regulation of heart rate in vertebrates during hypoxia: A comparative overview. Acta Physiol. (Oxf) 234, (2022).
Niewinski, P. et al. Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp. Physiol. 99, (2014).
Tubek, S. et al. Effects of selective carotid body stimulation with adenosine in conscious humans. J. Physiol. 594, (2016).
Kato, H., Menon, A. S. & Slutsky, A. S. Mechanisms mediating the heart rate response to hypoxemia. Circulation 77, 407–414 (1988).
Kumar, P. Systemic effects resulting from carotid body stimulation-invited article. Adv. Exp. Med. Biol. 648, 223–233 (2009).
de Daly, M., Scott, M. J. & B. & The effect of hypoxia on the heart rate of the dog with special reference to the contribution of the carotid body chemoreceptors. J. Physiol. 145, 440–446 (1959).
Siebenmann, C. & Lundby, C. Regulation of cardiac output in hypoxia. Scand. J. Med. Sci. Sports 25, 53–59 (2015).
Paleczny, B. et al. Hypoxic tachycardia is not a result of increased respiratory activity in healthy subjects. Exp. Physiol. 104, 476–489 (2019).
Cunningham, W. L., Becker, E. J. & Kreuzer, F. Catecholamines in plasma and urine at high altitude. J. Appl. Physiol. 20, 607–610 (1965).
Hoon, R. S., Sharma, S. C., Balasubramanian, V. & Chadha, K. S. & Mathew, O. P. Urinary catecholamine excretion on acute induction to high altitide (3658 m). J. Appl. Physiol. 41 (1976).
Hanada, A., Sander, M. & González-Alonso, J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J. Physiol. 551, 635–647 (2003).
Katayama, K. et al. Hypoxia augments muscle sympathetic neural response to leg cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301 (2011).
Rowell, L. B., Johnson, D. G., Chase, P. B., Comess, K. A. & Seals, D. R. Hypoxemia raises muscle sympathetic activity but not norepinephrine in resting humans. J. Appl. Physiol. (1985) 66, 1736–1743 (1989).
Grassi, G. et al. Heart rate as marker of sympathetic activity. J. Hypertens. 16, 1635–1639 (1998).
Hammill, S. C., Wagner, W. W., Latham, L. P., Frost, W. W. & Weil, J. V. Autonomic cardiovascular control during hypoxia in the dog. Circ. Res. 44, 569–575 (1979).
Koller, E. A., Drechsel, S., Hess, T., Macherel, P. & Boutellier, U. Effects of atropine and propranolol on the respiratory, circulatory, and ECG responses to high altitude in man. Eur. J. Appl. Physiol. Occup. Physiol. 57, 163–172 (1988).
Siebenmann, C. et al. Hypoxia increases exercise heart rate despite combined Inhibition of β-adrenergic and muscarinic receptors. Am. J. Physiol. Heart Circ. Physiol. 308, H1540–H1546 (2015).
Cooper, P. J. & Kohl, P. Species- and preparation-dependence of stretch effects on sino-atrial node pacemaking. Ann. N Y Acad. Sci. 1047, 324–335 (2005).
Forbes, L. M. et al. Right ventricular response to acute hypoxia among healthy humans. Am. J. Respir Crit. Care Med. 208, 333–336 (2023).
Euler, U. S. & Liljenstrand, G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol. Scand. 12, 301–320 (1946).
Siebenmann, C. et al. Parasympathetic withdrawal increases heart rate after 2 weeks at 3454 m altitude. J. Physiol. 595, 1619–1626 (2017).
Pachon, M. Cardioneuroablation’--new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace 7, 1–13 (2005).
Piotrowski, R., Baran, J., Sikorska, A., Krynski, T. & Kulakowski, P. Cardioneuroablation for reflex syncope: Efficacy and effects on autonomic cardiac regulation—a prospective randomized trial. JACC Clin. Electrophysiol. 9, 85–95 (2023).
Pachon-M, J. C. et al. Long-term evaluation of the vagal denervation by cardioneuroablation using Holter and heart rate variability. Circ. Arrhythm. Electrophysiol. 13, E008703 (2020).
Giannoni, A. et al. Chemoreflex and baroreflex sensitivity hold a strong prognostic value in chronic heart failure. JACC Heart Fail. 10, 662–676 (2022).
Ponikowski, P. et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 79, 1645–1650 (1997).
Niewinski, P. et al. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J. Card Fail. 19, 408–415 (2013).
Jose, A. D. & Taylor, R. R. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J. Clin. Invest. 48, 2019 (1969).
Niewinski, P., Tubek, S., Paton, J. F. R., Banasiak, W. & Ponikowski, P. Oxygenation pattern and compensatory responses to hypoxia and hypercapnia following bilateral carotid body resection in humans. J. Physiol. https://doi.org/10.1113/JP281319 (2021).
Chua, T. P. et al. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur. Heart J. 18, 480–486 (1997).
Ponikowski, P. et al. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation 96, 2586–2594 (1997).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public. Health 5, 258 (2017).
Malik, M. et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381 (1996).
Fang, S. C., Wu, Y. L. & Tsai, P. S. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: A meta-analysis of cohort studies. Biol. Res. Nurs. 22, 45–56 (2020).
Kleiger, R. E., Miller, J. P., Bigger, J. T. & Moss, A. J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 59, 256–262 (1987).
Jarczok, M. N. et al. Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations. Neurosci. Biobehav Rev. 143, 104907 (2022).
Ciccone, A. B. et al. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 56, 674–678 (2017).
Brennan, M., Palaniswami, M. & Kamen, P. Poincaré plot interpretation using a physiological model of HRV based on a network of oscillators. Am. J. Physiol. Heart Circ. Physiol. 283, (2002).
Di Rienzo, M., Bertinieri, G., Mancia, G. & Pedotti, A. A new method for evaluating the baroreflex role by a joint pattern analysis of pulse interval and systolic blood pressure series. Med. Biol. Eng. Comput. 3, 313–314 (1985).
Rosei, E. A., Chiarini, G. & Rizzoni, D. How important is blood pressure variability? Eur. Heart J. Suppl. 22, E1–E6 (2020).
Bertinieri, G. et al. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am. J. Physiol. 254, H377–H383 (1988).
Colombo, R. et al. Comparison between spectral analysis and the phenylephrine method for the assessment of baroreflex sensitivity in chronic heart failure. Clin. Sci. (Lond.) 97, 503–513 (1999).
La Rovere, M. T., Pinna, G. D. & Raczak, G. Baroreflex sensitivity: measurement and clinical implications. Ann. Noninvasive Electrocardiol. 13, 191–207 (2008).
Pinna, G. D., Maestri, R. & Raczak, G. La Rovere, M. T. Measuring baroreflex sensitivity from the gain function between arterial pressure and heart period. Clin. Sci. (Lond.)103, 81–88 (2002).
Debruyne, P. et al. Unifocal Right-Sided ablation treatment for neurally mediated Syncope and functional sinus node dysfunction under computed tomographic guidance. Circ. Arrhythm. Electrophysiol. 11, e006604 (2018).
Rivarola, E. W. R. et al. Long-Term outcome of cardiac denervation procedures: the anatomically guided septal approach. JACC Clin. Electrophysiol. 9, 1344–1353 (2023).
Li, L., Po, S. & Yao, Y. Cardioneuroablation for treating vasovagal syncope: Current status and future directions. Arrhythm. Electrophysiol. Rev. 12, (2023).
van Weperen, V. Y. H. & Vaseghi, M. Cardiac vagal afferent neurotransmission in health and disease: review and knowledge gaps. Front. Neurosci. 17, 1192188 (2023).
Pachon, J. C. et al. Cardioneuroablation: Where are we at? Heart Rhythm O2 4, 401–413 (2023).
Aksu, T. et al. Medium-term results of cardioneuroablation for clinical bradyarrhythmias and vasovagal syncope: Effects on QT interval and heart rate. J. Interv Card Electrophysiol. 60, 57–68 (2021).
Qin, M. et al. Atrial ganglionated plexus modification: A novel approach to treat symptomatic sinus bradycardia. JACC Clin. Electrophysiol. 3, 950–959 (2017).
Kulakowski, P. et al. Cardioneuroablation for reflex asystolic syncope: Mid-term safety, efficacy, and patient’s acceptance. Heart Rhythm. 21, 282–291 (2024).
Billman, G. E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4, (2013).
Laitinen, T., Hartikainen, J., Niskanen, L., Geelen, G. & Länsimies, E. Sympathovagal balance is major determinant of short-term blood pressure variability in healthy subjects. Am. J. Physiol. 276, (1999).
Takahashi, K. et al. Anatomical proximity between ganglionated plexi and epicardial adipose tissue in the left atrium: Implication for 3D reconstructed epicardial adipose tissue-based ablation. J. Interv Card Electrophysiol. 47, 203–212 (2016).
Armour, J. A., Murphy, D. A., Yuan, B. X., Macdonald, S. & Hopkins, D. A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. https://doi.org/10.1002/(SICI)1097-0185(199702)247:2
Aksu, T., Skeete, J. R. & Huang, H. H. Ganglionic plexus ablation: A Step-by-step guide for electrophysiologists and review of modalities for neuromodulation for the management of atrial fibrillation. Arrhythm. Electrophysiol. Rev. 12 (2023).
Narkiewicz, K. et al. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99, 1183–1189 (1999).
Richalet, J. P. et al. Decreased cardiac response to isoproterenol infusion in acute and chronic hypoxia. J. Appl. Physiol. (1985) 65, 1957–1961 (1988).
Kacimi, R., Richalet, J. P. & Crozatier, B. Hypoxia-induced differential modulation of adenosinergic and muscarinic receptors in rat heart. J. Appl. Physiol. (1985). 75, 1123–1128 (1993).
Calbet, J. A. L. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J. Physiol. 551, 379–386 (2003).
Severi, S., Cavalcanti, S., Mancini, E. & Santoro, A. Effect of electrolyte and pH changes on the sinus node pacemaking in humans. J. Electrocardiol. 35, 115–124 (2002).
Hughson, R. L., Yamamoto, Y., McCullough, R. E., Sutton, J. R. & Reeves, J. T. Sympathetic and parasympathetic indicators of heart rate control at altitude studied by spectral analysis. J. Appl. Physiol. (1985). 77, 2537–2542 (1994).
Wolfel, E. E., Selland, M. A., Mazzeo, R. S. & Reeves, J. T. Systemic hypertension at 4300 m is related to sympathoadrenal activity. J. Appl. Physiol. (1985). 76, 1643–1650 (1994).
Sundlof, G. & Wallin, B. G. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J. Physiol. 274, 621–637 (1978).
Somers, V. K., Mark, A. L. & Abboud, F. M. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J. Clin. Invest. 87, 1953–1957 (1991).
Cooper, V. L., Pearson, S. B., Bowker, C. M., Elliott, M. W. & Hainsworth, R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia—a mechanism for promoting hypertension in obstructive sleep Apnoea. J. Physiol. 568, 677–687 (2005).
Paton, J. F. R., Deuchars, J., Li, Y. W. & Kasparov, S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neuroscience 105, 231–248 (2001).
Goldstein, D. S., Bentho, O., Park, M. Y. & Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but May be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 96, 1255–1261 (2011).
Akselrod, S. et al. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 213, 220–222 (1981).
Berntson, G. G., Cacioppo, J. T. & Grossman, P. Whither vagal tone. Biol. Psychol. 74, 295–300 (2007).
Kiss, O. et al. Detailed heart rate variability analysis in athletes. Clin. Auton. Res. 26, 245–252 (2016).
Hayano, J., Yasuma, F., Okada, A., Mukai, S. & Fujinami, T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation 94, 842–847 (1996).
Hayano, J. & Yasuma, F. Hypothesis: respiratory sinus arrhythmia is an intrinsic resting function of cardiopulmonary system. Cardiovasc. Res. 58, 1–9 (2003).
Shanks, J. et al. Reverse re-modelling chronic heart failure by reinstating heart rate variability. Basic. Res. Cardiol. 117, 4 (2022).
Goldberger, J. J., Challapalli, S., Tung, R., Parker, M. A. & Kadish, A. H. Relationship of heart rate variability to parasympathetic effect. Circulation 103, 1977–1983 (2001).
Parlow, J., Viale, J. P., Annat, G., Hughson, R. & Quintin, L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension 25, 1058–1068 (1995).
Swenne, C. A. Baroreflex sensitivity: mechanisms and measurement. Neth. Heart J. 21, 58 (2013).
Thurber, C. J., Sneider, D. R., Sauer, W. H. & Kapur, S. Recurrent Vasovagal syncope following successful cardioneuroablation. HeartRhythm Case Rep. 8, 465–468 (2022).
Futyma, P. & Kułakowski, P. Reinnervation after cardioneuroablation: When on the run for best intraprocedural endpoints, be aware of possible ablation overdose. HeartRhythm Case Rep. 8, 469–470 (2022).
Robertson, D. et al. The diagnosis and treatment of baroreflex failure. N Engl. J. Med. 329, 1449–1455 (1993).
Biffi, M. et al. Malignant vasovagal syncope: A randomised trial of metoprolol and clonidine. Heart 77, 268–272 (1997).
Sandweiss, A. J., Morrison, C. M., Spichler, A. & Rozich, J. A case report of clonidine induced syncope: A review of central actions of an old cardiovascular drug. BMC Pharmacol. Toxicol. 19, (2018).
Toader, E., Cividjian, A. & Quintin, L. Recruitment of cardiac parasympathetic activity: effects of clonidine on cardiac vagal motoneurones, pressure lability, and cardiac baroreflex slope in rats. Br. J. Anaesth. 102, 322–330 (2009).
Castiglioni, P. et al. Scale exponents of blood pressure and heart rate during autonomic Blockade as assessed by detrended fluctuation analysis. J. Physiol. 589, 355–369 (2011).
Cox, M. M. et al. Acute and long-term beta-adrenergic Blockade for patients with neurocardiogenic syncope. J. Am. Coll. Cardiol. 26, 1293–1298 (1995).
Rongen, G. A. et al. Comparison of intrabrachial and finger blood pressure in healthy elderly volunteers. Am. J. Hypertens. 8, 237–248 (1995).
Author information
Authors and Affiliations
Contributions
P.N. designed the study, performed experiments, analysed data, wrote and revised the manuscript; S.T. analysed data, revised the manuscript; K.J. performed experiments, revised the manuscript; K.N consulted the study design, revised the manuscript and supervised the project; P.P. consulted the study design, revised the manuscript and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Niewinski, P., Tubek, S., Josiak, K. et al. Cardiac parasympathetic denervation reduces hypoxic tachycardia, baroreflex sensitivity and heart rate variability in humans. Sci Rep 15, 6633 (2025). https://doi.org/10.1038/s41598-025-91214-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91214-6