Abstract
This study aimed to investigate the potential relationship between baseline glucose to lymphocyte ratio (GLR) levels and the first episode of peritonitis in patients treated with peritoneal dialysis (PD). A total of 314 patients treated with PD were included and divided into three groups based on GLR tertiles: tertile 1 (GLR ≤ 4.23); tertile 2 (4.23 < GLR ≤ 5.96), and tertile 3 (GLR > 5.96). The relationships between GLR and the first peritonitis episode were analyzed using Kaplan–Meier curves and multivariable Cox regression models. Competitive risk analysis, subgroup and sensitivity analyses were performed to validate the robustness of the findings. During a median follow-up of 27 months, 123 (39.17%) patients developed the first episode of peritonitis. The incidence of the first peritonitis episode increased with the higher GLR tertiles (tertile 1: 32.08%, tertile 2: 37.50%, tertile 3: 48.08%). Kaplan–Meier curves revealed significant differences in the cumulative incidence of the first peritonitis episode among the GLR tertiles (Log-Rank test, P = 0.018). After full adjustment for confounding factors, patients in tertile 3 remained at significantly higher risk for the first episode of peritonitis compared to those in tertile 1 (HR 2.633, 95% CI 1.223–5.668, P = 0.013). Competitive risk models and sensitivity analysis further confirmed this association. Our study suggests that elevated GLR is associated with an increased risk of the first peritonitis episode in patients with PD.
Similar content being viewed by others
Introduction
Peritoneal dialysis (PD) is a widely utilized method of renal replacement therapy for patients with end-stage kidney disease (ESKD). Since 1985, Hong Kong has implemented the PD first policy, which had a positive impact on the promotion and application of PD therapy worldwide1. However, PD-associated peritonitis remains a significant complication, leading to poor clinical outcomes2 and posing a major challenge to the long-term treatment of PD. Therefore, to reduce the risk of peritonitis episodes, exploring novel biomarkers to identify patients at higher risk of peritonitis episodes is an important research direction.
Patients treated with PD commonly experience chronic inflammation3 and have an increased risk of developing disorders in glucose metabolism4 and immune function5. Both of these factors may be associated with the peritonitis episodes. The glucose to lymphocyte ratio (GLR) has emerged as a novel inflammatory biomarker, combining aspects of glucose metabolism and immune status. GLR has been shown to correlate with the development and prognosis of various diseases6,7, but its specific role in predicting PD-associated peritonitis remains underexplored. A study has demonstrated a positive correlation between GLR and neutrophil to lymphocyte ratio (NLR)8, while NLR has been proven useful in predicting the risk of PD-associated peritonitis9. Based on these findings, we hypothesize that GLR could serve as a predictive marker for peritonitis episodes in patients treated with PD. This study aims to explore the potential relationship between baseline GLR levels and the first episode of peritonitis in patients treated with PD. By evaluating whether GLR is a reliable early biomarker for identifying high-risk individuals, our research findings may facilitate personalized medical decision-making and patient-centered care to reduce the incidence of peritonitis, prolong the technical survival of patients, and ultimately improve patients’ quality of life and long-term outcomes.
Materials and methods
Study population
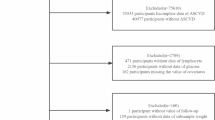
In this retrospective cohort study, a total of 430 patients undergoing initial PD at the Second Affiliated Hospital of Anhui Medical University were identified from existing medical records between January 1, 2010, and December 31, 2022, for inclusion in the study. The exclusion criteria included: (1) younger than 18 years; (2) transferred from maintenance hemodialysis (HD); (3) PD less than 3 months; (4) active infection, hematological diseases, or use of glucocorticoids, as these may influence the GLR levels; (5) Missing GLR or follow-up data. All patients and caregivers received standardized PD training and passed operational assessments at the hospital after catheterization. All patients used glucose-based dialysis solution and no patients used the PD cyclers. All data were fully anonymized and all participants were signed informed consent. This study conforms to the Declaration of Helsinki and was approved by the ethics committee of the Second Affiliated Hospital of Anhui Medical University (No. KY201302).
Data collection
All baseline characteristics and clinical information were collected at the initiation of PD therapy from the electronic medical records of our center. Demographic data including sex, age, body mass index (BMI), and causes of ESKD; comorbidities including diabetes mellitus (DM) and history of cardiovascular disease (CVD); laboratory parameters including hemoglobin, white blood cell (WBC) count, lymphocyte count, platelet count, high-sensitive C-reactive protein (hs-CRP), serum creatinine, serum urea nitrogen, serum uric acid, serum corrected calcium, serum phosphorus, serum albumin, fasting blood glucose, and intact parathyroid hormone. GLR was calculated by fasting blood glucose/lymphocyte count. Dialysis-related data were collected at least 1 month after the initiation of PD therapy, including daily ultrafiltration, 24-hour urine volume, total weekly Kt/V, and glucose exposure. Glucose exposure was calculated by multiplying the glucose concentration by the prescribed infusion volume on a regular dialysis day. For example, in a patient who receives 2 exchanges of 2 L 1.5% glucose and 2 exchanges of 2 L 2.5% glucose, the total glucose exposure is calculated as (2 × 2 × 15) + (2 × 2 × 25) = 160 g of glucose/24 h. Additionally, the use of antidiabetic drugs was also recorded.
Study outcome
The study outcome was the first episode of peritonitis, transfer to HD therapy, renal transplantation, death, transfer to other centers, loss of follow-up, or censoring on December 31, 2023. Peritonitis was diagnosed when at least 2 of the following conditions were present10: (1) abdominal pain and/or cloudy dialysis effluent; (2) dialysis effluent WBC count > 100/µL (after a dwell time of at least 2 h), with > 50% polymorphonuclear leukocytes; (3) positive dialysis effluent culture. When peritonitis was suspected, 5–10 mL effluent was injected into aerobic and anaerobic blood-culture bottles for microbiology tests. All peritonitis was treated using the recommended empirical antibiotic therapy and the medication regimen was adjusted based on the actual organism and antibiotic susceptibility. Dialysis effluent WBC count on day 1, causative organisms, and peritonitis outcomes were recorded.
Statistical analysis
The Shapiro-Wilk test was employed to assess whether the data conforms to a normal distribution. The results indicated that only albumin and daily ultrafiltration adhered to a normal distribution. Albumin and daily ultrafiltration were expressed as mean ± standard deviation, while other skewness distribution data were expressed by median (interquartile range). Categorical variables were expressed as frequency (percentage). All participants were divided into three groups based on the tertiles of GLR. Group differences were compared using the “CBCgrps” package in R. The difference in cumulative incidence of the first peritonitis episode was analyzed using the Kaplan-Meier curve. Cox proportional hazard models were used to investigate the association between GLR and the first peritonitis episode. The proportional hazards assumption was evaluated using Schoenfeld residuals, and no violations were identified. The variance inflation factor (VIF) was used to assess potential collinearity among the covariables. The use of antidiabetic drugs was excluded from the Cox regression models due to strong collinearity with DM, as indicated by a VIF greater than 10. The performance of the models was assessed using the concordance index. To ensure the reliability of the finding, we constructed four models based on meaningful covariates identified in univariate Cox regression analysis and clinically significant factors: model 1, adjusted for age, sex, BMI; model 2, adjusted for model 1 covariates plus DM and history of CVD; model 3, adjusted for model 2 covariates plus hemoglobin, platelet count, serum creatinine, serum uric acid, serum corrected calcium, serum phosphorus, serum albumin, intact parathyroid hormone, and hs-CRP; model 4, adjusted for model 3 covariates plus daily ultrafiltration, 24-hour urine volume, total weekly Kt/V, and glucose exposure. Additionally, as transfer to HD therapy, renal transplantation, death, transfer to other centers, and loss of follow-up were considered competing risk events for the first peritonitis episode, cumulative incidence considering competing risks was depicted using Gray’s test, and the Fine-Gray subdistribution hazards model was employed. Considering the missing values in hs-CRP, total weekly KTV, daily ultrafiltration, 24-hour urine volume, and intact parathyroid hormone, we employed multiple imputation methods and subsequently re-conducted the multivariate Cox regression models as a sensitivity analysis. The time-dependent receiver operating characteristic (ROC) curves and their corresponding area under the curve (AUC) were used to assess the predictive ability of fasting blood glucose, lymphocyte count, and GLR for the first episode of peritonitis. The “compare” function of the “timeROC” package was applied to compare the AUC values. Subgroups were stratified by age (< 60 vs. ≥60 years), sex (male vs. female), DM (yes vs. no), and history of CVD (yes vs. no). Interactions between subgroup variables and GLR were examined by conducting a formal test of interaction. A forest plot was constructed to depict the result of the subgroup analyses and interaction test. Power analysis for the study was performed using the “powerSurvEpi” package in R, yielding a power of 0.928. A 2-sided P < 0.05 was considered statistically significant. All statistical analyses were performed by R software (version 4.3.1).
Results
Study participants and clinical characteristics
As shown in Fig. 1, a total of 314 patients treated with PD were included in the analysis. The patients were divided into three groups based on GLR tertiles: tertile 1 (GLR ≤ 4.23); tertile 2 (4.23 < GLR ≤ 5.96), and tertile 3 (GLR > 5.96). Baseline demographic and clinical characteristics are presented in Table 1. The median age of the patients was 52 (42, 61) years, with 145 males (46.18%) and 59 patients (18.79%) having diabetes. There were significant differences in age, hemoglobin, platelet count, serum creatinine, serum urea nitrogen, serum corrected calcium, serum albumin, intact parathyroid hormone, and 24-hour urine volume among the three groups. During a median follow-up of 27 months, 123 patients (39.17%) developed the first episode of peritonitis. The incidence of the first peritonitis episode increased with the higher GLR tertiles (tertile 1: 32.08%, tertile 2: 37.50%, tertile 3: 48.08%). The overall peritonitis rate was 0.13 episodes per year at risk. The causative organisms and outcomes of peritonitis are presented in Table 2. Gram-positive bacteria were the most common causative organisms in the first peritonitis episode, observed in 59 (47.97%) patients. Among other culture-positive organisms, 28 (22.76%) peritonitis episodes involved gram-negative bacteria, 5 (4.07%) peritonitis episodes involved fungus, and 1 (0.81%) peritonitis episode involved polymicrobial bacteria. Additionally, culture-negative peritonitis was observed in 30 (24.39%) patients. Of the total patients with peritonitis, 109 (88.62%) were completely cured, 10 (8.13%) transferred to HD therapy, and 4 (3.25%) died.
GLR and the first episode of peritonitis in patients treated with PD
The time-dependent ROC curves for predicting the first peritonitis episode using fasting blood glucose, lymphocyte count, and GLR were showed in Fig. 2. At 1 year, the AUC values were 0.576 for GLR, 0.557 for fasting blood glucose, and 0.553 for lymphocyte count. At 3 years, the AUC values were 0.605, 0.563, and 0.591, respectively. At 5 years, the AUC values were 0.596, 0.522, and 0.604. Although there were no statistically significant differences in AUC between GLR and fasting blood glucose or lymphocyte count, the AUC for GLR was higher than that for fasting blood glucose and lymphocyte count at 1 and 3 years, and the AUC for GLR was slightly lower than that for lymphocyte count but remained higher than that for fasting blood glucose at 5 years, indicating that the predictive ability of GLR may be superior to that of fasting blood glucose or lymphocyte count alone. The Kaplan-Meier curves for GLR tertiles was shown in Fig. 3. The results indicate significant differences in the cumulative incidence of the first peritonitis episode (Log-Rank test, P = 0.018). Further comparisons between groups revealed that the cumulative incidence of the first episode of peritonitis in tertile 3 was significantly higher than in tertile 1. In the unadjusted Cox proportional hazards model, patients in tertile 3, as compared with those in tertile 1, were associated with a higher cumulative risk of the first episode of peritonitis (HR: 1.824, 95% CI: 1.178–2.824, P = 0.007). After full adjustment for confounding factors, patients in tertile 3 remained at significantly higher risk for the first episode of peritonitis compared to those in tertile 1 (HR: 2.633, 95% CI: 1.223–5.668, P = 0.013) (Table 3).
Competitive risk analysis
The Fine-Gray competing risk model demonstrated that the cumulative incidence of the first episode of peritonitis was higher in tertile 3 compared to tertile 1 and 2 (Fig. 4). The cumulative incidence of peritonitis increased progressively across tertiles, with tertile 3 showing the highest risk. Gray’s test revealed a statistically significant difference among the three groups (P = 0.021). In the multivariate Fine-Gray analysis, the subdistribution hazard ratio (SHR) across adjusted models consistently showed that patients in tertile 3 were independently associated with a higher risk of developing the first episode of peritonitis compared to those in tertile 1 (Table 3). These findings are consistent with the results observed in the multivariate Cox regression analysis.
Subgroup and sensitivity analyses
The potential association between GLR and the first episode of peritonitis was further evaluated through subgroup analyses stratified by sex, age, DM, and history of CVD. The forest plot revealed no significant interaction effects between GLR and these subgroup variables (Fig. 5).
After performing sensitivity analysis using multiple imputation methods to address missing data, the results of the multivariate Cox regression analysis remained consistent. A statistically significant difference in the incidence of the first peritonitis episode was observed between tertile 3 and tertile 1 (HR: 1.811, 95% CI: 1.117–2.939, P = 0.016).
Discussion
In this study, we observed a consistent increase in the cumulative incidence of peritonitis across GLR tertiles, with tertile 3 showing the highest risk. Elevated GLR in tertile 3, as compared to tertile 1, was independently associated with an increased risk of the first peritonitis episode in patients treated with PD. This association remained significant even after fully adjusting for potential confounding factors. The robustness of this finding was further confirmed by the competitive risk analysis, subgroup and sensitivity analyses.
GLR is calculated based on fasting blood glucose and lymphocyte count, which reflect glucose metabolism and systemic immune status11, and is a novel biomarker associated with chronic inflammation8. Previous research has primarily focused on its association with the prognosis of various diseases. Several studies have indicated that GLR serves as a predictive factor for the prognosis of patients with malignant tumors, including hepatocellular carcinoma12, breast cancer13, metastatic gastric cancer14, colorectal cancer15, renal cell carcinoma16, and pancreatic cancer11. Additionally, GLR has been identified as a clinical biomarker for in-hospital mortality in intensive care unit (ICU) patients with acute myocardial infarction7, acute respiratory distress syndrome17, sepsis18, and acute exacerbation chronic obstructive pulmonary disease19. Li et al. found that GLR was an independent predictor for the development of acute kidney injury after cardiac surgery in ICU patients6. Furthermore, a recent cohort study conducted among PD patients in China indicated that GLR was an independent prognostic factor for all-cause and CVD mortality8.
Our research indicated that GLR was associated with the first peritonitis episode in patients treated with PD. The underlying mechanisms between them remain unclear. We speculate that it may be related to chronic inflammation, glucose metabolism disorder, and impaired immune function. Inflammation is common in patients treated with PD, and its reasons are multifactorial, interacting synergistically. These include the accumulation of uremic toxins, elevated endotoxin levels, decreased clearance of proinflammatory cytokines, oxidative stress, the presence of the PD catheter as a foreign body, and continuous exposure of the peritoneum to biologically incompatible glucose-based dialysis solution, among others3,20. GLR is a biomarker of chronic inflammation. In this study, patients in GLR tertile 3 had higher levels of hs-CRP compared to those in tertile 1, and elevated hs-CRP levels have been shown to predict the risk of peritonitis in patients treated with PD21.
Elevated GLR indicates an increase in glucose levels and/or a decrease in lymphocyte counts. Patients with CKD often experience disorders in glucose and insulin homeostasis, which may be further aggravated in patients treated with PD through exposure to glucose from dialysis solution4. A meta-analysis revealed an elevated risk of glucose metabolism disorders among patients treated with PD in China22. Elevated fasting blood glucose levels were associated with an increased risk of all-cause mortality in PD patients with diabetes23. A study involving 3075 older black and white participants showed that dysglycemia was related to inflammation, and this relationship was consistent in both diabetic individuals and those with impaired fasting glucose and impaired glucose tolerance24. Hyperglycemia can maintain a chronic inflammation status by upregulating the expression of proinflammatory cytokines and synergizing with hypoxia25, while chronic inflammation is a risk factor for peritonitis episodes. Moreover, patients treated with PD generally use glucose-based dialysis solutions. The high glucose environment brought about by the glucose-based dialysis solution can induce microenvironmental hypoxia, drive increased levels of proinflammatory cytokine in the abdominal cavity, activate chronic inflammatory and oxidative stress, result in changes in the structure and function of the peritoneum, impair host defenses, and ultimately increase susceptibility to peritonitis26,27. Additionally, high glucose exposure can also cause intestinal barrier dysfunction by altering its morphological, structural, and functional properties, increasing intestinal wall permeability, and making it easier for bacteria to invade the abdominal cavity, which may be a partial reason for the increased risk of peritonitis episode28.
Lymphocytes serve as integral constituents of the human immune system and can reflect the systemic immune status. Previous studies have indicated that a decreased lymphocyte count could be used to predict poor treatment response in peritonitis29 and was a predictive factor for mortality and technique failure in patients treated with PD30. Patients with CKD commonly exhibit impaired immune function31. This impairment can be attributed to reasons such as impaired kidney function, the accumulation of uremic toxins32, elevated fibroblast growth factor 23, which suppresses vitamin D and proinflammatory responses33, and more. For CKD patients undergoing PD, prolonged exposure of the peritoneum to dialysis solution containing supraphysiologic concentrations of glucose can exacerbate inflammation34 and impair immune defense in the peritoneal cavity5. Decreased lymphocyte counts indicate abnormal immune function35, making patients more susceptible to bacterial infections and unable to clear bacteria in a timely and effective manner, leading to an increased risk of peritonitis. Additionally, Hu et al. pointed out that T lymphocyte cells could improve intestinal barrier dysfunction in heatstroke, implying that decreased lymphocyte counts may exacerbate intestinal barrier dysfunction and increase the incidence of peritonitis36.
The culture-negative peritonitis rate in this study was 24.39%, exceeding the 15% recommended by the ISPD peritonitis guideline10. This disparity may be attributed to differences in the definition and technique of microbiological isolation. Additionally, some patients had already received antibiotic therapy before dialysis effluent specimens were collected.
Dai et al. confirmed that DM was an independent predictor for peritonitis episodes9. However, in our study, we did not observe a similar association between DM and peritonitis episodes, which may be due to regional and demographic differences. Moreover, no statistically significant difference in the proportion of DM was observed in this study, and subgroup analyses indicated no interaction between DM and GLR. GLR was identified as a risk factor for peritonitis episodes in the non-diabetes group, but this relationship was not observed in the diabetes group. Nevertheless, the sample size of our study was relatively small, and caution is required in interpreting the results of subgroup analysis.
To the best of our knowledge, this study is the first to explore the potential relationship between GLR and the first peritonitis episode in patients treated with PD. As a cost-effective and readily available biomarker, GLR simultaneously reflects both glucose metabolism and systemic immune status, offering a more comprehensive predictive marker for the risk of the first peritonitis episode in patients treated with PD. This may contribute to developing personalized medical decision-making and improving patient prognosis. Moreover, since patients treated with PD are prone to disorders in glucose metabolism and immune function, GLR holds greater clinical significance in this special population. However, several limitations must be acknowledged. First, as a retrospective study, it can only establish an association between GLR and the first peritonitis episode in patients treated with PD, without clarifying any causal relationship. Second, the AUC for GLR in predicting the first peritonitis episode was relatively low, and the small sample size limits the generalizability of our findings, which requires further validation in larger, multi-center cohorts. Third, we only considered baseline GLR levels and did not account for potential changes in GLR over the follow-up period. Fourth, due to significant collinearity between DM and the use of antidiabetic drugs, the latter was not included in the multivariate Cox regression models to avoid model instability. Finally, we cannot eliminate all potential confounding factors that may affect the peritonitis episode, such as living environment, education levels, and economic conditions.
Conclusion
In conclusion, our study suggests that elevated GLR is associated with an increased risk of the first peritonitis episode in patients with PD. Further large-scale prospective studies and exploration of underlying mechanisms are needed to confirm this association.
Data availability
The data used during this study are available from the corresponding author on reasonable request.
References
Li, P. K. et al. Peritoneal dialysis first policy in Hong Kong for 35 years: Global impact. Nephrol. (Carlton Vic). 27, 787–794. https://doi.org/10.1111/nep.14042 (2022).
Ma, X. et al. Analysis of risk factors and outcome in peritoneal dialysis patients with early-onset peritonitis: A multicentre, retrospective cohort study. BMJ Open. 10, e029949. https://doi.org/10.1136/bmjopen-2019-029949 (2020).
Li, P. K., Ng, J. K. & McIntyre, C. W. Inflammation and peritoneal Dialysis. Semin. Nephrol. 37, 54–65. https://doi.org/10.1016/j.semnephrol.2016.10.007 (2017).
Fortes, P. C. et al. Insulin resistance and glucose homeostasis in peritoneal dialysis. Perit. dialysis Int.: J. Int. Soc. Perit. Dial.. 29 (Suppl 2), S145–148 (2009).
Herzog, R. et al. Functional and transcriptomic characterization of peritoneal Immune-Modulation by addition of Alanyl-Glutamine to Dialysis fluid. Sci. Rep. 7, 6229. https://doi.org/10.1038/s41598-017-05872-2 (2017).
Li, L., Zou, G. & Liu, J. Preoperative Glucose-to-Lymphocyte ratio is an independent predictor for acute kidney injury after cardiac surgery in patients in intensive care unit. Int. J. Gen. Med. 14, 6529–6537. https://doi.org/10.2147/ijgm.S335896 (2021).
Liu, J. & Hu, X. Association between glucose-to-lymphocyte ratio and in-hospital mortality in acute myocardial infarction patients. PLoS One. 18, e0295602. https://doi.org/10.1371/journal.pone.0295602 (2023).
Chen, J. et al. Clinical significance of serum glucose to lymphocyte ratio as a prognostic marker in peritoneal dialysis patients. Ren. Fail. 45 https://doi.org/10.1080/0886022x.2023.2224893 (2023).
Dai, R. et al. Construction and validation of a predictive model for the risk of peritoneal dialysis-associated peritonitis after peritoneal dialysis catheterization. Front. Med. 10, 1193754. https://doi.org/10.3389/fmed.2023.1193754 (2023).
Li, P. K. et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int.: J. Int. Soc. Perit. Dialysis. 42, 110–153. https://doi.org/10.1177/08968608221080586 (2022).
Zhong, A., Cheng, C. S., Kai, J., Lu, R. & Guo, L. Clinical significance of glucose to lymphocyte ratio (GLR) as a prognostic marker for patients with pancreatic Cancer. Front. Oncol. 10, 520330. https://doi.org/10.3389/fonc.2020.520330 (2020).
Yılmaz, A. et al. The importance of the glucose-to-lymphocyte ratio in patients with hepatocellular carcinoma treated with Sorafenib. Future Oncol. (London England). 17, 4545–4559. https://doi.org/10.2217/fon-2021-0457 (2021).
Zhang, X. W. et al. Prognostic power of Nutrition-Inflammation indicators in patients with breast Cancer. Clin. Breast. Cancer. 23, e312–e321. https://doi.org/10.1016/j.clbc.2023.04.009 (2023).
Hannarici, Z. et al. The value of pretreatment glucose-to-lymphocyte ratio for predicting survival of metastatic gastric cancer. Future Oncol. (London England). 19, 315–325. https://doi.org/10.2217/fon-2022-0579 (2023).
Yang, M. et al. Glucose to lymphocyte ratio predicts prognoses in patients with colorectal cancer. Asia-Pac. J. Clin. Oncol. 19, 542–548. https://doi.org/10.1111/ajco.13904 (2023).
Ni, J. et al. Prognostic value of glucose to lymphocyte ratio for patients with renal cell carcinoma undergoing laparoscopic nephrectomy: A multi-institutional, propensity score matching cohort study. Front. Surg. 9, 911411. https://doi.org/10.3389/fsurg.2022.911411 (2022).
Zhang, Y. & Zhang, S. Prognostic value of glucose-to-lymphocyte ratio in critically ill patients with acute respiratory distress syndrome: A retrospective cohort study. J. Clin. Lab. Anal. 36, e24397. https://doi.org/10.1002/jcla.24397 (2022).
Cai, S. et al. Association between glucose-to-lymphocyte ratio and in-hospital mortality in intensive care patients with sepsis: A retrospective observational study based on medical information Mart for intensive care IV. Front. Med. 9, 922280. https://doi.org/10.3389/fmed.2022.922280 (2022).
Hu, T., Liu, X. & Liu, Y. Usefulness of glucose to lymphocyte ratio to predict in-Hospital mortality in patients with AECOPD admitted to the intensive care unit. Copd 19, 158–165. https://doi.org/10.1080/15412555.2022.2052272 (2022).
Kadoya, H. et al. Activation of the inflammasome drives peritoneal deterioration in a mouse model of peritoneal fibrosis. FASEB Journal: Official Publication Federation Am. Soc. Experimental Biology. 37, e23129. https://doi.org/10.1096/fj.202201777RRR (2023).
Su, Y. J., Liao, S. C., Cheng, B. C., Hwang, J. C. & Chen, J. B. Increasing high-sensitive C-reactive protein level predicts peritonitis risk in chronic peritoneal dialysis patients. BMC Nephrol. 14, 185. https://doi.org/10.1186/1471-2369-14-185 (2013).
Shi, Y., Cai, J., Shi, C., Liu, C. & Li, Z. Incidence and mortality of new-onset glucose disorders in peritoneal dialysis patients in China: A meta-analysis. BMC Nephrol. 21, 152. https://doi.org/10.1186/s12882-020-01820-x (2020).
Lim, S. J. et al. Fasting blood glucose level and risk of all-cause and cause-specific mortality in peritoneal dialysis patients. J. Diabetes 16, e13601, (2024). https://doi.org/10.1111/1753-0407.13601
de Rekeneire, N. et al. Diabetes, hyperglycemia, and inflammation in older individuals: The health, aging and body composition study. Diabetes Care. 29, 1902–1908. https://doi.org/10.2337/dc05-2327 (2006).
Morey, M., O’Gaora, P., Pandit, A. & Hélary, C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS One. 14, e0220577. https://doi.org/10.1371/journal.pone.0220577 (2019).
Zhao, H., Zhang, H. L. & Jia, L. High glucose dialysate-induced peritoneal fibrosis: Pathophysiology, underlying mechanisms and potential therapeutic strategies. Biomed. Pharmacotherapy = Biomedecine Pharmacotherapie. 165, 115246. https://doi.org/10.1016/j.biopha.2023.115246 (2023).
Uiterwijk, H., Franssen, C. F. M., Kuipers, J., Westerhuis, R. & Nauta, F. L. Glucose exposure in peritoneal Dialysis is a significant factor predicting peritonitis. Am. J. Nephrol. 51, 237–243. https://doi.org/10.1159/000506324 (2020).
Dubois, N., Muñoz-Garcia, J., Heymann, D. & Renodon-Cornière, A. High glucose exposure drives intestinal barrier dysfunction by altering its morphological, structural and functional properties. Biochem. Pharmacol. 216, 115765. https://doi.org/10.1016/j.bcp.2023.115765 (2023).
He, Y. et al. Decreased peripheral blood lymphocyte count predicts poor treatment response in peritoneal Dialysis-Associated peritonitis. J. Inflamm. Res. 16, 5327–5338. https://doi.org/10.2147/jir.S438674 (2023).
Cueto-Manzano, A. M., Quintana-Piña, E. & Correa-Rotter, R. Long-term CAPD survival and analysis of mortality risk factors: 12-year experience of a single Mexican center. Perit. dialysis Int.: J. Int. Soc. Perit. Dial.. 21, 148–153 (2001).
Highton, P. J., Neale, J., Wilkinson, T. J., Bishop, N. C. & Smith, A. C. Physical activity, immune function and inflammation in kidney patients (the PINK study): A feasibility trial protocol. BMJ Open. 7, e014713. https://doi.org/10.1136/bmjopen-2016-014713 (2017).
Maksić, D., Colić, M., Stanković-Popović, V., Radojević, M. & Bokonjić, D. Systemic and intraperitoneal Proinflammatory cytokines profiles in patients on chronic peritoneal dialysis. Med. Pregl. 60(Suppl 2), 53–57 (2007).
Ishigami, J. et al. Fibroblast growth factor 23 and risk of hospitalization with infection in chronic kidney disease: the chronic renal insufficiency cohort (CRIC) study. J. Am. Soc. Nephrol.: JASN. 31, 1836–1846. https://doi.org/10.1681/asn.2019101106 (2020).
Trionfetti, F. et al. Novel aspects of the immune response involved in the peritoneal damage in chronic kidney disease patients under dialysis. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms24065763 (2023).
Luo, Y. et al. Combination of lymphocyte number and function in evaluating host immunity. Aging 11, 12685–12707. https://doi.org/10.18632/aging.102595 (2019).
Hu, J. et al. Regulatory T cells could improve intestinal barrier dysfunction in heatstroke. Inflammation 42, 1228–1238. https://doi.org/10.1007/s10753-019-00983-6 (2019).
Acknowledgements
We would like to thank all participants in this work.
Funding
This work was supported by the Natural Science Foundation of Anhui Province (2008085MH244), Incubation Program of National Natural Science Foundation of China of the Second Affiliated Hospital of Anhui Medical University (2020GMFY04), and Clinical Research and Cultivation Plan Project of the Second Affiliated Hospital of Anhui Medical University (2021LCZD16).
Author information
Authors and Affiliations
Contributions
Q.Y. designed study, performed analysis, and drafted the manuscript. D.W. designed and directed the study. G.L., R.W., D.L., X.C. and J.C. collected data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethnic approval
This study was approved by the ethics committee of the Second Affiliated Hospital of Anhui Medical University (authorization number: KY201302).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, Q., Liu, G., Wang, R. et al. Relationship between glucose to lymphocyte ratio and the first peritonitis episode in patients treated with peritoneal dialysis. Sci Rep 15, 6834 (2025). https://doi.org/10.1038/s41598-025-91252-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91252-0