Abstract
Venous thromboembolism (VTE) is a common complication in patients with traumatic brain injury (TBI). This study aimed to assess the predictive ability of the Caprini score, Risk Assessment Profile for Thromboembolism (RAPT), and Trauma Embolic Scoring System(TESS) for VTE risk assessments in TBI patients. A retrospective analysis of 460 TBI patients was conducted, categorizing them into VTE and non-VTE groups based on imaging results. The three scales were applied to assess VTE risk, and their performance was compared using receiver operating characteristic(ROC) curves and area under the curve(AUC) values. The VTE incidence was 31.7%. The RAPT scale demonstrated the highest AUC (0.826) and optimal cutoff (9.5) with balanced sensitivity (0.753) and specificity (0.771). The Caprini and TESS scales also showed moderate to high predictive value but had lower AUCs. All three scoring scales showed medium to high predictive value for the risk of VTE in patients with TBI. Among them, the RAPT scoring scale offered the highest predictive value for VTE risk in TBI patients, with fewer items, making it easier for clinical implementation. It stands as the most appropriate VTE risk assessment scale for TBI patients at present.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis and pulmonary embolism, is the third most common cardiovascular disease in the world1. In China, the population incidence of VTE is 17.5 per 100,000 individuals2, representing a significant cause of unanticipated mortality among inpatients across all levels of hospitals. Traumatic brain injury (TBI) is brain damage caused by external impacts or penetrations to the head, which can result from various causes3. Each year, around 69 million people worldwide experience TBI, TBI is the leading cause of trauma-related deaths and disabilities globally4 and is also a separate risk factor for VTE5,Patients with TBI experience prolonged bed rest caused by trauma, blood vessel damage from surgery, and increased tissue factor release in the vascular system post-trauma, which elevates procoagulant factors and contributes to the onset of VTE6,7. VTE in TBI patients is characterized by high incidence, high mortality, high misdiagnosis rate, and high missed diagnosis rate8, Relevant studies indicate that after the occurrence of VTE in TBI patients, the risk of adverse outcomes such as mortality, prolonged hospital stays, increased surgical procedures, ventilator dependency, prolonged bed rest, invasive intracranial monitoring, and surgery significantly increase9. Therefore, early prophylaxis of VTE is crucial for improving the prognosis of patients with TBI.
Assessing the risk of VTE is essential for early risk-benefit analysis in VTE prevention decision-making. The American College of Chest Physicians (ACCP) recommends this assessment as a standard practice to prevent thrombosis10. Accurate assessment and identification of high-risk patients through VTE risk assessment tools can facilitate targeted interventions for TBI patients, thereby reducing the incidence of VTE. Currently, there is no universally accepted VTE risk assessment scale tailored for TBI patients. The Caprini score is the most widely used tool for evaluating thrombosis risk in hospitalized patients undergoing surgeries other than orthopedic ones11, while the RAPT score and the TESS score are often used for assessing risk in hospitalized trauma patients12,13.
ACCP recommends using the Caprini scale to assess the risk of VTE in hospitalized patients11. The Caprini Risk Assessment Scale was first published by Professor Caprini in 199114 and the currently most widely used version, Caprini 2005, was formulated in 200515. It was developed based on evidence-based medical evidence, expert consensus, and clinical experience. This scale has been recommended by ACCP guidelines11 for assessing thrombosis risk in hospitalized patients undergoing surgeries other than orthopedic surgeries and has been clinically validated in populations such as internal medicine, oncology, ICU, trauma, and orthopedic surgery patients16,17,18,19; The European Society for Trauma and Emergency Surgery (ESTES) guideline20 recommend the use of the RAPT scale or the TESS scale for assessing the risk of VTE in trauma patients. Additionally, the RAPT scale is also recommended by the European Association of Neurosurgical Societies (EANS) guideline for assessing VTE risk in neurosurgical patients. Both the RAPT scale and the TESS scale are tools for assessing VTE risk in trauma patients and have good predictive power and clinical practicality; The RAPT score, developed by Greenfield et al.12, is a VTE risk assessment tool for trauma patients, developed based on clinical experience and literature reviews. Previous researchers have already validated the RAPT scale21,22, consistently demonstrating its predictive capability for VTE in trauma patients. The TESS score scale was developed by Rogers et al.13 in 2012, drawing upon clinical experience and significant risk factors for VTE identified in retrospective studies.Although the TESS scale was validated using data from the National Trauma Database (NTDB)13, subsequent research is relatively scarce. Only a few studies, such as those by Ho KM et al. in severely injured patients23 and Walker PF et al. in military trauma patients24, have further explored its application, indicating that it still lacks extensive clinical research in other aspects and its generalizability remains to be confirmed.
The above-mentioned scales have been utilized in the context of surgical or trauma patients; however, their efficacy in predicting outcomes for individuals with TBI remains uncertain. This study is designed to investigate the application potential of the well-established Caprini, RAPT, and TESS risk assessment scales in TBI patients, aiming to expand their usability rather than developing a novel prediction model. Therefore, we retrospectively analyzed the clinical data of 460 patients with TBI admitted to a regional trauma medical center from July 2019 to July 2022. The objective of this study was to rigorously evaluate and compare the Caprini, RAPT, and TESS risk assessment scales in terms of their ability to precisely predict the risk of VTE in patients with TBI.
Materials and methods
Patients
This investigation constitutes a retrospective cohort study, wherein the hospital’s medical record information system was employed to identify cases with discharge diagnoses encompassing “traumatic brain injury”, “traumatic intracerebral hemorrhage” and “traumatic craniocerebral trauma” among patients admitted to a regional trauma medical center from July 2019 to July 2022.
Inclusion criteria
(1) Patients admitted to hospital due to trauma and diagnosed with TBI based on head CT or MRI results for the first time; (2) Age ≥ 18 years; (3) No previous history of anticoagulant drugs, antiplatelet therapy, or thrombolytic medication; (4) Ultrasound and/or CTPA examination results during hospitalization.
Exclusion criteria
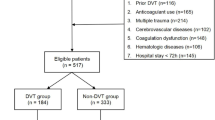
(1) VTE was diagnosed at admission; (2) Patients who died and voluntarily discharged within 3 days after admission; (3) Cases with incomplete clinical data. Based on the aforementioned diagnostic criteria, a total of 460 cases met the criteria (Fig. 1). The hospital ethics committee approved this retrospective study (IRB number: 2023 − 1888); The procedures used in this study adhere to the Declaration of Helsinki; as this study is retrospective and does not involve patient privacy information, the informed consent was waived.
Diagnostic criteria for VTE
The diagnosis of VTE was based on Doppler vascular ultrasound and CT pulmonary angiography (CTPA). At our institution, it is a routine practice to perform weekly ultrasound screening of the limb vessels for bedridden TBI patients, The Doppler color ultrasound was performed using a CX50 bedside portable ultrasound device (Philips Company, Amsterdam, the Netherlands) with an L12-3 probe operating at a frequency of 3-12 MHz. Experienced ultrasound physicians routinely screened the limb vessels of patients with traumatic brain injuries weekly, examining the venous lumens for abnormal echo fillings, any filling defects in the blood flow signals, and the ability of the probe to occlude the vessels upon pressure. Findings such as weak, iso-, or slightly hyperechoic areas filling the deep venous lumens, absent or deficient blood flow signals, or partial occlusion of the vessels upon probe pressure, all suggest the presence of deep venous thrombosis. Patients exhibiting symptoms suggestive of pulmonary embolism, such as dyspnea and chest pain, along with elevated D-dimer levels, were promptly subjected to CTPA. The radiology technician employed dual-source CT (Siemens Somatom Definition Flash, Germany) for localization and contrast-enhanced scanning. Following the procedure, the images were transferred to a Siemens workstation, where two experienced radiologists assessed the quality of the post-processing images. The presence of filling defects in the pulmonary arteries was observed to exclude the presence of PE.The primary outcome of this study is to determine whether patients with TBI develop VTE. The presence of VTE in patients is identified through Doppler vascular ultrasound and CT pulmonary angiography. If the test results show deep vein thrombosis or pulmonary embolism, it is judged that VTE has occurred and is recorded as a positive outcome.
Data collection
The patients’ general information, including their sex, age, body mass index(BMI), injury severity score(ISS), total hospital length of stay (LOS), Glascow coma scale(GCS), GCS level, cause of injury, surgery, central venous catheter(CVC), mechanical prophylaxis, chemoprophylaxis, and incidence of VTE were collected. The patients’ VTE risk was assessed using the Caprini 2005 score, the RAPT score and the TESS score. The corresponding scores were calculated based on the patient information after reviewing the medical records. Prior to the commencement of the study, we provided unified training to the researchers involved in data collection and assessment. We clearly defined the specific definitions and judgment criteria for each risk factor. Additionally, for some controversial cases, group discussions and expert consultations were carried out to ensure the consistency of scoring.
The Caprini 2005 Risk Assessment Scale encompasses a comprehensive set of criteria, including patient demographics, comorbidities, trauma history, surgical procedures, medical history, laboratory findings, and additional considerations, totaling 38 individual risk factors. Each factor is allocated a specific score, with the cumulative score then translated into one of four risk tiers: a score of 0–1 results from low risk, 2 points indicate moderate risk, 3–4 points classify as high risk, and a score of 5 or above is categorized as very high risk25.
The RAPT scoring scale classifies recognized risk factors into four categories: underlying medical conditions, iatrogenic factors, injury-associated factors, and age. Comprising 19 individual scoring items, the scale yields a cumulative score ranging from 0 to 46, with scores exceeding 5 denoting a high-risk category for thromboembolic events12.
The TESS scoring scale classifies risk factors into five key areas: obesity, mechanical ventilation, lower limb trauma, age, and ISS26. Comprising 13 scoring items in total, the scale yields a cumulative score ranging from 0 to 14. The final scores are stratified into four risk levels: 0–3 points suggest no risk, 4–6 points denote moderate risk, 7–10 points indicate high risk, and scores exceeding 10 points are classified as very high risk13.
Data processing and statistical analysis
Two researchers independently verified data included in the study and corrected any errors. Data were stored using Excel 365(Microsoft Corporation, Redmond, Washington, USA) and Data analysis was performed using IBM SPSS 25.0 statistical software. [https://www.ibm.com/products/spss-statistical-software](IBM Corporation, Armonk, New York, USA). Measurement data were tested for normality using the Shapiro-Wilk method, and those conforming to a normal distribution were described using the mean ± standard deviation. The comparison between the VTE group and the non-VTE group was carried out using the independent sample t - test. Data with a non-normal distribution were expressed as M (Q1, Q3), and the Mann-Whitney U test was used for the comparisons between the VTE group and the non-VTE group. Count data were described using frequency and percentages and analyzed with the chi-square test. Receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) with 95% confidence interval (95%CI) were calculated based on the scores from three risk scales(). Sensitivity and specificity were calculated to assess the predictive accuracy of these scales for VTE in TBI patients. An AUC 0.9 indicates high discrimination ability27; Pairwise comparisons of AUC were conducted using the Delong method, with a significance level of a = 0.05;Furthermore, we fitted logistic regression models based on the scores of the three scales and the VTE outcome respectively, used the calibration curve to identify model calibration, and used the decision curve analysis (DCA) curve to analyze the differences in the clinical utility of different scales.The above analysis was completed using R 4.4.2 and the rms and ggDCA packages.
Results
Demographics of subjects
A total of 460 TBI patients were included based on the inclusion and exclusion criteria, as shown in Fig. 1. Of these, 349 were male [75.9%] and 111 were female [24.1%], with ages ranging from 18 to 101 years and a median age of 54 years. The average BMI was 22.83. The ISS had a median of 25 points. The average length of hospital stay was 12 days.The average GCS score was 9 points. The causes of injury were traffic accidents in 164 cases (35.6%), falls/high falls in 224 cases (48.6%), and other causes in 72 cases (15.6%). Medication for prevention was used in 75 cases (16.3%), and physical prevention methods were used in 70 cases (15.2%). The Caprini risk score ranged from 6 to 28 points, with a median of 12 points The RAPT risk score ranged from 2 to 22 points, with a median of 8 points. The TESS risk score ranged from 0 to 14 points, with a median of 8 points. Of these patients, 146 cases developed VTE, accounting for 31.7%, Among the 146 cases with thrombosis, according to the site of occurrence, there were 120 cases (80.2%) of calf intermuscular veins, 22 cases (15%) of posterior tibial veins, 16 cases (11.0%) of femoral veins, 11 cases (7.5%) of axillary veins, 7 cases (4.8%) of popliteal veins, 7 cases (4.8%) of peroneal veins and 2 cases (1.4%) of pulmonary embolism. When comparing the characteristics of the two groups of patients, there were no statistically significant differences in sex, age, BMI, and scores on various physical prophylaxis scales (P > 0.05). However, there were statistically significant differences in ISS, length of hospital stay, GCS, GCS level, causes of injury, surgical treatment, placement of central venous catheters, chemoprophylaxis and scores on various scales (P < 0.05) as shown in Table 1.
In our study, VTEs were detected through weekly surveillance. Among them, the number of symptomatic cases was relatively small. Specifically, there were 12 cases with extremity swelling and only 2 cases with symptoms such as dyspnea and chest pain. The relatively low incidence of symptomatic VTE might be due to the fact that many VTEs were detected at an earlier stage during the regular surveillance before they developed into more severe conditions with obvious symptoms. Previous studies have also suggested that the risk factors for symptomatic and asymptomatic VTE are generally consistent. For example, in a study by Knudson et al.28, they found that factors such as age, ISS, and presence of lower extremity fracture were significant risk factors for both symptomatic and asymptomatic VTE in trauma patients. The main difference between them lies in whether there are clinical manifestations, which may be related to the time and severity of VTE when it is discovered. Symptomatic VTE is more likely to be detected when the thrombus has already caused a certain degree of obstruction or embolization, while asymptomatic VTE is usually found earlier in the course of the disease. This further emphasizes the importance of regular screening for early detection of VTE, regardless of whether it is symptomatic or not, to take appropriate preventive and treatment measures in a timely manner.
The correlation between the risk assessment score and the incidence of VTE
Comparison of ROC curves for three scales
ROC curves were shown in Fig. 5. Among these three scales, the AUC for RAPT (0.826) was greater than that for Caprini (0.783) and TESS (0.765). RAPT and Caprini scales both had the highest sensitivity of 0.753, but the RAPT scale had the highest specificity of 0.771; For the Caprini scale, using 12.5 as the cutoff point, the sensitivity and specificity were 0.726 and 0.729 respectively, with a Youden’s index of 0.455 and an AUC of 0.783; For the RAPT scale, using 9.5 as the cutoff point, the sensitivity and specificity were 0.753 and 0.771 respectively, with a Youden’s index of 0.524 and an AUC of 0.826; For the TESS scale, using 8.5 as the cutoff point, the sensitivity and specificity were 0.753 and 0.672 respectively, with a Youden’s index of 0.425 and an AUC of 0.765, as shown in Table 2. The AUCs of the three scales were compared using the Delong test. showing that there was a statistically significant difference in the AUC between the RAPT scale and the Caprini risk assessment scale, as well as between the RAPT risk assessment scale and the TESS scale (P < 0.05). However, as seen in Table 3, there was no statistically significant difference in the AUC between the Caprini and TESS scales (P > 0.05) (Figs 6, 7, 8).
Model calibration curve comparison: See Figs. 6, 7, 8. Among the three scales, the calibration curves of RAPT and TESS were close to the ideal curve, and both had relatively low MAE (the MAE of RAPT was 0.031, and that of TESS was 0.015), indicating that the deviation between the predicted risk and the actual risk of these two scales was relatively small. However, the calibration curve of the Caprini scale showed an obvious tendency to overestimate the actual risk at higher predicted probabilities, and its MAE value was 0.055.
Model clinical decision curve comparison: See Fig. 9. It can be seen that the RAPT scale had a clinical net benefit in a wider range, as well as a greater net benefit than the Caprini scale and the TESS scale. This means that in clinical practice, using the RAPT scale for VTE risk assessment can more effectively identify high-risk patients while avoiding unnecessary interventions, thus providing more valuable guidance for clinical decision-making.
Discussion
TBI patients are a high-risk population for VTE. This study showed that the incidence of VTE in patients was 31.7%, which is generally consistent with previous studies reporting a VTE incidence rate of 5-54% in TBI patients29. In the management of TBI, patients are at a significantly elevated risk of VTE, while also facing the potential danger of intracranial hemorrhage30. The delay in administering anticoagulation therapy has been identified as a crucial factor contributing to the onset of VTE in TBI patients31. In the current study, only 16.3% of patients received pharmacological prophylaxis. Despite a majority of studies suggesting that early prophylactic use of low molecular weight heparin (LMWH) in TBI patients is safe32,33,34,35,36, many authoritative guidelines do not firmly advocate for pharmacological prevention during hospital stay for these patients20,37,38 due to the lack of sufficient high-quality evidence. The optimal timing for initiating postoperative pharmacological anticoagulation in TBI patients who have undergone surgical intervention remains a topic of clinical debate. The more conservative approach to prevention may explain the higher incidence of VTE observed in this study. Moreover, the high prevalence of VTE is gaining attention, and the significance of risk assessment and periodic screening is underscored10. Periodic screening may also enhance the detection of asymptomatic VTE. The incidence of symptomatic VTE and its concrete impact on patient outcomes warrant further exploration.
A recent retrospective analysis of 75,570 cases reported that penetrating injuries, increasing age (> 75 years), male sex, obesity, tachycardia, increased head AIS, moderate abdominal-related injuries (AIS = 2), spinal injuries, upper limb injuries, lower limb injuries, craniotomy or intracranial pressure monitoring and previous history of hypertension are all independent risk factors for VTE in TBI patients39. However, there is currently no specifically developed risk assessment scale for TBI patients, and existing risk assessment scales do not adequately cover the above risk factors. Therefore, the choice of risk assessment scale for TBI patients remains an unresolved issue. In previous studies, the dedicated thrombotic risk assessment scales for trauma patients mainly included the RAPT and TESS scoring systems12,13, while the Caprini scoring scale is widely used for risk assessment of all surgical inpatients. Therefore, this study evaluated TBI patients using the existing Caprini, RAPT, and TESS thrombotic assessment scales and compared the predictive ability of the three scales.
The results of this study indicated that the AUC values for all three scoring systems were greater than 0.5, suggesting predictive value for each. Among them, the RAPT scoring system exhibits the highest predictive value with an AUC of 0.826, followed by the Caprini scoring system (0.783) and the TESS scoring system (0.765). In this study, in addition to the traditional AUC index, we further employed calibration curves and DCA curves to deeply evaluate the applicability of risk assessment scales in clinical practice. As previous studies have pointed out40, relying solely on the AUC index can reflect the discriminatory ability of the model to a certain extent, but it lacks sufficient and detailed insights into the performance of the model in actual clinical decision-making. The calibration curve can intuitively display the calibration degree of the model by comparing the predicted risk with the actually observed risk. We found that when analyzing the Caprini, RAPT, and TESS scales, they each exhibited different calibration characteristics. For example, the RAPT scale performed relatively better in the calibration curve, with a relatively smaller mean absolute error, indicating that the deviation between the risk predicted by this scale and the actually occurring risk is relatively small, which to some extent reflects its advantages in the accuracy and reliability of risk estimation. This is consistent with our expectations in actual clinical applications, that is, a good risk assessment scale should be able to accurately quantify the risk of VTE occurrence in patients, avoid overestimating or underestimating the risk, and thus provide a more reliable basis for clinical decision-making41. Through the DCA curve42, we can clearly see the changes in the net benefit brought by each scale within different risk threshold ranges. In this study, the RAPT scale showed a higher net benefit within multiple reasonable risk threshold ranges, which means that in clinical practice, using this scale for VTE risk assessment can effectively identify high-risk patients while avoiding unnecessary interventions, thus providing more valuable guidance for clinical decision-making. In contrast, the Caprini and TESS scales had relatively lower net benefits within certain threshold ranges, further highlighting the advantages of the RAPT scale in clinical utility. The RAPT scoring system is primarily used for trauma patients, but there has been some controversy regarding its predictive reliability in previous studies43. In previous research, Gearhart et al.21 reported a sensitivity of 100% and a specificity of 39% when the cutoff score was 5. In contrast, Hegsted et al.22 reported a sensitivity of 82% and a specificity of 57%, while Zander et al.43,reported a sensitivity of 83% and a specificity of 37%, with an AUC of 0.66. These studies focused on trauma populations. However, our study found that the optimal cutoff score for using RAPT to assess TBI patients is 9.5, at which point the sensitivity of RAPT is 0.753 and the specificity is 0.771, indicating a relatively balanced ability to identify both high-risk and low-risk patients. Compared to the Caprini and TESS scoring systems, The RAPT scoring system has the highest predictive value for VTE occurrence in TBI patients.
Further analysis suggests several possible reasons for the superior predictive ability of the RAPT scoring system in TBI patients. The RAPT scoring system includes assessments related to head injury (head AIS > 2 & GCS < 8), which are more effective in identifying critically ill TBI patients who are prone to prolonged bed rest and thus more susceptible to VTE. The other two scoring systems lack relevant descriptions of craniocerebral injury. The high prevalence of abnormal coagulation is another important factor. Studies have reported that approximately 20% of TBI patients exhibit coagulation disorders on admission44. This may be related to post-traumatic hyperfibrinolysis, vascular endothelial injury, acidosis, platelet dysfunction etc45. When the prothrombin time is shortened, indicating a hypercoagulable state, the risk of VTE is higher. Therefore, close monitoring of coagulation function during treatment is crucial for the prognosis of TBI patients. While effectively controlling further bleeding, it is also necessary to be vigilant about hypercoagulability. Additionally, surgery, blood transfusion, and deep venous catheterization are commonly used treatments for TBI patients. Relevant studies have found that these are all high-risk factors for VTE occurrence46,47. Therefore, such patients require high vigilance for the occurrence of VTE.
The findings indicate that a cutoff score of 9.5 is optimal for the RAPT scoring system. Patients scoring below 5 have a low incidence of VTE at only 3.9%. The RAPT scale is designed with practicality in mind, featuring four dimensions and 19 items, offering a more streamlined and clinically applicable approach compared to the 38-item Caprini risk assessment scale. This tool enables clinical nursing staff to efficiently evaluate VTE risk, thereby facilitating the implementation of preventive strategies and potentially reducing the prevalence of VTE in patients suffering from traumatic brain injury.
The Caprini scoring system has been less frequently used in trauma and TBI populations. Kirill et al. reported48 that in trauma and general surgical patients, the VTE incidence was 15.5–15.9% when the Caprini score was 9–10, and 47.1% when the score was ≥ 11. The current study found that the average Caprini score for TBI patients was 12, with a VTE incidence of 16% for scores in the 10–11 range and 52.8% for scores ≥ 12, similar to Kirill’s findings. Previous studies have shown that the Caprini scoring system has acceptable predictive performance, with AUC values ranging from 0.738 to 0.9249,50,51. Our study also found that it has good discrimination, and when the cutoff score is 12.5, the sensitivity (72.6%) and specificity (72.9%) are optimal. However, compared to the RAPT scoring system, the Caprini scoring system has too many assessment items, some of which are not suitable for Asian populations. For example, the assessment items such as Positive Factor V Leiden and Positive Prothrombin 20,210 A are the main hereditary thrombotic factors in Western countries. Since these items are not relevant to the Asian population characteristics, the Caprini assessment scale lacks specificity for the target population52,53,54,55. According to the risk stratification recommended by ACCP guidelines, all TBI patients in this study are at high risk of VTE, indicating that a specific cutoff score needs to be established for the TBI population to guide clinical practice. The TESS scoring system, designed explicitly for trauma patients as a thrombosis assessment tool, exhibits certain limitations. In our application, it has shown relatively lower accuracy compared to other scales. Although it has been validated to some extent, it still lacks comprehensive clinical research across diverse scenarios. Findings from this study suggest that when predicting the occurrence of VTE in TBI patients, the TESS scoring system demonstrates the weakest predictive ability among the compared scoring systems. For example, its AUC values were lower, and its risk stratification was less effective, with a notable percentage of VTE events occurring even in the low - risk group it defined. Given these findings, future efforts might be directed towards modifying its assessment items. This could involve incorporating variables more relevant to TBI patients, such as specific markers related to brain injury - induced hypercoagulability, to enhance its predictive power and applicability in this patient population.
Shortcomings and prospects
This study comprehensively compared the application effects of three risk assessment scales in patients with TBI, providing a reference for VTE risk assessment practices in TBI patients. However, there are still several limitations: (1) This study constitutes a single-center, retrospective analysis with a limited sample size, which may not fully represent the broader population. Future research will involve multi-center, prospective studies to enlarge the sample size, delving deeper into the long-term efficacy of the RAPT scale for VTE prevention in TBI patients. (2) The current study’s scope did not permit a detailed analysis of the specific items within the scales that influence VTE outcomes. Future endeavors may focus on identifying unique risk factors for VTE in TBI patients and explore the potential of combining the RAPT scale with additional factors such as coagulation profiles and imaging results to develop a more tailored risk assessment tool for TBI-related VTE. (3) The assessment method employed in this study was based on human judgment, which could introduce bias. To improve efficiency and accuracy, future assessments will aim to integrate the scale into electronic medical record systems. (4) The limitations of this study is the absence of a comprehensive validation analysis across different datasets as typically required for prediction models. Since our focus was on the exploration of scale applicability in TBI patients, we did not conduct such an analysis. However, this may introduce uncertainties regarding the generalizability of our results to other populations. Future studies should consider validating these scales in more diverse datasets to enhance the reliability of their application.(5)One of the limitations of this study is that our institution’s practice of using weekly routine ultrasound screening for TBI patients may not be consistent with the VTE screening routines in other institutions.
Conclusion
In summary, TBI patients have a higher risk of developing VTE, and it is worth exploring which risk assessment scale is effective for this population. This study found that compared to the Caprini Risk Assessment Model and the TESS scale, the RAPT scale has better predictive effectiveness for VTE risk in TBI patients. It is recommended to further promote its clinical application and, in the future, combine it with stratified precision interventions to provide support for reducing the incidence of VTE in TBI patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Wendelboe, A. M. & Raskob, G. E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 118, 1340–1347 (2016).
Zhang, Z. et al. Trends in hospitalization and In-Hospital mortality from VTE, 2007 to 2016, in China. Chest 155, 342–353 (2019).
Dams-O’Connor, K. et al. Traumatic brain injury as a chronic disease: Insights from the united States traumatic brain injury model systems research program. Lancet Neurol. 22, 517–528 (2023).
Dewan, M. C. et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130, 1080–1097 (2018).
Shibuya, N., Frost, C. H., Campbell, J. D., Davis, M. L. & Jupiter, D. C. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: Analysis of the National trauma data bank. J. Foot Ankle Surg. 51, 63–68 (2012).
Annen, J. et al. Function-structure connectivity in patients with severe brain injury as measured by MRI-DWI and FDG-PET. Hum. Brain Mapp. 37, 3707–3720 (2016).
Foreman, P. M., Schmalz, P. G. & Griessenauer, C. J. Chemoprophylaxis for venous thromboembolism in traumatic brain injury: A review and evidence-based protocol. Clin. Neurol. Neurosurg. 123, 109–116 (2014).
Ekeh, A. P., Dominguez, K. M., Markert, R. J. & McCarthy, M. C. Incidence and risk factors for deep venous thrombosis after moderate and severe brain injury. J. Trauma. 68, 912–915 (2010).
Cole, K. L. et al. Factors associated with venous thromboembolism development in patients with traumatic brain injury. Neurocrit Care. 40, 568–576 (2024).
Obi, A. T. et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 150, 941–948 (2015).
Kearon, C. et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians Evidence-Based clinical practice guidelines. Chest 141, e419S–e496S (2012).
greenfield, L. J. et al. Posttrauma Thromboembolism Prophylaxis January 42, 100–103 (1997).
Rogers, F. B. et al. Determining venous thromboembolic risk assessment for patients with trauma: The trauma embolic scoring system. J. Trauma. Acute Care Surg. 73, 511–515 (2012).
Caprini, J. A., Arcelus, J. I., Hasty, J. H., Tamhane, A. C. & Fabrega, F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb. Hemost. 17 (Suppl 3), 304–312 (1991).
Li, S., Wang, L. & Lu, Q. Comparison of the predictive power of the 2005 and 2010 Caprini risk assessment models for deep vein thrombosis in Chinese orthopedic patients at admission: A prospective cohort study. Thromb. Res. 222, 1–6 (2023).
Hazeltine, M. D., Guber, R. D., Buettner, H. & Dorfman, J. D. Venous thromboembolism risk stratification in trauma using the Caprini risk assessment model. Thromb. Res. 208, 52–57 (2021).
Zhou, H. et al. Assessment of the risk of venous thromboembolism in medical inpatients using the Padua prediction score and Caprini risk assessment model. J. Atheroscler Thromb. 25, 1091–1104 (2018).
Sterbling, H. M. et al. Caprini risk model decreases venous thromboembolism rates in thoracic surgery Cancer patients. Ann. Thorac. Surg. 105, 879–885 (2018).
Zhang, X. Q., He, D., Li, J. J. & Huang, X. B. Validity of Caprini risk assessment scale for assessing risk of venous thromboembolism in hospitalized critically ill patients. Sichuan Da Xue Xue Bao Yi Xue Ban. 46, 732–735 (2015).
Ley, E. J. et al. Updated guidelines to reduce venous thromboembolism in trauma patients: A Western trauma association critical decisions algorithm. J. Trauma. Acute Care Surg. 89, 971–981 (2020).
Gearhart, M. M. et al. The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery 128, 631–640 (2000).
Hegsted, D., Gritsiouk, Y., Schlesinger, P., Gardiner, S. & Gubler, K. D. Utility of the risk assessment profile for risk stratification of venous thrombotic events for trauma patients. Am J Surg 205, 517–520; discussion 520 (2013).
Ho, K. M., Rao, S., Rittenhouse, K. J. & Rogers, F. B. Use of the trauma embolic scoring system (TESS) to predict symptomatic deep vein thrombosis and fatal and non-fatal pulmonary embolism in severely injured patients. Anaesth. Intensive Care. 42, 709–714 (2014).
Walker, P. F. et al. Trauma embolic scoring system in military trauma: A sensitive predictor of venous thromboembolism. Trauma. Surg. Acute Care Open. 4, e000367 (2019).
Caprini, J. A. Thrombosis risk assessment as a guide to quality patient care. Dis. Mon. 51, 70–78 (2005).
Yorkgitis, B. K. et al. American association for the surgery of trauma/american college of Surgeons-Committee on trauma clinical protocol for inpatient venous thromboembolism prophylaxis after trauma. J. Trauma. Acute Care Surg. 92, 597–604 (2022).
Park, S. H., Goo, J. M. & Jo, C. H. Receiver operating characteristic (ROC) curve: Practical review for radiologists. Korean J. Radiol. 5, 11–18 (2004).
Knudson, M. M., Ikossi, D. G., Khaw, L., Morabito, D. & Speetzen, L. S. Thromboembolism after trauma: An analysis of 1602 episodes from the American college of surgeons National trauma data bank. Ann. Surg. 240, 490–496 (2004). discussion 496–498.
Hachem, L. D., Mansouri, A., Scales, D. C., Geerts, W. & Pirouzmand, F. Anticoagulant prophylaxis against venous thromboembolism following severe traumatic brain injury: A prospective observational study and systematic review of the literature. Clin. Neurol. Neurosurg. 175, 68–73 (2018).
Heim, C., Bruder, N., Davenport, R., Duranteau, J. & Gaarder, C. European guidelines on peri-operative venous thromboembolism prophylaxis: First update.: chap. 11: trauma. Eur. J. Anaesthesiol. 41, 612–617 (2024).
Dhillon, N. K. et al. Characterizing the delays in adequate thromboprophylaxis after TBI. Trauma. Surg. Acute Care Open. 6, e000686 (2021).
Coleman, J. R. et al. A stitch in time saves clots: Venous thromboembolism chemoprophylaxis in traumatic brain injury. J. Surg. Res. 258, 289–298 (2021).
Eck, R. J. et al. Low dose Low-Molecular-Weight heparin for thrombosis prophylaxis: Systematic review with Meta-Analysis and trial sequential analysis. J. Clin. Med. 8 (2019).
Ratnasekera, A. et al. Implementation science approaches to optimizing venous thromboembolism prevention in patients with traumatic injuries: Findings from the 2022 consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J. Trauma. Acute Care Surg. 94, 490–494 (2023).
Schellenberg, M. et al. Timing of venous thromboembolism prophylaxis initiation after injury: Findings from the consensus conference to implement optimal VTE prophylaxis in trauma. J Trauma Acute Care Surg 94, 484–489 (2023).
Wu, Y. T. et al. Early venous thromboembolism prophylaxis in patients with trauma intracranial hemorrhage: Analysis of the prospective multicenter consortium of leaders in traumatic thromboembolism study. J. Trauma. Acute Care Surg. 95, 649–656 (2023).
Carney, N. et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 (2017).
Nyquist, P. et al. Prophylaxis of venous thrombosis in neurocritical care patients: An Evidence-Based guideline: A statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 24, 47–60 (2016).
Jakob, D. A. et al. Risk factors for thromboembolic complications in isolated severe head injury. Eur. J. Trauma. Emerg. Surg. 50, 185–195 (2024).
Cook, N. R. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 115, 928–935 (2007).
Van Calster, B. et al. Reporting and interpreting decision curve analysis: A guide for investigators. Eur. Urol. 74, 796–804 (2018).
Huang, Y., Li, W., Macheret, F. & Gabriel, R. A. Ohno-Machado, L. A tutorial on calibration measurements and calibration models for clinical prediction models. J. Am. Med. Inf. Assoc. 27, 621–633 (2020).
Zander, A. L. et al. Venous thromboembolic risk assessment models should not solely guide prophylaxis and surveillance in trauma patients. J. Trauma. Acute Care Surg. 79, 194–198 (2015).
Maegele, M. Coagulopathy and progression of intracranial hemorrhage in traumatic brain injury: Mechanisms, impact, and therapeutic considerations. Neurosurgery 89, 954–966 (2021).
Ibrahim, N. A., Hassan, F. M., Elgari, M. M. & Abdalla, S. E. Risk factors for deep vein thrombosis of lower extremities in Sudanese women. Vasc Health Risk Manag. 14, 157–164 (2018).
A, D. L. et al. Assessing the use ofvenous thromboembolism risk assessment profiles in the trauma population: Is it necessary?? Am. Surg. 77, 783–789 (2011).
Goel, R. et al. Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg. 153, 826–833 (2018).
Khaldi, A., Helo, N., Schneck, M. J. & Origitano, T. C. Venous thromboembolism: Deep venous thrombosis and pulmonary embolism in a neurosurgical population. J. Neurosurg. 114, 40–46 (2011).
Lobastov, K. et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J. Vasc Surg. Venous Lymphat Disord. 4, 153–160 (2016).
Fang, X. et al. Predictive value of Caprini risk assessment model, D-dimer, and fibrinogen levels on lower extremity deep vein thrombosis in patients with spontaneous intracerebral hemorrhage. Front. Neurol. 15, 1370029 (2024).
He, L. et al. Predicting venous thromboembolism in hospitalized trauma patients: A combination of the Caprini score and data-driven machine learning model. BMC Emerg. Med. 21, 60 (2021).
Lyons, M. D., Pope, B. & Alexander, J. Perioperative management of antithrombotic therapy. Jama 332, 420–421 (2024).
Dowling, N. F. et al. The epidemiology of venous thromboembolism in Caucasians and African-Americans: The GATE study. J. Thromb. Haemost. 1, 80–87 (2003).
Lu, Y. et al. Factor V gene G1691A mutation, prothrombin gene G20210A mutation, and MTHFR gene C677T mutation are not risk factors for pulmonary thromboembolism in Chinese population. Thromb. Res. 106, 7–12 (2002).
Jun, Z. J. et al. Prevalence of factor V Leiden and prothrombin G20210A mutations in Chinese patients with deep venous thrombosis and pulmonary embolism. Clin. Lab. Haematol. 28, 111–116 (2006).
Funding
This research is supported by The Science and Technology Department of Sichuan Province (Project No. 2023YFS0238).
Author information
Authors and Affiliations
Contributions
D.Z. and L.H. wrote the main manuscript text. Y.W. and C.O. prepared figures and tables. D.Z. and D.L. designed the studv. Q.N. and C.O. collected and analysed the data.D, Z wrote the manuscript, D.L. critically revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Biomedical Research Ethics Committee, West China Hospital of Sichuan University (IRB number: 2023 − 1888). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. The Biomedical Research Ethics Committee, West China Hospital of Sichuan University waived the need for informed consent due to retrospective nature of the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, D., He, L., Ouyang, C. et al. A comparative analysis of three risk assessment scales for predicting venous thromboembolism in traumatic brain injury patients. Sci Rep 15, 11623 (2025). https://doi.org/10.1038/s41598-025-91290-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91290-8