Abstract
Traditionally, completion axillary lymph node dissection (ALND) has been standard for breast cancer patients with positive sentinel lymph nodes (SLNs). However, ALND poses risks of morbidity. Recent trials suggest omission of ALND may be safe in selected cases. Controversy exists regarding ALND omission in mastectomy patients with micrometastases. We retrospectively analyzed data from 12 centers in China and the Surveillance, Epidemiology, and End Results (SEER) database. Patients with T1-2 breast cancer and SLN micrometastases who underwent up-front mastectomy were included. Patients were categorized into two groups: Non-ALND and ALND. Clinicopathological factors and survival outcomes were compared between the two groups. A total of 118 patients from 12 centers in China and 4,884 patients from the SEER database were included in the analysis. The Non-ALND group demonstrated non-inferiority in terms of recurrence-free survival (RFS), locoregional recurrence-free survival (LRFS), breast cancer-specific survival (BCSS), and overall survival (OS) when compared to the ALND group. Multivariable analysis identified significant predictors of survival outcomes. This study supports the omission of ALND in T1-2N1mi breast cancer patients undergoing mastectomy, demonstrating comparable survival outcomes to those undergoing ALND. Proper patient selection is essential for tailored treatment strategies.
Similar content being viewed by others
Introduction
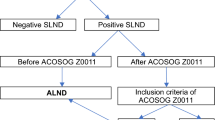
In the surgical management of axilla in breast cancer, completion axillary lymph node dissection (ALND) has traditionally been the standard of care for patients with a sentinel lymph node (SLN) positive for malignancy1. However, ALND is associated with significantly higher risks of morbidities such as lymphedema, shoulder stiffness, and sensory neuropathy compared to sentinel lymph node biopsy (SLNB)2,3. In the bid to reduce surgical morbidity, several studies have been conducted to investigate if patients with early-stage breast cancer and low nodal disease burden may be spared an ALND without compromise to oncological outcomes.
The ACOSOG Z0011 trial and the IBCSG 23-01 trial demonstrated that patients with cT1-2N0 breast cancer, presenting with one to two positive sentinel lymph nodes (SLNs) containing either micro- or macrometastases, who underwent breast-conserving surgery and whole breast irradiation, could safely forego ALND4,5. Additionally, findings from the AMAROS trial showed that both ALND and axillary radiotherapy following a positive SLN yielded excellent and comparable axillary control in patients with T1–2 primary breast cancer6.
These trial findings have led to significant changes in clinical practice, advocating for the omission of ALND in patients with tumor-involved SLNs, thereby reducing the extent of surgical intervention7. However, the majority of participants in these trials underwent breast-conserving surgery and received radiation therapy, leaving limited evidence for patients who underwent mastectomy. Of particular controversy is whether omitting axillary dissection and radiotherapy is appropriate for early-stage breast cancer patients with micrometastases in the SLNs following total mastectomy with or without chest wall postmastectomy radiotherapy.
To address this issue, we conducted a retrospective study to investigate whether the omission of further axillary treatment, including ALND or adjuvant radiation therapy, in selected patients undergoing total mastectomy for early-stage clinically node-negative breast cancer identified as having node micrometastases by SLNB, has any adverse effects on regional recurrence risk or survival.
Patients and methods
Study population
We retrospectively analyzed data from 12 centers in China and the Surveillance, Epidemiology, and End Results (SEER) database. Case data from the 12 Chinese medical centers were manually entered into the Shanghai Jiao Tong University Breast Cancer Database (SJTUBCDB). The study included patients with T1-2 breast cancer, no distant metastasis, and micrometastases in the SLNs. All patients underwent total mastectomy, with or without ALND. Adjuvant radiotherapy was administered if axillary dissection revealed metastasis beyond the SLN; otherwise, it was omitted. Data for the study were sourced from the SJTUBCDB spanning from 2013 to 2020 and the SEER database covering the period from 2010 to 2020. N1mi was defined as the presence of a metastatic deposit larger than 0.2 mm and up to 2 mm, detected in one or more lymph nodes on hematoxylin and eosin (H&E) or immunohistochemical evaluation. Patients were stratified based on their axillary dissection into two groups: the Non-ALND group and the ALND group. The number of lymph nodes removed was used as a surrogate for the type of axillary surgery which was defined as in previous similar studies8,9,10,11, that is, patients with 5 or fewer lymph nodes resected were categorized as receiving SLNB while 6 or more as undergoing ALND.
Clinicopathological information and outcomes
In the SJTUBCDB, we collected comprehensive patient information, including age at diagnosis, year of diagnosis, histological type, tumor grade, T stage, number of SLN with micrometastasis, Ki67 expression, lymphovascular invasion, tumor molecular subtype, recurrence-free survival (RFS), local recurrence-free survival (LRFS), breast cancer-specific survival (BCSS), overall survival (OS), as well as treatment information, including surgery, chemotherapy, endocrine therapy, targeted therapy. In the SEER database, we collected age at diagnosis, year of diagnosis, marital status, race, income, T stage, number of SLN with micrometastasis, histological type, tumor grade, primary tumor site, tumor molecular subtype, surgery, chemotherapy, BCSS, and OS.
LRFS was defined as the time from surgery to local and/or axillary relapse. RFS was defined as the time from surgery to any relapse. OS and BCSS were defined as the time from surgery to death and to death from breast cancer, respectively. The study received thorough review and approval from the Institutional Review Board of Shaoxing Second Hospital.
Statistical analysis
To compare clinicopathological factors between the different subgroups, we used Pearson or Mantel-Haenszel x2 tests for categorical and ordinal factors, respectively. To handle missing data in the SEER database, we employed multiple imputation with a multivariate logistic regression model. This process was repeated for 10 cycles to generate a final imputed dataset. Variables included in the imputation model encompassed age (continuous), race (white, black, or other), marital status (single, married, separated), year of diagnosis, histology (IDC, ILC, and other), T stage, number of SLN with micrometastasis, nuclear grade (I, II, III), primary tumor site, tumor molecular subtype, chemotherapy. To minimize the effects of potential modifiers, a 1-to-1 propensity score matching (PSM) method without replacement was performed for the comparisons using the nearest-neighbor method with a stringent caliper of 0.05 based on the R package MatchIt (Supplemental Fig. 1). After PSM adjustment, Kaplan-Meier survival curves were used to estimate RFS, LRFS, BCSS, and OS of patients in Non-ALND and ALND groups. Factors associated with BCSS and OS were assessed using multivariable proportional hazards regression. Subsequently, hazard ratios (HRs) and confidence interval (CI) for BCSS, and OS were calculated in subgroup analyses using multivariable models.
All P-values were calculated from 2-sided tests, with a threshold of 0.05 used to evaluate the statistical significance of survival benefits related to surgery. All statistical analyses were performed using R software (version 4.3.0).
Results
Patient demographic and clinical information
In the SJTUBCDB, we identified a total of 118 patients diagnosed with T1-2 and SLN micrometastases who underwent mastectomy between 2013 and 2020. The follow-up period for these patients extended up to 106.93 months, with a mean follow-up time of 50.65 months. Table 1 presents a summary of the demographic and clinicopathological characteristics of the patients included in the SJTUBCDB dataset. The patient distribution in year of diagnosis, age, histological type, T stage, Ki67, lymphovascular invasion, tumor molecular subtype, and receipt of chemotherapy, endocrine therapy or targeted therapy was similar in the different subgroups. The distribution of patients with tumor grade III is significantly higher in the ALND group compared to the Non-ALND group. The tumor grade III was 34.7% in the ALND group and 28.3% in the Non-ALND group. Among the patients who underwent ALND, 14 cases showed metastasis in non-sentinel lymph nodes, and these patients received postoperative radiotherapy” will be revised to: “Among the 72 patients who underwent ALND, 14 cases showed metastasis in non-sentinel lymph nodes, and these patients received postoperative radiotherapy (19.4%). No radiotherapy was given to the 46 patients who did not undergo ALND.
In the SEER database, we identified a larger cohort of 4,884 patients diagnosed with T1-2 and SLN micrometastases who underwent mastectomy without radiotherapy between 2010 and 2020. Patients with lymph node micrometastases and chest wall radiotherapy were excluded. The follow-up period for these patients ranged up to 131 months, with a mean follow-up time of 64.47 months. Table 2 presents a summary of the demographic and clinicopathological characteristics of the patients included in the SEER dataset. Balance in patient characteristics was achieved after multiple imputations and PSM adjustments between the Non-ALND and the ALND subgroups, as shown in Fig. 1. The research findings indicate that patients diagnosed earlier years (2010–2013) and younger age groups (< 65 years) are more inclined towards undergoing ALND. Additionally, individuals from low-income backgrounds (<$75,000) and those of African descent show a higher propensity for ALND. Furthermore, tumors characterized as IDC, Grade III, and at T2 stage are more likely to result in ALND. Moreover, our results also demonstrate that individuals undergoing chemotherapy exhibit a greater preference for axillary dissection.
Survival Analysis Comparing Non-ALND and ALND Groups: RFS and LRFS in SJTUBCDB (A,B), BCSS and OS in SEER Database Matched Cohort (C,D) and Whole Cohort (E,F). ALND axillary lymph node dissection, RFS recurrence-free survival, LRFS locoregional recurrence-free survival, BCSS breast cancer-specific survival, OS overall survival, mo months, HR hazard ratios, CI confidence interval.
Survival analysis of patients undergoing non-ALND versus ALND
We compared the RFS, LRFS, BCSS and OS between the Non-ALND group and the ALND group using Kaplan-Meier curves. Our analyses of both SJTUBCDB and SEER database data consistently indicated that the Non-ALND group exhibited non-inferiority to the ALND group in terms of RFS, LRFS, BCSS and OS. In the SJTUBCDB dataset, the 5-year RFS was 94.1% in the Non-ALND group and 93.1% in the ALND group. The RFS in the Non-ALND group was non-inferior to the ALND group (HR 0.57, 95% CI 0.11–2.81; p = 0.487; Fig. 1A). Similarly, the 5-year LRFS was 94.1% in the Non-ALND group and 94.5% in the ALND group, with non-inferiority observed in the Non-ALND group (HR 0.37, 95% CI 0.06–2.23; p = 0.28; Fig. 1B). In the population matched through PSM in the SEER database, the 5-year BCSS was 94.9% in the Non-ALND group and 95.7% in the ALND group (HR 0.83, 95% CI 0.62–1.10, p = 0.19; Fig. 1C). Additionally, the 5-year OS was 89.5% in the Non-ALND group and 91.1% in the ALND group (HR 0.89, 95% CI 0.73–1.07; p = 0.22; Fig. 1D). Similar results were observed in the unmatched population (BCSS HR 0.80, 95% CI 0.62–1.02, p = 0.08; Fig. 1E; OS HR 0.86, 95% CI 0.73–1.02, p = 0.09; Fig. 1F).
Multivariable proportional hazards regression analysis of BCSS and OS, based on the 4,884 patients in the SEER database without PSM, revealed that age over 65 years, Grade III, T2 stage, HR-/HER2- status, and having more than 2 positive sentinel lymph nodes were significant predictors of BCSS and OS. Conversely, being in a good marital status and having the tumor located in the upper outer quadrant were protective factors for predicting BCSS and OS (Table 3). Subgroup analyses were conducted for the included variables, revealing that apart from subgroups with income less than $75,000, Black race, and HR-/HER2- status, which showed better outcomes in the ALND group, almost all other subgroup results indicated non-inferiority of the Non-ALND group compared to the ALND group (Fig. 2).
Comparison of Hazard Ratios for BCSS and OS Across Subgroups Between Non-ALND and ALND Groups in SEER Database Matched Cohort. ALND axillary lymph node dissection, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, HR hormone receptor, HER2 human epidermal growth factor 2, DSW divorced widowed separated.
Discussion
In early breast cancer, assessing axillary lymph node involvement is critical for prognosis and treatment planning. ALND, as a standard treatment approach, enables accurate assessment of axillary lymph node metastasis in patients. However, despite the clear therapeutic efficacy of ALND, its associated complications such as lymphedema, nerve injury, and shoulder dysfunction need to be considered12,13,14. In recent decades, there has been significant progress in developing less invasive approaches for axillary staging, facilitating more tailored treatments for breast cancer patients15,16. Consequently, the utility of ALND has been undergoing transformation. While omitting ALND in cases of node-positive disease has become increasingly accepted, the question persists as to whether the findings from the ACOSOG Z0011 trial can be extrapolated to broader patient cohorts, particularly those undergoing total mastectomy.
Furthermore, the occurrence of lymph node metastases outside the SLN in the axilla is notable. The residual tumor burden in the axilla poses a significant risk factor for axillary failure, warranting increased attention. While patients with SLN micrometastases may present with a low axillary disease burden, it does not preclude the possibility of additional axillary node involvement17. Data from the SJTUBCDB in our study reveal that among patients with SLN showing N1mi status, further axillary dissection resulted in an additional non-sentinel lymph node metastasis rate of 19.4%. This incidence is comparable to previous reports of non-sentinel lymph node metastases ranging from 10 to 20%5,18,19,20,23. In our study, despite the presence of non-sentinel lymph node metastases, the incidence of local recurrence was reassuringly low in cases where the axilla remained undissected, comparable to that in patients who underwent axillary dissection (p = 0.28). This outcome was consistent with findings from other studies, underscoring the importance of these observations21,22.
Patients with SLN micrometastases who did not undergo axillary dissection exhibit not only a lower rate of axillary recurrence but also similar survival outcomes compared to those undergoing axillary dissection. Studies such as IBCSG 23-01 and AATRM, which exclusively enrolled patients with SLN micrometastases, compared observation with ALND5,23. Among these, 86 patients and 18 patients, respectively, underwent mastectomy. These trials revealed a 5-year OS exceeding 97% across all groups, with no significant increase in axillary recurrence at the 5-year mark. Research indicates that tumor biology plays a pivotal role in disease progression among patients with micrometastases, rather than merely the extent of nodal involvement24,25. Therefore, these trials support the idea that contemporary adjuvant therapies for breast cancer, encompassing chemotherapy, endocrine therapy, and whole-breast radiotherapy, effectively control regional axillary disease and yield excellent long-term survival for women with SLN micrometastases who forego ALND26. Based on these findings, it is deemed safe to observe women with only micrometastatic disease identified during SLNB evaluation, provided they have access to adjuvant therapy24.
In comparison to the well-established efficacy of chemotherapy, endocrine therapy, and targeted treatment, the utilization of postmastectomy radiotherapy (PMRT) in this cohort remains a subject of debate25. Current guidelines suggest strong consideration of PMRT to the chest wall and regional lymph nodes for mastectomy patients with one to three positive nodes7. However, uncertainty persists regarding whether micrometastases should be factored into the node count. Moreover, both ALND and axillary radiotherapy are linked to heightened risks of complications such as ipsilateral upper limb lymphedema and shoulder stiffness when compared to SLNB alone. Moreover, when chest wall PMRT is performed, the incidental radiotherapy dose to the axillary basin has been shown to be as effective as the radiotherapy used in breast-conserving surgery with breast radiotherapy27. Therefore, it is imperative to identify the subset of patients who stand to benefit from the judicious reduction of axillary therapy.
In multivariable survival analysis, the receipt of adjuvant chemotherapy emerged as an independent predictor for improved OS in breast cancer patients. Additionally, various other factors were identified as influential on survival outcomes, including age, marital status, income, race, tumor stage (T2), tumor location in the upper outer quadrant, Grade III tumor, molecular subtype (HR-/HER2-), and the number of metastatic sentinel nodes. Subgroup survival analysis revealed that only patients with incomes below $75,000, Black race, and HR-/HER2- subtype exhibited survival benefits among those with SLN micrometastases treated with Non-ALND or ALND. These findings contribute to the surgical management of the axilla in patients with early-stage breast cancer undergoing mastectomy and provide support for less extensive axillary surgery in select patients. They provide valuable support for the implementation of less extensive axillary surgery in appropriately selected patient cohorts.
The current study has several limitations that warrant acknowledgment. Firstly, as it is a retrospective analysis, it lacks the randomization typically found in prospective studies. Moreover, utilizing telephone follow-up may introduce the potential for missing information. Additionally, due to the prolonged study duration, some patients were lost to follow-up, leading to a lack of contact with the clinic. Furthermore, the limited data within the SJTUBCDB dataset resulted in a low number of events, which precluded subgroup analysis. Secondly, concerning the SEER database, patients have not been categorized based on the type of axillary surgery. Consequently, we relied on the number of regional lymph nodes examined as a proxy, which may introduce errors. Finally, although the average follow-up time in our study was approximately five years, this may not be sufficient to capture late recurrences, particularly in early-stage breast cancer. Longer follow-up is needed to further validate our findings.
Conclusions
In this study of T1-2N1mi breast cancer, patients who underwent mastectomy without ALND showed comparable outcomes in terms of RFS, LRFS, BCSS, and OS to those who underwent completion ALND. Our findings indicate that these patients can be safely managed without axillary therapy, including completion ALND or axillary radiotherapy.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
16 April 2025
The original online version of this Article was revised: In the original version of this Article Wu Ding and YongTian Chen were omitted as equally contributing authors. The statement has now been added and reads: “Wu Ding and YongTian Chen contributed equally to this work.”.
References
O’Dwyer, P. J. Axillary dissection in primary breast cancer. BMJ 302(6773), 360–361 (1991).
Hladiuk, M. et al. Arm function after axillary dissection for breast cancer: a pilot study to provide parameter estimates. J. Surg. Oncol. 50(1), 47–52 (1992).
Ivens, D. et al. Assessment of morbidity from complete axillary dissection. Br. J. Cancer. 66(1), 136–138 (1992).
Giuliano, A. E. et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and Sentinel node metastasis: a randomized clinical trial. JAMA 305(6), 569–575 (2011).
Galimberti, V. et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 19(10), 1385–1393 (2018).
Donker, M. et al. Radiotherapy or surgery of the axilla after a positive Sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 15(12), 1303–1310 (2014).
Gradishar, W. J. et al. NCCN guidelines insights: breast cancer, version 4.2023. J. Natl. Compr. Canc Netw. 21(6), 594–608 (2023).
Amin, M. B. et al. (eds) American Joint Committee on Cancer Staging Manual 8th edn (Springer, 2017).
Weiser, R. et al. Prognosis and chemotherapy use in breast cancer patients with multiple lymphatic micrometastases: an NCDB analysis. Ann. Surg. Oncol. 28(13), 8717–8727 (2021).
Weiser, R. et al. The 21-gene recurrence score in node-positive, hormone receptor-positive, HER2-negative breast cancer: a cautionary Tale from an NCDB analysis. Breast Cancer Res. Treat. 185(3), 667–676 (2021).
Luo, S. P. et al. Association of axillary lymph node evaluation with survival in women aged 70 years or older with breast cancer. Front. Oncol. 10, 596545 (2021).
Lucci, A. et al. Surgical complications associated with Sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American college of surgeons oncology group trial Z0011. J. Clin. Oncol. 25(24), 3657–3663 (2007).
Husted Madsen, A. et al. Arm morbidity following Sentinel lymph node biopsy or axillary lymph node dissection: a study from the Danish breast Cancer cooperative group. Breast 17(2), 138–147 (2008).
Del Bianco, P. et al. Morbidity comparison of Sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the Sentinella-GIVOM Italian randomised clinical trial. Eur. J. Surg. Oncol. 34(5), 508–513 (2008).
Krag, D. N. et al. Surgical resection and radiolocalization of the Sentinel lymph node in breast cancer using a gamma probe. Surg. Oncol. 2(6), 335–339 (1993). discussion 340.
Giuliano, A. E. et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 220(3), 391–398 (1994).
Cong, B. B., Yu, J. M. & Wang, Y. S. Axillary management still needed for patients with Sentinel node micrometastases. Cancer Manag Res. 11, 2097–2100 (2019).
Cserni, G. et al. Variations in Sentinel node isolated tumour cells/micrometastasis and non-sentinel node involvement rates according to different interpretations of the TNM definitions. Eur. J. Cancer. 44(15), 2185–2191 (2008).
Tallet, A. et al. Locoregional treatment of early breast cancer with isolated tumor cells or micrometastases on Sentinel lymph node biopsy. World J. Clin. Oncol. 7(2), 243–252 (2016).
Pilewskie, M. L. & Morrow, M. Management of the clinically nodenegative axilla: what have we learned from the clinical trials? Oncol. (Williston Park). 28(5), 371–378 (2014).
Veronesi, U. et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl. J. Med. 349(6), 546–553 (2003).
Giuliano, A. E. et al. Locoregional recurrence after Sentinel lymph node dissection with or without axillary dissection in patients with Sentinel lymph node metastases: the American college of surgeons oncology group Z0011 randomized trial. Ann. Surg. 264(3), 413–420 (2016).
Sola, M. et al. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with Sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann. Surg. Oncol. 20(1), 120–127 (2013).
Ozcan, L. C. & Giuliano, A. E. Is axillary lymph node dissection necessary after a positive Sentinel lymph node biopsy?? Adv. Surg. 51(1), 165–178 (2017).
Mamtani, A. et al. Axillary micrometastases and isolated tumor cells are not an indication for Post-Mastectomy radiotherapy in stage 1 and 2 breast Cancer. Ann. Surg. Oncol. 24(8), 2182–2188 (2017).
Castelo, M. et al. Comparing observation, axillary radiotherapy, and completion axillary lymph node dissection for management of axilla in breast Cancer in patients with positive Sentinel nodes: A systematic review. Ann. Surg. Oncol. 27(8), 2664–2676 (2020).
Nicolas, C. et al. Does breast surgery type alter incidental axillary irradiation?? A dosimetric analysis of the Sentinel Envahi et randomisation du curage SERC trial. Cancers (Basel). 16(6), 1198 (2024).
Author information
Authors and Affiliations
Contributions
Conception and design: Wu Ding, Xiaoliang ChenAdministrative support: Yongtian Chen, Yingli LinCollection and assembly of data: Wu Ding, Yongtian Chen, Yingli LinData analysis and interpretation: Wu Ding, Xiaoliang ChenManuscript writing: All authors Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Our study was approved by the independent ethics committee/institutional review board at Shaoxing Second Hospital Ethical Committee. The data from the SEER datasets are publicly available and therefore do not require informed patient consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, W., Chen, Y., Lin, Y. et al. The impact of axillary lymph node dissection on the prognosis of breast cancer patients undergoing up-front mastectomy with Sentinel lymph node micrometastases. Sci Rep 15, 10525 (2025). https://doi.org/10.1038/s41598-025-91405-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91405-1