Abstract
The effects of alloying elements (Al and Zn) on the second-phase transformation and deformation mechanisms were revealed using the microstructure characteristics, and the mechanical behaviour of Mg–4Y–(4 − x)Al–xZn was systematically investigated. The results showed that the Al‒Y phase (Al2Y and Al11Y3) was formed in the Mg‒4Y‒4Al alloy. When both Al and Zn were contained, the AlZnY phase was produced, and correspondingly, as the Al–Y phase decreases. In addition, as the Zn content exceeded 2 wt%, the W phase was formed in the alloy system. In the alloy under investigation, the axial ratio (c/a) of α-Mg was lower than that of pure Mg. These results indicated a reduction in the disparity between the slip systems along the a-axis and c-axis directions. The yield strength and ultimate tensile strength initially decreased and then increased as the Zn content increased. Additionally, the elongation decreased with the incorporation of Zn, and the Mg–4Y–4Al formulation exhibited the highest ductility at 11.6%. The primary mechanism contributing to the strengthening of Mg–4Y–(4 − x)Al–xZn was the influence of second-phase strengthening and solid-solution strengthening.
Similar content being viewed by others
Introduction
Owing to the lightweight structure of Mg, it has emerged in the industrial field and caused groundbreaking changes in various fields. Nonetheless, its utility is often hindered by its low ductility and limited workability at room temperature1. The inadequate performance of Mg can be attributed to its hexagonal close-packed (HCP) crystal structure, which features only two slip systems that are available for activation under ambient conditions. In contrast, the von Mises criterion indicates that effective uniform deformation needs five independent slip systems, which is a characteristic typically associated with body-centred cubic (BCC) and face-centred cubic (FCC) structures2. Consequently, researchers have focused on enhancing the mechanical properties of Mg alloys by reducing the critical shear stress needed to activate these slip systems. One significant strategy to achieve this enhancement is through the reduction of the axial ratio via of alloying3,4.
The incorporation of rare earth (RE) elements such as Y, Nd, and Gd into Mg alloys has been demonstrated to significantly enhance their properties because of the high solid solubility of these elements and their ability to form a strengthening second phase5,6,7. In particular, Y, which is a crucial rare earth element, has a pronounced solid solution strengthening effect caused by its substantial difference in atomic radius with respect to that of Mg8. Furthermore, the mechanical performance of Mg-Y alloys can be further increased by the reasonable addition of Al and Zn. Previous studies have shown that even a small inclusion of Al in Mg–10Y9 and Mg–4Y10 alloys facilitates the formation of phases such as Al2Y and Al11Y3 while also refining the grain structure, which subsequently enhances the mechanical properties. Additionally, the Al‒Y phase effectively pins dislocations during the alloy deformation process, thereby impeding the movement of the grain boundaries10,11. The introduction of Zn into Mg–Y-based alloys generates numerous ternary phases, including the I-phase (Mg3Zn6Y), X-phase (Mg12YZn), W-phase (Mg3Y2Zn3), and Z-phase (Mg28Y7Zn65)12. These phases demonstrate significant strengthening or synergistic reinforcement effects through their combined action12,13,14,15. For example, the Mg97Y2Zn1 alloy, produced via rapid solidified powder metallurgy, results in the formation of the X phase with a nanoscale long-period stacking ordered (LPSO) structure; this process inhibits twin growth during the deformation of the alloy. Consequently, the Mg97Y2Zn1 alloy exhibits remarkable strength and ductility, with Elongation(EL)and yield strength (YS) values of 5% and 610 MPa, respectively, at ambient temperature.
In recent years, there has been increasing interest among researchers in the simultaneous incorporation of Al and Zn into Mg–Y-based alloys. Studies have revealed that the Mg‒Y‒Al‒Zn alloy systems feature ternary and Al‒Y phases analogous to those found in the Mg‒Y‒Zn and Mg‒Y‒Al alloy systems, respectively. For example, the Mg–4Zn–0.8Y–0.2Al alloy contains both Al2Y and I-phase16, whereas the Mg–2Zn–4.5Y–3Al alloy incorporates Al2Y, W-phase, and X-phase17; additionally, all these alloys exhibit commendable mechanical properties. However, the emergence of certain quaternary phases in the Mg‒Y‒Al‒Zn alloy systems has been documented in recent studies. Zeng et al. noted that the addition of 1.5 wt% RE to a ZA84 alloy resulted in the precipitation of the Mg3Al4Zn2RE phase within the β-Mg matrix; thus, the formation of this phase was attributed to the dissolution of RE within the Al2Mg5Zn2 phase18. Tan et al.19 identified an additional quaternary phase, Mg4Y2ZnAl3, within the Mg93.8Y3.76Zn2Al0.5 alloy. This phase has also been reported by other researchers, including those examining the Mg97Zn1Y2Al alloy20, the MgY1.65Zn0.74Al0.53 alloy21, and the MgY5.5Zn2.3Al0.9 alloy22. In summary, the phase composition within the Mg–Y–Al–Zn system remains unclear and represents a scientific challenge that warrants further investigation. Consequently, conducting a systematic study of the microstructure of this alloy system and exploring its strengthening mechanisms in greater depth have attracted increased research interest.
In this study, we systematically investigated the evolution of the second phase, elemental solution, and mechanical properties of the Mg–Y–Al–Zn system in relation to varying Al and Zn contents. Alloys were designed with the composition Mg–4Y–(4 − x)Al–xZn, where x was set to 1, 2, 3 and 4 wt%. Additionally, we examined the strengthening mechanisms underlying these alloys. The findings of this research provide a novel strategy for the composition design and performance optimization of Mg–Y–Al–Zn alloys.
Experiment process and method
Mg–4Y–(4 − x)Al–xZn alloys (where x = 0, 1, 2, 3 and 4 wt%) were synthesized utilizing high-purity Mg (99.9%), Al (99.9%), and Zn (99.9%), in addition to Mg–30 wt% Y master alloys. The raw materials were melted in a protective atmosphere composed of SF6 and CO2 at an approximate melting temperature of 740 °C, with the volume ratio of SF6 to CO2 maintained at 1:100. Casting was performed at a temperature of 720 °C, and the molten alloy was poured into a preheated iron mould at 200 °C. The resulting ingots measured 25 mm by 100 mm by 150 mm. The Mg–4Y–(4 − x)Al–xZn alloys were subsequently designated WA44 (x = 0 wt%), WAZ431 (x = 1 wt%), WAZ422 (x = 2 wt%), WAZ413 (x = 3 wt%) and WZ44 (x = 4 wt%), and the designation reflected increasing Zn content.
The morphology and composition of the secondary phases were investigated using scanning electron microscopy (SEM) (FEl Helios 5 CX) equipped with energy-dispersive X-ray spectrometry (EDS). To identify the phase types, X-ray diffraction (XRD) analysis was conducted using a Rigaku X-2000 diffractometer with Cu Kα radiation at 40 kV and 150 mA. The XRD data were subsequently analysed via Rietveld software. The microstructure was characterized using a JEOL JEM-2100 F field-emission transmission electron microscope (TEM). The specimen was mechanically polished to approximately 40 μm and subsequently thinned via low-angle, low-energy ion beam milling on a cold stage using a Gatan 691 Precision Ion-Polishing System. Phase transformations within the alloy systems were predicted and analysed using JMatPro 7.0 software. The thermodynamic properties of the alloys were characterized using differential scanning calorimetry (DSC) on a DSC 214 Polyma system, in which a sample weighing 10 mg was subjected to a temperature gradient ranging from 25 °C to 700 °C under a continuous flow of argon, with a heating rate of 10 °C/min.
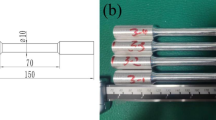
The tensile properties were evaluated using a CMT5105 mechanical testing machine, and the tests were performed at a crosshead speed of 0.45 mm/min. At least 3 samples of each component were tested to ensure the reproducibility of the data. As shown in Fig. 1, the tensile sample had a dog-bone shape, and the dimensions of the dog-bone-shaped sample were as follows: thickness of 2 mm, width of 3.5 mm, and length of 14 mm. The sample follows the ASTM E8/E8M-24 standard.
Results and discussion
Analysis of the phase composition and crystal structure
Figure 2 presents the X-ray diffraction (XRD) patterns obtained from the as-cast alloy samples, and a comprehensive analysis of the Rietveld refinement pertaining to the α-Mg matrix is provided in Table 1. The XRD patterns of these as-cast alloys are shown in Fig. 2, and the Rietveld refinement results for -Mg can be found in Table 1. Notably, the WA44 alloy is composed of α-Mg (ICDD PDF#35–0821), Al2Y (ICDD PDF#29–0103), and Al11Y3 (ICDD PDF#48-1841). When Al was replaced with Zn, the type of phase in the alloy changed. The WZ44 alloy contains 4 wt% Zn and no Al, and only α‒Mg and W‒phases (ICDD PDF#36-1275) are present in the alloy. However, when the alloys contain both Al and Zn, such as WAZ431, WAZ422 and WAZ413, a new phase appears and corresponds to 33.7°, 38.1° and 72.5°, respectively, in the XRD patterns. The diffraction pattern of the new phase clearly does not match any phase of Mg–RE–Al–Zn that has been reported in the literature. WAZ431 and WAZ422 alloys are composed of Al2Y, Al11Y3, a new phase and α-Mg. As the Al content decreases to 1 wt%, the Al2Y phase disappears. Consequently, WAZ413 was composed solely of the α-Mg phase, Al11Y3, and a newly identified phase.
The lattice parameters of pure Mg and the α‒Mg phase of the alloy system are listed in Table 1. The results indicate that both the cell volume and the lattice parameters (a and c) of the α-Mg in the Mg–4Y–(4 − x)Al–xZn alloy are smaller than those of pure Mg. This observation indicates lattice distortion of Mg within the alloy system. The atomic radii of Mg, Y, Al and Zn were previously established in Ref17, and the following result were attained: rAl=0.143 nm, rY = 0.178 nm, rZn = 0.139 nm, and rMg = 0.160 nm. Consequently, determining the atomic radius ratios for Y to Mg, Mg to Al, and Mg to Zn is straightforward, with values of 1.11, 1.12 and 1.15, respectively. According to the Hume‒Rothery rules, a substitutional solid solution is likely to form without additional vacancies when the atomic ratio of solute to solvent falls within the range of 1 to 1.1523. This mechanism can be used to explain the decrease in lattice parameter of the solid solution when the atomic radius of the alloying element is smaller than that of the α‒Mg matrix; conversely, the lattice parameter increases when the atomic radius is larger18. This behaviour indicates that both Al and Zn can dissolve into the Mg matrix under nonequilibrium solidification conditions, resulting in lattice distortion of Mg. As indicated in Table 1, the axial ratio (c/a) of α-Mg in the alloy under investigation is lower than that of pure Mg; these results indicate a reduction in the difference between the slip systems functioning along the c-axis versus those functioning along the a-axis. This leads to a reduction in the I1 stacking fault energy and cross-slip energy barrier, promoting dislocation nucleation and cross-slip, thereby activating non-basal slip24,25. Additionally, by reducing the critical resolved shear stress (CRSS) difference between basal and non-basal slip systems, the activation of non-basal slip is further facilitated26. Therefore, the incorporation of Al and Zn into the Mg matrix contributes to an improvement in the ductility of the alloy20. Additionally, monitoring changes in the c/a ratio serves as a valuable approach for assessing the lattice distortion of α-Mg.
Analysis of the microstructure evolution and solidification behaviour
The distribution of secondary phases and the microstructure of the alloys under investigation were analysed using SEM. As illustrated in Fig. 3, the WA44 alloy consists of an α-Mg matrix, with a blocky phase that displays considerable contrast and an acicular-shaped phase. However, the WAZ431 alloy not only contains three phases, similar to WA44 but also produces a new phase that has an irregular blocky shape and weaker contrast. For the WAZ422 and WAZ413 alloys, the blocky shape phase, which has a strong contrast, disappears, and white eutectic networks appear. Furthermore, the WZ44 alloy has a Zn content of 4 wt% and exclusively consists of a white eutectic network phase. Here, the change in the Al and Zn contents causes a change in the phase constitution of the alloy system, and these results are in general agreement with the XRD results.
Figures 3 and 4 show the results from the EDS analysis conducted on distinct phases within the samples. The findings presented in Fig. 4a,b indicate that the blocky-shaped phase has a pronounced contrast, whereas the acicular-shaped phase is composed of Y, Al, Zn and Mg. The atomic ratios of Al to Y are approximately 2:1 for the blocky-shaped phase and 11:3 for the acicular-shaped phase. These phases are likely associated with the Al2Y and Al11Y3 phases identified via X-ray diffraction (XRD) analysis. Additionally, the peaks attributed to Mg and Zn observed in the energy dispersive spectroscopy (EDS) analysis could arise from the surrounding matrix or measurement errors during the test. As shown in Fig. 4c, more Zn atoms are found in the new phase than in Al2Y and Al11Y3, and the proposed stoichiometric formula Al31.6Y27.17Zn33.03 is close to that of AlZnY, which corresponds to the new phase in the XRD results. The elemental compositions of the white eutectic network phase are identified using the EDS spectra in Fig. 4d. The atomic ratio of Y to Zn in the alloy is approximately 2:3, which aligns with the characteristics of the W-phase. Additionally, the Al peaks detected in the EDS analysis were potentially caused by experimental error. Furthermore, the content of the Mg atoms in the EDS results is high and likely caused by the numerical deviation from the penetration of the electron beam; here, the beam penetrates the phase and interacts with the matrix. This is a normal phenomenon of the experiment and does not affect the results of the experiment27.
Figure 5 shows the TEM image along with the associated selected area electron diffraction (SAED) pattern obtained from WZ413. The needle-like phase (Fig. 5a,d) was confirmed to be the Al11Y3 phase with an orthorhombic structure (space group Immm, a = 4.431, b = 10.132, c = 13.142). The network eutectic phase, as illustrated in Fig. 5b and e, was confirmed to be the W-phase; this phase was characterized by a cubic structure belonging to the space group Fm\(\:\stackrel{-}{3}\)m, with lattice parameters a = b = c = 6.848. These results were also confirmed by the XRD patterns and EDS results, as shown in Figs. 2 and 4. Among the previously reported Al–Y–Zn phases, only two are present with compositions similar to Al31.6Y27.17Zn33.03, namely, the AlZnY phase28 and the Al3Y4Zn3 phase29. However, the Al3Y4Zn3 phase has a long-term stacking ordered structure. However, the SAED pattern of the Al31.6Y27.17Zn33.03 phase in the WAZ413 alloy, as shown in Fig. 5c,f, does not exhibit the characteristics of a long-period phase but is consistent with the crystal characteristics of the AlZnY phase. Furthermore, an atomic structural model of the AlZnY phase has been developed and reveals that the phase possesses a hexagonal structure characterized by lattice constants of a = b = 8.9443 Å and c = 7.2111 Å, with angles of α = β = 90° and γ = 120°. The space group associated with this structure is P63/mmc. The specific analysis was described in our previous work30. Therefore, a new phase is produced in WAZ413 and is the AlZnY phase.
JMatPro 7.0 software was used to simulate the behaviour of the alloys during equilibrium solidification, as depicted in Fig. 6. Additionally, Fig. 7 shows the solubilities of various elements within the α-Mg matrix. Table 2 lists the solubilities of the elements in the matrix calculated at 300 °C and 550 °C. In the equilibrium solidification process, when the alloy does not contain Zn, e.g., WA44. Al2Y precipitated from the liquid phase above 700 °C, and solidification of the Mg matrix started at 630 °C and ended at 618 °C. As the temperature decreased, the Al2Y phase abundance started to decrease, accompanied by Al3Y precipitating from the Mg matrix at approximately 490 °C and Mg17Al12 precipitating from the Mg matrix at 38 °C. This result was likely associated with the concentration of the alloying elements during the solidification process of the alloy system. As shown in Fig. 7, after solidification of the alloy, approximately 1.72 wt% of the Al was dissolved in the α-Mg matrix. The solid solubility of Al decreased as the temperature decreased; thus, Al precipitated from the alloy during the cooling process. When the temperature was high, Al reacted with Al2Y to precipitate the Al3Y phase from the matrix; thus, the content of Al2Y decreased. When the Al2Y completely disappeared, the precipitated Al and Mg matrix reacted to precipitate the Mg17Al12 phase at lower temperatures. For the WAZ431 alloy, Al2Y precipitated from the liquid phase. As the temperature decreased, Al, with its decreased solid solubility, precipitated from the Mg matrix at a temperature of 408 °C and reacted with Al2Y to form Al3Y; this led to a reduction in the abundance of the Al2Y phase during the cooling process. When the temperature was reduced to 369 °C, Zn began to precipitate from the Mg matrix because of the decrease in solid solubility, and the I-phase correspondingly precipitated from the matrix. This occurred because Al2Y was generated at high temperatures and consumed a large amount of Y, resulting in a relatively large Zn/Y ratio in the Mg matrix and thus generating the I-phase. Nonetheless, with the formation of the I- phase, the ratio of Zn/Y gradually decreased, and the W-phase was subsequently formed at 277 °C. Focusing on the WAZ422 alloy, when the Al content was reduced to 2 wt%, the Al2Y phase crystallized out of the liquid phase, and the Y consumed in this process was correspondingly reduced because of the reduction in the Al content. The ratio of Zn to Y in the residual liquid phase was sufficiently high to induce the precipitation of a portion of the W-phase from the liquid. Following this, the solidification of the alloy was completed. Moreover, the solid solubility decreased with decreasing temperature such that the solute elements were precipitated from the Mg matrix. The precipitated Zn and Y reacted with the Mg matrix, leading to the formation of the W phase. Additionally, the precipitated Al reacted with Al2Y at a temperature of 263 °C, resulting in the production of Al3Y. In the case of the WAZ413 alloy, an increase in the Zn concentration to 3 wt% was accompanied by a decrease in the Al content to 1 wt%. During this process, the Al2Y phase precipitated from the liquid phase, with a portion of the W phase, which formed because of the elevated Zn/Y ratio present in the liquid phase. As the temperature gradually decreased, the alloy solidified. Subsequently, Zn and Y precipitated from the matrix, leading to the continuous formation of the W-phase from the Mg matrix. Throughout this solidification process, the Zn/Y ratio gradually decreased. Eventually, some of the W-phase was reincorporated back into the Mg matrix, whereas the X-phase concurrently crystallized from the matrix at 378 °C. When the Zn content continued to increase to 4 wt%, the alloy contained no Al. Most W phases were directly formed from the melt, but some W phases precipitated from the Mg matrix because Zn and Y precipitated from the matrix as the temperature decreased. When the Zn/Y ratio decreases, the W-phase cannot be generated, a portion of the W-phase starts to dissolve within the Mg matrix, and the X-phase precipitates from the matrix at 451 °C. The results in Table 1 indicate that Al and Zn dissolve in the Mg matrix under nonequilibrium solidification conditions in the Mg–4Y–(4 − x)Al–xZn alloys. In conjunction with the findings provided in Table 2, when the Zn content is less than 2 wt%, Y fails to become incorporated into the Mg matrix. Conversely, as the Zn content increases beyond 3 wt%, Y becomes the predominant solute within the alloy matrix. This observation appears to contradict the data outlined in Table 1; however, the findings in Table 1 were acquired under conditions of equilibrium solidification, whereas those in Table 2 were derived from nonequilibrium solidification conditions.
The results obtained from the JMatPro analysis are predicated using equilibrium solidification and indicate a reduction in the simulated cooling rate. Compared with the actual solidification scenarios, this scenario results in a more extensive diffusion of solutes, leading to discrepancies between the simulated and real solidification processes. In the alloy system, when the Zn content is less than 2 wt% and the Al content exceeds 2 wt%, actively nucleated Al2Y particles are generated in the melt because of the elevated concentration of Al in the liquid phase. Consequently, the following peritectic reaction is observed: L + Al2Y→α-Mg (ss)31. During this process, the Al concentration in the residual liquid phase gradually increases. This leads to a pseudo-binary eutectic reaction, L→α-Mg + Al2Y32, and another type of Al2Y at the boundary is formed. The majority of Y is consumed above the process, and then a part of the Al2Y and liquid phase undergoes another peritectic reaction: 3Al2Y + L→Al11Y3 33. This process mainly consumes Al in the remaining liquid phase. As the alloy solidifies, the volume of the residual liquid phase decreases, leading to an increase in the Zn/Y ratio. This change potentially facilitates the formation of a new phase, due to the interaction of Al2Y with the concentrated Zn and Y elements present in the residual liquid phase. Moreover, as the Zn content increased, the blocky-shape phase in the WAZ422 and WAZ413 alloys disappeared, and granular Al2Y and Al11Y3 formed in the WA44 alloy and WAZ431 alloy. Figure 8 shows the DSC curves of the WAZ413 and WZ44 alloys. In the temperature range of 510–530 °C, an endothermic peak was observed in the DSC curves for both the WAZ413 and WZ44 alloys. The initiation temperatures were recorded at 518 °C and 522 °C. This phenomenon corresponded to the phase transition temperature of the W phase. Therefore, the W-phase directly precipitated in the WAZ413 alloy through a eutectic reaction at 518 °C: L→α-Mg + W34. Notably, the new phase in the WAZ413 alloy appeared to cut off the W phase; these results indicated that the new phase could be generated at 510 °C and was as the W phase, and the reaction was L + Al2Y→New phase. In the WZ44 alloy, the W-phase was formed by a eutectic reaction as the only second phase. However, the temperature reached 522 °C; this value exceeded that of the WAZ413 alloy because of an increase in the Zn content.
Room-temperature mechanical properties
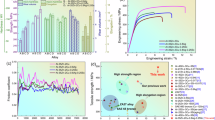
Figure 9 shows a functional diagram of the Zn content and stress. The mechanical behaviour is strongly influenced by the presence of Zn and Al. With increasing Zn content, the YS and UTS initially decrease and then increase. The Mg–4Y–4Zn alloy has optimal UTS and YS values of 232 and 122 MPa, respectively. EL decreases with the addition of Zn. The ductility of the WZ44 alloy is the lowest at 9.7%, and the ductility of WA44 is the highest at 11.6% of all samples. In addition, the ELs of the WAZ431, WAZ422 and WAZ413 alloys are similar. These results indicate that the EL of the alloy system is marginally influenced as the Zn content increases from 1 wt% to 3 wt%, whereas the Al content simultaneously decreases from 3 wt% to 1 wt%. This phenomenon is likely attributed to variations in the concentrations of the alloying elements, which subsequently initiate alterations in both the types and amounts of the secondary phases present within the alloy system.
As shown in Table 1, the solid solution elements Al and Zn cause α‒Mg lattice distortion and a smaller axial ratio (c/a) than pure Mg in the Mg–4Y–(4 − x)Al–xZn alloy system. Therefore, the slip system along the prismatic cone direction is easily activated, and the anisotropy of the deformation diminishes. Consequently, the EL of the alloy system at room temperature correspondingly increases. However, the pattern of change in the alloy EL is not consistent with the pattern of change in the c/a values; these results indicate that solid solution strengthening is not the main exaggeration factor in the studied alloys. Figure 3 shows the microstructure characteristics of the samples. The incorporation of Zn clearly results in a reduction in the Al-rare earth (Al-RE) phase. Furthermore, the increase in the Zn concentration stimulates the formation of the AlZnY phase. Additionally, the W-phase is also observed to develop, and the amounts of both phases increase in conjunction with increasing Zn content. In the WZ44 alloy, only the W-phase is present. Previous studies have shown that the bulk Al2Y in the gain centre and needle-like Al11Y3 phase can effectively impede the grain boundary migration and pin dislocations under tension. The Al11Y3 phase acts as an effective barrier to the sliding system11, and both the bulk Al2Y and needle-like Al11Y3 phases are beneficial to the mechanical properties. The small Al2Y phase distributed along the gain boundary easily causes stress concentration during deformation, resulting in poor performance10. As noted by Zou et al.31, a significant increase in the size of the Al2Y phase can lead to its intersection with the matrix, consequently facilitating the initiation or propagation of cracks and resulting in a deterioration of mechanical properties. Conversely, the W-phase is prone to cracking during deformation because of the incoherent interface between the cubic W-phase and the α-Mg matrix, which is characterized by weak bonding forces. This situation is not advantageous35,36.
Figure 10 shows the fracture morphologies of the tensile-tested Mg–4Y–(4 − x)Al–xZn alloys. The fracture behaviour greatly changes with changes in the Al and Zn contents. The fracture surfaces of the WA44 alloy display a significant number of plastic dimples and tear ridges. The dimples are characterized by their considerable size and depth, thereby illustrating the hallmark features of ductile fracture. For the WAZ431, WAZ422 and WAZ413 samples, with the addition of Zn and the decrease in the Al content, the fracture of the alloys appears as a destructive surface, and a large amount of fractured W phase is observed. Moreover, dimples can still be observed in the fractures, and the number of dimples initially decreases and then increases with increasing content. Therefore, these alloys experience quasi-dissociative fractures. The fracture surface of the WZ44 alloy exhibited numerous cleavage planes and fragmented eutectic tungsten phases; these results indicate a fracturing mechanism characterized by deconstructive fracture. Thus, the WA44 alloy is dominated by dimple-type transgranular cracking, and WZ44 cracks along the grain boundary; meanwhile, both fracture modes appear in the WAZ431, WAZ422 and WAZ413 alloys. Notably, broken second phases in the dimples were observed in the WA44, WAZ431, WAZ422 and WAZ413 alloys, and evident secondary cracks were observed on the surface of the large second phase in the WA44 and WAZ413 alloys. In general, the strengthening mechanism of the second phase mainly occurs when the slip of dislocations are hindered and microcracks are generated around the second phase. The expansion of the microcracks then absorbs strain energy during the tensile process. The broken second phase confirms that these second phases easily cause stress concentrations; these phases initially break and then act as a microcrack source for secondary crack initiation during tensile deformation. Figure 3 shows that the sizes of the Al2Y phase in the WA44 sample and the AlZnY phase in the WAZ422 and WAZ413 alloys are greater than that of the AlZnY phase in the WAZ431 alloy. Thus, for the aforementioned reasons, as the Zn content in the Mg–4Y–(4 − x)Al–xZn alloys increases, the size of the AlZnY phase progressively increases. This increase facilitates the occurrence of stress concentrations during tensile deformation and ultimately reduces the toughness of the alloy. In the case of the WZ44 alloy, the fractured W-phase is observed on the surface; these results indicate that the fracture initiates at the interface between the W-phase and the matrix. This phenomenon is attributed to the limited symmetry of the W-phase and its weak bonding strength with the matrix.
Conclusions
-
(1)
In the Mg–4Y–(4 − x)Al–xZn alloy, the phase composition varied with the contents of Al and Zn. When the Al content was 4%, the microstructures were mainly α‒Mg and the Al‒Y phases (Al2Y and Al11Y3). When both Al and Zn were present, and the AlZnY phase was produced; additionally, the Al‒Y phase decreased with decreasing Al content and increasing Zn content, whereas the W phase started to be produced as the Zn content increased to 2%. As the Zn content increased to 4%, the alloy contained only the W phase.
-
(2)
The UTS and YS of the alloys initially decreased and then increased with increasing Zn content. The Mg–4Y–4Zn alloy had the best UTS and YS at 232 MPa and 122 MPa, respectively. Moreover, EL decreased with the addition of Zn, and Mg–4Y–4Al had the highest ductility of 11.6%.
-
(3)
The Al2Y and AlZnY phases could improve the mechanical properties by hindering the slip during plastic deformation. However, when these phases grew in size, they could cause stress concentrations during tensile deformation, fracture first and act as microcrack sources to initiate secondary cracking; thus, due to these factors, the EL of the alloy decreased. The incoherent interface between the W phase and the α‒Mg matrix was the cause of fracture, which reduced the EL.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to technical or time limitations but are available from the corresponding author on reasonable request.
References
Wu, Z., Ahmad, R., Yin, B., Sandlöbes, S. & Curtin, W. A. Mechanistic origin and prediction of enhanced ductility in magnesium alloys. Sci 359, 447–452. https://doi.org/10.1126/science.aap8716 (2018).
Yin, B., Wu, Z. & Curtin, W. A. First-principles calculations of stacking fault energies in Mg–Y, Mg–Al and Mg–Zn alloys and implications for〈c + a〉activity. Acta Mater. 136, 249–261. https://doi.org/10.1016/j.actamat.2017.06.062 (2017).
Holm, E. A. & Mangal, A. Applied machine learning to predict stress hotspots II: Hexagonal close packed materials. Int. J. Plast. 114, 1–14. https://doi.org/10.1016/j.ijplas.2018.08.003 (2019).
Ahmad, R., Wu, Z., Groh, S. & Curtin, W. A. Pyramidal II to basal transformation of〈c + a〉edge dislocations in Mg–Y alloys. Scr. Mater. 155, 114–118. https://doi.org/10.1016/j.scriptamat.2018.06.026 (2018).
Stanford, N. Micro-alloying Mg with Y, Ce, Gd and La for texture modification: A comparative study. Mater. Sci. Eng. A 527 (10–11), 2669–2677. https://doi.org/10.1016/j.msea.2009.12.036 (2010).
Liao, Q., Hu, W., Chen, R. & Le, Q. Effect of Zn/Y atomic ratio on precipitation behavior and dynamic recrystallization behavior of Mg–Zn–Y alloy under different extrusion temperature. J. Mater. Res. Technol. 27, 48–62. https://doi.org/10.1016/j.jmrt.2023.09.228 (2023).
Chen, X. et al. Tensile creep behavior and microstructure evolution of Mg–11Gd–2Y–0.5Zr alloy. Vacuum 167, 421–427. https://doi.org/10.1016/j.vacuum.2019.06.040 (2019).
Sudholz, A. D. et al. Electrochemical behaviour and corrosion of Mg–Y alloys. Corros. Sci. 53 (6), 2277–2282. https://doi.org/10.1016/j.corsci.2011.03.010 (2011).
Qiu, D., Zhang, M. X., Taylor, J. A. & Kelly, P. M. A new approach to designing a grain refiner for Mg casting alloys and its use in Mg–Y-based alloys. Acta Mater. 57 (10), 3052–3059. https://doi.org/10.1016/j.actamat.2009.03.011 (2009).
Xie, H. et al. Effect of aluminum addition on lattice parameter, formation of intermetallic compounds and mechanical properties of Mg–4Y alloys. Mater. Sci. Eng. A 766, 138359. https://doi.org/10.1016/j.msea.2019.138359 (2019).
Nami, B., Razavi, H., Miresmaeili, S. M., Mirdamadi, S. & Shabestari, S. G. Impression creep properties of a semi-solid processed magnesium–aluminum alloy containing calcium and rare Earth elements. Scr. Mater. 65 (3), 221–224. https://doi.org/10.1016/j.scriptamat.2011.04.011 (2011).
Jiang, W., Zou, C., Huang, H. T., Ran, Z. & Wei, Z. Crystal structure and mechanical properties of a new ternary phase in Mg–Zn–Y alloy solidified under high pressure. J. All Comp. 717, 214–218. https://doi.org/10.1016/j.jallcom.2017.05.088 (2017).
Shao, X. H., Yang, Z. Q. & Ma, X. L. Strengthening and toughening mechanisms in Mg–Zn–Y alloy with a long period stacking ordered structure. Acta Mater. 58 (14), 4760–4771. https://doi.org/10.1016/j.actamat.2010.05.012 (2010).
Zhu, J. et al. High strength Mg-Zn-Y alloys reinforced synergistically by Mg12ZnY phase and Mg3Zn3Y2 particle. J. All Comp. 703, 508–516. https://doi.org/10.1016/j.jallcom.2017.02.012 (2017).
Cao, F. R., Liu, R. J., Kong, S. T. & Xu, G. M. Microstructural evolution, high temperature tensile deformation behavior, and deformation mechanism in an Mg–Zn–Y–Ca–Zr alloy processed by multidirectional forging and hot rolling. J. Mater. Res. Technol. 27, 6729–6743. https://doi.org/10.1016/j.jmrt.2023.11.145 (2023).
Kim, D. H., Lee, J. Y., Lim, H. K., Kim, W. T. & Kim, D. H. Effect of al addition on the elevated temperature deformation behavior of Mg–Zn–Y alloy. Mater. Sci. Eng. A 487 (1–2), 481–487. https://doi.org/10.1016/j.msea.2007.10.031 (2008).
Zhang, G., Wang, Y., Liu, Z. & Liu, S. Influence of al addition on solidification path and hot tearing susceptibility of Mg–2Zn–(3 + 0.5x)Y–xAl alloys. J. Mg Alloys. 7 (2), 272–282. https://doi.org/10.1016/j.jma.2019.04.001 (2019).
Wang, Y., Guan, S., Zeng, X. & Ding, W. Effects of RE on the microstructure and mechanical properties of Mg–8Zn–4Al magnesium alloy. Mater. Sci. Eng. A 416 (1–2), 109–118. https://doi.org/10.1016/j.msea.2005.09.104 (2006).
Tan, X., Chee, K. H. W., Chan, K. W. J., Richard Kwok, W. O. & Gupta, M. Effect of homogenization on enhancing the failure strain of high strength quaternary LPSO Mg–Y–Zn–Al alloy. Mater. Sci. Eng. A. 644, 405–412. https://doi.org/10.1016/j.msea.2015.07.079 (2015).
Wang, D. et al. Microstructure and corrosion behavior of Mg–Zn–Y–Al alloys with long-period stacking ordered structures. J. Mg Alloys 2 (1), 78–84. https://doi.org/10.1016/j.jma.2014.01.008 (2014).
Tan, X. H., Winston, C. K. H., Jimmy, C. K. W. & Gupta, M. Development of high-performance quaternary LPSO Mg–Y–Zn–Al alloys by disintegrated melt deposition technique. Mater. Des. 83, 443–450. https://doi.org/10.1016/j.matdes.2015.06.041 (2015).
Liu, Z. L. et al. Design of composition for Mg–Y–Zn–Al alloys using the Taguchi design and analysis of experiments. Mater. Res. Expr. 6, 106562. https://doi.org/10.1088/2053-1591/ab3964 (2019).
Uichiro, M. Hume-Rothery rules for structurally complex alloy phases. MRS Bull. 37 (2), 169–169. https://doi.org/10.1557/mrs.2012.45 (2012).
Ahmad, R., Yin, B., Wu, Z. & Curtin, W. A. Designing high ductility in magnesium alloys. Acta Mater. 172, 161–184. https://doi.org/10.1016/j.actamat.2019.04.019 (2019).
Jang, H. et al. Activation of non-basal < c + a > slip in multicomponent Mg alloys. J. Mg Alloys 10, 585–597. https://doi.org/10.1016/j.jma.2021.03.007 (2022).
Wang, L. et al. Study of slip activity in a Mg–Y alloy by in situ high energy X-ray diffraction microscopy and elastic viscoplastic self-consistent modeling. Acta Mater. 155, 138–152. https://doi.org/10.1016/j.actamat.2018.05.065 (2018).
Zhang, Z., Tremblay, R., Dube, D. & Couture, A. Solidification microstructure of Za102, ZA104 and ZA106 magnesium alloys and its effect on creep deformation. Can. Metall. Quart. 39 (4), 503–512. https://doi.org/10.1179/cmq.2000.39.4.503 (2013).
Iaudelli, A. Crystallographic studies of some pseudobinary intermetallic systems. J. Less-Common Met. 169, 187–196. https://doi.org/10.1016/0022-5088(91)90067-E (1991).
Yamasaki, M., Izumi, S., Kawamura, Y. & Habazaki, H. Corrosion and passivation behavior of Mg–Zn–Y–Al alloys prepared by cooling rate-controlled solidification. Appl. Surf. Sci. 257 (19), 8258–8267. https://doi.org/10.1016/j.apsusc.2011.01.046 (2011).
Xie, H. J. et al. A new hexagonal AlZnY phase and the nano-hardness in Mg–Y–Al–Zn alloy. Mater. Lett. 317, 132111. https://doi.org/10.1016/j.matlet.2022.132111 (2022).
Zou, H., Zeng, X., Zhai, C. & Ding, W. The effects of yttrium element on microstructure and mechanical properties of Mg–5wt.% Zn–2wt.% al alloy. Mater. Sci. Eng. A 402 (1–2), 142–148. https://doi.org/10.1016/j.msea.2005.04.011 (2005).
Chang, H. W., Qiu, D., Taylor, J. A., Easton, M. A. & Zhang, M. X. The role of Al2Y in grain refinement in Mg–Al–Y alloy system. J. Mg Alloys. 1 (2), 115–121. https://doi.org/10.1016/j.jma.2013.07.006 (2013).
Su, C. et al. Enhanced ductility in high-pressure die casting Mg–4Ce–xAl–0.5Mn alloys via modifying second phase. Mater. Sci. Eng. A. 773 https://doi.org/10.1016/j.msea.2019.138870 (2020).
Zhu, Z. & Pelton, A. D. Thermodynamic modeling of the Y–Mg–Zn, Gd–Mg–Zn, Tb–Mg–Zn, Dy–Mg–Zn, Ho–Mg–Zn, Er–Mg–Zn, Tm–Mg–Zn and Lu–Mg-Zn systems. J. All Comp. 652, 426–443. https://doi.org/10.1016/j.jallcom.2015.08.214 (2015).
Xu, D. K., Tang, W. N., Liu, L., Xu, Y. B. & Han, E. H. Effect of Y concentration on the microstructure and mechanical properties of as-cast Mg–Zn–Y–Zr alloys. J. All Comp. 432 (1–2), 129–134. https://doi.org/10.1016/j.jallcom.2006.05.123 (2007).
Wang, J. et al. Effects of Zn on the microstructure, mechanical properties, and damping capacity of Mg–Zn–Y–Zr alloys. Mater. Sci. Eng. A 528 (18), 5914–5920. https://doi.org/10.1016/j.msea.2011.04.002 (2011).
Acknowledgments
This research was fnanclally supported by Sctence and Technology Tackling Project of Henan Province (No.252102230067), Key Scientific Research Project of Higher Education Institutions In Henan Province (No. 25A430040).
Author information
Authors and Affiliations
Contributions
H. X.: investigation, methodology, data curation, formal analysis, writing—original draf, funding acquisition. X. Y.: investigation, methodology investigation, data curation. L. X.: formal analysis, writing—original draf. H. S.: investigation. Z. Y.: writing—review & editing. C. Z.: investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, H., Yin, X., Xu, L. et al. Effect of substituting Al with Zn on the microstructure and mechanical properties of the as-cast Mg–4Y–4Al alloy. Sci Rep 15, 6711 (2025). https://doi.org/10.1038/s41598-025-91320-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91320-5