Abstract
This study aimed to examine feasibility of inspiratory muscle strength tests, such as maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP) in adult patients affected by spinal muscular atrophy (SMA), as well as their ability, along with forced vital capacity (FVC), to predict noninvasive ventilation (NIV) needs. Additionally, we evaluated feasibility and effectiveness of respiratory oscillometric measurements in the same patients. Twenty patients were retrospectively evaluated. NIV requirement was considered as peak nocturnal transcutaneous PCO2 > 49 mmHg, according to the cut-off used by Ward et al. MIP and SNIP were feasible in all patients. SNIP had significantly higher values (p < 0.001) and poorly agreed with MIP. ROC analysis revealed MIP as a weak predictor for NIV initiation (AUC = 0.57), while FVC (AUC 0.78, best cut-off 20% of predicted) and SNIP (AUC 0.84, best cut-off 61 cmH2O) were more effective. Oscillometry was performed in 11 patients. Reactance was abnormal in six of them and was significantly correlated with FVC (ρ = 0.70, p = 0.01) and SNIP (ρ = 0.72, p = 0.007), but not with MIP. In adult SMA patients, both MIP and SNIP are feasible, but SNIP is a better predictor of NIV needs, similar to FVC. Optimal predictive thresholds differed from those previously observed in neuromuscular disease. Oscillometric measurements may help to estimate FVC and SNIP in poorly collaborating patients.
Similar content being viewed by others
Introduction
5q-associated spinal muscular atrophy (SMA) is a rare autosomal recessive neuromuscular disease characterized by a progressive degeneration of motor neurons in the spinal cord. The clinical spectrum of SMA is very broad1. According to the age of onset and best-achieved motor milestones, types 0–4 are classified. Management of SMA has primarily focused on standard respiratory care in the pediatric population. However, today, adults represent one-third of all patients, and their proportion will keep growing with new effective available treatments. Hence, it is imperative to concentrate on these patients, who may exhibit distinct characteristics from children.
In SMA, respiratory function decline is the most important cause of morbidity and mortality. A slowing down in the progression of respiratory impairment may be obtained with a timely initiation of noninvasive mechanical ventilation (NIV). NIV should be started as soon as nocturnal hypoventilation appears, which is usually estimated based on outcomes of respiratory and muscular function tests.
The most commonly performed respiratory function test in neuromuscular diseases, including SMA, is spirometry, whose most important parameter is forced vital capacity (FVC). An FVC < 50% of predicted is commonly adopted as a threshold to start NIV2. In the management of neuromuscular patients, muscular function tests are often considered less important than spirometry. However, these patients may have normal lung volumes despite weak respiratory muscles or disproportionately reduced lung volumes in relation to the loss of respiratory muscle force3,4,5. Hence, without precise information on respiratory muscle function, the best therapy for each patient may not be chosen. Respiratory muscle strength is commonly assessed with maximal inspiratory and expiratory pressures (respectively MIP and MEP). In addition to MIP, today sniff nasal inspiratory pressure (SNIP) is increasingly used in the assessment of inspiratory muscle strength. A MIP < 60 cmH2O and a SNIP < 40 cmH2O are considered an indication for NIV therapy in neuromuscular patients2. However, feasibility and usefulness of SNIP in patients with SMA are poorly known.
Further indications of respiratory function can be given by respiratory oscillometry, which explores mechanical properties of the lung parenchyma and chest wall measuring the impedance of the respiratory system6. The most important measures obtained with oscillometry are those of respiratory resistance (Rrs) and respiratory reactance (Xrs). Rrs largely reflects airway openness. Xrs reflects the combined effects of elastance (thoracic and lung stiffness) and inertance (pressure loss due to acceleration of gas in the central airway plus a possible chest wall component). Xrs decreases with increasing elastance while it increases with increasing inertance. In neuromuscular diseases, weakness of thoracic muscles can lead to microatelectasis and severe scoliosis, with an increase in airway resistance and marked reduction in lung volumes and chest wall compliance; therefore, an increase in Rrs and decrease in Xrs, due to increased elastance, are expected. Although oscillometry is intended to complement, and not replace more, commonly performed respiratory tests, it could provide information somehow correlated to spirometry measurements and respiratory muscle strength. It requires only minimal patient collaboration and could prove particularly valuable in subjects who are not able to perform the more common respiratory function tests, often encountered among neuromuscular patients.
We retrospectively studied adult patients with SMA with the following aims: (1) to assess feasibility and performance of MIP and SNIP; (2) to evaluate the suitability of the MIP, SNIP, and FVC values commonly used as thresholds to start NIV therapy in adult SMA patients; (3) to explore the role of oscillometric measurements in the assessment of respiratory function.
Methods
Study design and setting
A retrospective cross-sectional design was adopted. The Regional Centre for Diagnosis and Treatment of Neuromuscular Disease of the University of Palermo was the setting for the study, where our subjects were evaluated as outpatients.
Population and data collection
Patients with genetically proven SMA type 2 or 3 were studied. Data collected between January 2022 and December 2023 were retrospectively analysed. Some patients were followed periodically in the regional centre, while other ones had come to our observation for the first time. No one was treated by NIV. If during that period more than one visit was performed on a patient, only data from the last visit were considered. All tests described below were perforned at each visit. Patients’ age was between 18 and 67 years old. Therefore, patients at various stages of their disease were studied. The inability to give informed consent was considered an exclusion criterion.
Procedures
Height, weight, blood pressure, heart rate, and oxygen saturation (SpO2) were measured. Ulna length was used to predict height7. Respiratory tests were performed as described below, and respiratory measures were expressed as absolute values and as percentages of normal reference values (See supplementary methods).
Spirometry (Pony express, COSMED, Rome, Italy) was performed to measure FVC; it was executed in a sitting position with a flanged rubber mouthpiece and with the nose occluded, in accordance with ATS/ERS society recommendations8,9. MIP and SNIP were measured using a pressure meter (MicroRPM device, Micro Medical/ CareFusion, Kent, UK), following recent European Respiratory Society task force indications10; however, SNIP was performed with the contralateral nostril closed11. For sake of simplicity, both MIP and SNIP were expressed with positive values.
Nocturnal oxygen saturation (SpO2) and transcutaneous PCO2 (PtcCO2) were recorded simultaneously at home within 3 months from the diurnal respiratory function assessment using a digital monitoring instrument (SenTec AG, Therwil, Switzerland). A peak PtcCO2 > 49 mmHg was considered a sign of hypoventilation and of requirement of NIV therapy, in agreement with Ward et al.12,13.
Oscillometric measurements were performed using a commercial forced oscillation technique (FOT) system (Resmon pro-full RESTECH) following current guidelines14; Rrs and Xrs at 5 Hz were measured (respectively Rrs5 and Xrs5) (See Supplementary file for methodological details).
This study, including any relevant details, was approved by our ethics committee Palermo 1, on 31.01.2024 (N° 02/2024) Written informed consent was obtained from all patients. All procedures were performed in accordance with relevant guidelines and regulations, as well as the 1964 Helsinki Declaration and its subsequent revisions, or comparable ethical standards.
Statistical analysis
Normally distributed data were expressed as mean ± SD, and non-normally distributed data as median [IQR]. MIP and SNIP measurements were compared by the paired t-test, and their relationship was evaluated using Pearson’s correlation coefficient. Besides, their agreement was evaluated by Bland–Altman plots, where bias was represented by the mean difference between SNIP and MIP, while upper and lower limits of agreement were defined as the 2.5 and 97.5% limits of the distribution of the differences. ROC curve analysis was used to compare the diagnostic performance of FVC, SNIP and MIP at different thresholds as predictors of NIV requirement. For oscillometric measurements, values with z-scores ranging between − 1.64 and 1.64 were considered normal15. To describe the relation between results from oscillometry and the other respiratory tests, the Spearman correlation coefficient (ρ) was used. A p-value < 0.05 was considered significant. Statistical analysis was performed using IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp; https://www.ibm.com/spss) and MedCalc® Statistical Software version 22.014 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2024).
Results
The cohort included 20 patients. None of them complained of symptoms of nocturnal hypoventilation. Feasibility of all tests was very good. All the 20 patients were able to perform FVC, MIP and SNIP correctly, following the procedures described in the supplemental material. Only 11 patients were requested to undergo the oscillometric evaluation, which was always performed correctly according to standard procedures.
Characteristics of patients and respiratory tests
Table 1 shows that mean SNIP was higher than MIP, whether expressed as absolute or as percentage of predicted values (p = 0.001 and p = 0.01 respectively). The correlation (scatter plot) and agreement (Bland Altman plot) between MIP and SNIP are shown in Fig. 1. Correlation was moderate (r = 0.71, p < 0.001). Agreement between measures was poor; the Bland–Altman plot showed a mean difference of 17.4 and wide limits of agreement (–12.8 to 47.5 cm H2O).
Prediction of NIV
PtcCO2 peak was > 49 mmHg in 10 patients and ≤ 49 mmHg in the remaining 10. Thresholds commonly used to assess the requirement of NIV were exceeded in a variable proportion of patients. FVC was < 50% in 65% of patients, and MIP was < 60 cmH2O in 90% of patients. When used as predictors of NIV requirement, both thresholds were associated with a high false positive rate and a low specificity. SNIP was < 40 cm H2O in 25% of patients; it was associated with a high false negative rate and a low sensitivity (Table 2).
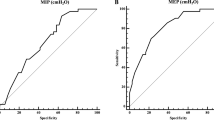
ROC curve analyses (Fig. 2) showed that the accuracy of MIP as a predictor of NIV was low (AUC = 0.57). Instead, AUC values were high for both FVC (AUC = 0.78) and SNIP (AUC = 0.84). This analysis showed that the best cut-off values for FVC were < 20% of predicted due to the disappearance of false positives below this threshold (100% positive predictive value), with a large improvement in specificity at the expense of some decrease in sensitivity. The best cut-off for SNIP was < 61 cmH2O due to the disappearance of false negatives (100% negative predictive value), with a large improvement in sensitivity at the expense of a decrease in specificity.
Oscillometric measurements
FOT measurements were available in 11 patients. In the group, values were normal (median 5.4 cm H2O/(L/s), z-score 1.4 for Rrs5; median − 1.9 cmH2O/(L/s), z-score − 1.5 for Xrs5). However, abnormal values were found in 1 patient for Rrs5, in 2 patients for Xrs5, and in 4 patients for both parameters. Rrs5 was not correlated with FVC (ρ=−0.45), MIP (ρ= −0.03), or SNIP (ρ=−0.44). Similarly, Xrs5 was not correlated with MIP (ρ = 0.29), but it showed a significant correlation with both FVC (ρ = 0.730, p = 0.009) and SNIP (ρ = 0.76, p = 0.006 (Fig. 3).
Discussion
This study explored the role of respiratory and respiratory muscle function tests in adult SMA. The main results were the following. In adult SMA: inspiratory muscle strength can be assessed with both MIP and SNIP, but SNIP shows higher values than, and a poor degree of agreement with, MIP; FVC and SNIP values other than those commonly used in neuromuscular disorders perform better as predictors of NIV requirement; FOT can be used to have additional information on respiratory function and to roughly estimate some aspects of respiratory and muscular function.
Both MIP and SNIP are measures of inspiratory muscle strength, but the strength of the diaphragm is better reflected by SNIP than by MIP16. That should be kept in mind when evaluating patients with SMA. This disease is characterized by weakness of intercostal muscles and relative saving of the diaphragm, which remains the muscle actually responsible for alveolar ventilation. Therefore, in SMA, SNIP may be more appropriate than MIP to estimate the ability of the patients to ventilate. That may warrant our findings of the better performance of SNIP than MIP in predicting nocturnal hypoventilation, and of the large difference between SNIP and MIP values in our patients. An additional reason to prefer SNIP to MIP in SMA, is that SNIP does not require to grip a mouthpiece. SMA patients, especially in adulthood, may present important limitations in maximal mouth opening due to atrophy and fatty degeneration of the lateral pterygoid muscles, which can affect the performance of MIP17,18.
In the literature, there is not much information about usefulness of SNIP in SMA. In children with SMA, SNIP has been utilized with inconsistent outcomes. Skirani et al. reported that in children SNIP is feasible, reliable, and more reproducible than MIP19. However, Veldohen et al. observed that SNIP was not suited to discriminate between SMA types or as an outcome measure for longitudinal follow-up20. Finally, it was concluded that it does not predict the need for NIV.
For adults, some information comes from a study in a heterogeneous group of subjects with neuromuscular disorders that included a large number of patients with SMA21, which reported good feasibility and usefulness of SNIP. Our results indicate that in adult SMA SNIP is easily feasible and is an important measure for the assessment of muscular function and the clinical management of the patients. Its significant difference from MIP may indicate that evaluating MIP alone in SMA could lead to a considerable underestimate of patients’ ability to generate inspiratory forces.
Spirometry and muscular strength measures in neuromuscular disorders are used not only to evaluate the severity of respiratory and muscular impairment but also to predict nocturnal hypoventilation and the requirement of NIV therapy. For that purpose, threshold values for FVC, MIP, and SNIP have been proposed22,23,24. In this study, we used a definition of nocturnal hypoventilation provided by the literature in neuromuscular patients12,13. No MIP value showed good predictive ability, including the commonly used 60 cmH2O threshold. In particular, that threshold was associated with a very high false positive rate. Early start of NIV can be associated with poor compliance to ventilatory treatment, which may persist when NIV is really needed. For that reason, false positivity of tests for prediction of NIV requirement should be reduced as much as possible. Instead, the ROC curve analysis showed that accuracy of both FVC and SNIP was higher than accuracy of MIP, but FVC and SNIP thresholds that best predicted NIV requirement differed from the commonly accepted values. The commonly used FVC threshold of 50% was associated with a high false positive rate, which decreased its specificity and positive predictive value. Indeed, the loss of lung volume in SMA may be disproportionate to the loss of respiratory muscle strength. Alterations in chest wall properties can be responsible for scoliosis, which in patients with SMA 2 and 3 tends to be the worst among neuromuscular patients, as it develops early and progresses rapidly25. In turn, scoliosis can cause severe lung volume restriction with a decrease in FVC while the diaphragm is not yet severely affected and is still able to warrant an acceptable arterial oxygenation. Contrary to FVC, we observed that the traditional predictive threshold value for SNIP (i.e. 40 cm H2O) was associated with a high false negative rate, low sensitivity, and negative predictive value. The use of a higher threshold greatly decreased false negatives, although causing a decrease in specificity. The relative preservation of diaphragm function, which allows SMA patients to maintain a relatively high SNIP, can account for this finding. FOT revealed easy to perform in all subjects. In SMA, due to the large spectrum of functional impairment that can be found, various degrees of alteration in oscillometric indices may be expected. In most of our patients, Rrs5 and Xrs5measurements came out normal. In this respect, our data differed from those reported in samples of SMA children in previous studies26,27but were more similar to those found in a study in adult neuromuscular patients including subjects with SMA28. Although oscillometry should be complementary, and not alternative, to other respiratory function tests, it is generally considered particularly useful for patients who cannot perform tests requiring active collaboration. In the literature, significant correlations between spirometry and oscillometry measurements were found in SMA children and a group of adult neuromuscular patients including some SMA subjects29,30. However, different findings were reported in patients with other neuromuscular disorders where impulse oscillometry (IOS) was used instead of FOT31. More studies in homogeneous groups of patients would be necessary to better understand the role of oscillometry in the management of SMA patients. Our study supports the role of impedance measurements by FOT as a correlate of FVC and SNIP among adult patients with SMA.
This study has some limitations. The number of patients is small, due to the rarity of the disease. However, we found significant differences between SNIP and MIP. Additionally, as we report an experience from a single centre, the results may be not fully generalizable. Future multicenter studies would be appropriate to confirm our findings. Different criteria for hypoventilation may produce different results. Finally, we did not monitor electromyograms during SNIP and MIP, which could provide important electrophysiological data.
To conclude, in adult SMA patients with severe reduction of respiratory function, SNIP performed better than MIP. Besides, in the prediction of NIV requirement, the thresholds of FVC, MIP, and SNIP commonly used in neuromuscular diseases did not fit well. In that regard, MIP appeared of little usefulness, while SNIP and FVC, at new thresholds, proved much more effective.
Oscillometry can be a useful complement in the functional evaluation of SMA patients. Our findings suggest that it may be used in poorly collaborating patients to estimate lung mechanics and respiratory muscle strength, but further studies are required on larger samples of subjects.
Due to the nature of this study, we have not yet collected data to monitor respiratory and muscular function after NIV application. An evaluation of the evolution of the results of each test in relation to variations in PtcCO2 following the initiation of ventilatory therapy could confirm the significance of our observations and clarify their usability for a personalized follow-up of each patient.
Data availability
The datasets used and analysed during the current study are available from the corresponding author (G.C.) upon reasonable request.
Abbreviations
- FVC:
-
Forced vital capacity
- FOT:
-
Forced oscillation technique
- HFSME:
-
Hammersmith Functional Motor Scale
- MIP:
-
Maximum inspiratory pressure
- NIV:
-
Noninvasive ventilation
- Rrs5 :
-
Resistance at 5 Hz
- RULM:
-
Revised Upper Limb Module
- SNIP:
-
Sniff nasal pressure
- Xrs5 :
-
Reactance at 5 Hz
References
Mercuri, E., Sumner, C. J., Muntoni, F., Darras, B. T. & Finkel, R. S. Spinal muscular atrophy. Nat. Rev. Dis. Primers. 8, 52 (2022).
Khan, A. et al. Respiratory management of patients with neuromuscular weakness: an American college of chest physicians clinical practice guideline and expert panel report. Chest 164, 394–413 (2023).
Crescimanno, G., Romano, M., Spataro, R., La Bella, V. & Marrone, O. Early and rapidly progressing respiratory failure in a patient with amyotrophic lateral sclerosis: when FVC% is misleading. Neurol. Sci. 40, 421–422 (2019).
De Troyer, A., Borenstein, S. & Cordier, R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax 35, 603–610 (1990).
Sheers, N. L. et al. Rapidly and slowly progressive neuromuscular disease: differences in pulmonary function, respiratory tract infections and response to lung volume recruitment therapy (LVR). BMJ Open. Respir Res. 9. https://doi.org/10.1136/bmjresp-2022-001241 (2022).
King, G. G. et al. Technical standards for respiratory oscillometry. Eur. Respir J. 55, 1900753 (2020).
Gauld, L. M., Kappers, J., Carlin, J. B. & Robertson, C. F. Height prediction from ulna length. Dev. Med. Child. Neurol. 46, 475–480 (2004).
Graham, B. L. et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am. J. Respir Crit. Care Med. 200, e70–e88. https://doi.org/10.1164/rccm.201908-1590st (2019).
Stanojevic, S. et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir J. 60, 2101499 (2022).
Laveneziana, P. et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir J. 53, 1801214 (2019).
Kaminska, M., Noel, F. & Petrof, B. J. Optimal method for assessment of respiratory muscle strength in neuromuscular disorders using Sniff nasal inspiratory pressure (SNIP). PLoS One. 12, e0177723. https://doi.org/10.1371/journal.pone.0177723 (2017).
Ward, S., Chatwin, M., Heather, S. & Simonds, A. K. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax 60, 1019–1024 (2005).
Ogna, A. et al. Nocturnal hypoventilation in neuromuscular disease: prevalence according to different definitions issued from the literature. Sleep. Breath. 20, 575–581 (2016).
Oostveen, E. et al. The forced Oscillation technique in clinical practice: methodology, recommendations, and future developments. Eur. Respir J. 22, 1026–1041 (2003).
Veneroni, C. et al. Diagnostic potential of oscillometry: A Population-based approach. Am. J. Respir Crit. Care Med. 209, 444–453 (2024).
Nava, S., Ambrosino, N., Crotti, P., Fracchia, C. & Rampulla, C. Recruitment of some respiratory muscles during three maximal inspiratory manoeuvres. Thorax 48, 702–707 (1993).
Wadman, R. I. et al. Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia. Neurology 83, 1060–1066 (2014).
van Bruggen, H. W. et al. Mandibular dysfunction as a reflection of bulbar involvement in SMA type 2 and 3. Neurology 86, 552–559 (2016).
Khirani, S. et al. Longitudinal course of lung function and respiratory muscle strength in spinal muscular atrophy type 2 and 3. Eur. J. Paediatr. Neurol. 17, 552–560 (2013).
Veldhoen, E. S. et al. Natural history of respiratory muscle strength in spinal muscular atrophy: a prospective National cohort study. Orphanet J. Rare Dis. 17, 70 (2022).
Stefanutti, D., Benoist, M. R., Scheinmann, P., Chaussain, M. & Fitting, J. W. Usefulness of Sniff nasal pressure in patients with neuromuscular or skeletal disorders. Am. J. Respir Crit. Care Med. 162, 1507–1511 (2000).
EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)--revised report of an EFNS task force. Eur. J. Neurol. 19, 360–375 (2012).
Gonzalez-Bermejo, J. et al. Prognostic value of efficiently correcting nocturnal desaturations after one month of non-invasive ventilation in amyotrophic lateral sclerosis: a retrospective monocentre observational cohort study. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 373–379 (2013).
Morgan, R. K. et al. Use of Sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am. J. Respir Crit. Care Med. 171, 269–274 (2005).
Merlini, L. et al. Scoliosis in spinal muscular atrophy: natural history and management. Dev. Med. Child. Neurol. 31, 501–508 (1989).
Gauld, L. M., Keeling, L. A., Shackleton, C. E. & Sly, P. D. Forced Oscillation technique in spinal muscular atrophy. Chest 146, 795–803 (2014).
Kapur, N., Deegan, S., Parakh, A. & Gauld, L. Relationship between respiratory function and need for NIV in childhood SMA. Pediatr. Pulmonol. 54, 1774–1780 (2019).
Wesseling, G., Quaedvlieg, F. C. & Wouters, E. F. Oscillatory mechanics of the respiratory system in neuromuscular disease. Chest 102, 1752–1757 (1992).
Veldhoen, E. S. et al. A substitute of spirometry in children with neuromuscular diseases? Pediatr. Pulmonol. 57, 1618–1624 (2022).
Gochicoa-Rangel, L. et al. Respiratory impedance in patients with Duchenne muscular dystrophy. Pediatr. Pulmonol. 51, 1072–1079 (2016).
Iliaz, S. et al. The clinical use of impulse oscillometry in neuromuscular diseases. Respir Med. 200, 106931 (2022).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
GC conceptualized the study, interpreted data, and prepared the initial manuscript. AL, VT, and VD conducted clinical examinations of the patients described in the study and interpreted the data. OM interpreted the data and critically revised the manuscript for important intellectual content. All authors contributed equally to the study design and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by our ethics committee Palermo 1 on 31.01.2024 (N° 02/2024). Written informed consent was obtained from patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Crescimanno, G., Lupica, A., Tomasello, V. et al. Multiple respiratory assessment and thresholds for noninvasive ventilation in adult patients with spinal muscular atrophy. Sci Rep 15, 6478 (2025). https://doi.org/10.1038/s41598-025-91276-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91276-6
Keywords
This article is cited by
-
Rehabilitation management for patients with spinal muscular atrophy: a review
Orphanet Journal of Rare Diseases (2025)