Abstract
Butylated hydroxytoluene (BHT) is among the most widely used synthetic phenolic antioxidants. However, BHT and its metabolites have been detected in aquatic ecosystems, posing potential risks to aquatic organisms. The present study aimed to investigate the effects of BHT metabolites on embryonic development in zebrafish. To this end, embryos were exposed to BHT metabolites, including 3,5-di-tert-butyl-4 hydroxybenzaldehyde (BHT-CHO), 2,6-di-tert-butyl-4-(hydroxymethyl) phenol (BHT-OH), 3,5-di-tert-butyl-4 hydroxybenzoic acid (BHT-COOH), 2,6-di-tert-butyl-P-benzoquinone (BHT-Q), and 2,6-di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one (BHT-quinol), from 1–120 h post-fertilization (hpf). BHT-CHO, -OH, -COOH, -Q, and -quinol were toxic to zebrafish larvae with 96 h LC50 values of > 0.10, 15.85, 4.51, > 1.30, and 3.46 mg/L, respectively. Moreover, the acute toxicity of BHT metabolites to zebrafish larvae was indicated by morphological abnormalities, changes in heart rate, and alterations in locomotory behavior. The results indicated that exposure to BHT-COOH and BHT-OH caused intestinal developmental abnormalities, blood coagulation, tail deformities, and pericardial edema. Exposure to BHT-Q and BHT-quinol resulted in abnormal swim bladder development. Moreover, alterations in heart rate and locomotory behavior were observed in zebrafish larvae exposed to BHT-COOH, BHT-OH, and BHT-quinol. These findings demonstrate that exposure to BHT metabolites significantly affects the early growth and developmental stages of zebrafish larvae.
Similar content being viewed by others
Introduction
Synthetic phenolic antioxidants (SPAs) are widely used as additives in consumer products such as plastics, rubber, mineral oil, fuel additives, cosmetics, food, and pharmaceuticals. They neutralize and capture free radicals to protect these products from oxidation, thus extending their shelf life and enhancing their properties1,2,3,4,5. One of the most commonly used SPAs is 2,6-di-tert-butyl hydroxytoluene, also known as butylated hydroxytoluene (BHT). BHT is unstable in the environment and can easily convert into BHT-quinol, BHT-CHO, and BHT-OH via the loss of alkyl groups, addition or loss of hydroxyl groups, isomerization, and oxidation6,7. In addition, BHT can be converted into various metabolites through metabolic processes such as oxidation of the alkyl substituents and oxidation of the aromatic ring system. The oxidation of BHT, which is catalyzed by cytochrome P450, is a major degradation pathway in BHT metabolism. The oxidation of its p-methyl group results in the formation of BHT-COOH, the principal metabolite, while oxidation of its aromatic ring leads to the formation of BHT-Q.
Owing to its extensive use, BHT and its metabolites have been frequently detected in the environmental and biological matrices. For example, BHT was detected at concentrations ranging from 0.02 to 3.02 µg/g in indoor dust, with total concentrations calculated8,9. Moreover, BHT four metabolites were detected in the indoor dust samples: BHT-Q (0.28–1.77 μg/g), BHT-CHO (0.20–1.07 μg/g), BHT-OH (0.02–0.14 μg/g), and BHT-COOH (0.01–0.18 μg/g). In Germany, BHT-CHO was identified at a concentration of 102 ng/L in the Oder River10. In sediments, BHT-Q, BHT-CHO, and BHT-OH were detected at 0.02–1.36, 0.04–0.14, and 0.02–0.20 μg/g, respectively11. BHT metabolites have also been reported in sewage, sludge, and mollusks12,13, as well as in human biological samples such as fingernails, blood, and urine14.
Concerns regarding the potential toxicity of BHT and its metabolites to humans have increased15. In mice, BHT induces lung damage and melanoma metastasis16. In rats, it causes liver necrosis, nephrotoxicity, and hemorrhagic death17,18. Notably, BHT can also inhibit the basal expression of estrogen-responsive genes19 and promote the development of tumors induced by dimethylhydrazine20. Oikawa et al.21 reported that BHT and its BHT metabolites induce the production of peroxides and cause cellular DNA damage in mice and rats. Moreover, low concentrations of BHT-Q can induce fragmentation of supercoiled DNA by generating oxygen radicals in vitro. Similarly, BHT-CHO also promotes DNA damage, but to a lesser extent than BHT-Q, whereas BHT-OH, BHT-COOH, and BHT-quinol do not22. Similar to the various toxic effects observed in humans and rodents, BHT decreases the heart rate by 25–30% in zebrafish at 48 and 72 h post-exposure23. It also causes various morphological abnormalities, including uninflated swim bladder, pericardial edema, spinal curvature, severe yolk deformation, and abnormal pigmentation24. Although BHT has been extensively studied in terms of its carcinogenicity, reproductive toxicity, genotoxicity, and behavioral properties in fish and mammals, research on the toxicity of its metabolites and their effects on living organisms, particularly under environmental stress, remains limited in aquatic organisms such as fish.
In the present study, we aimed to investigate the toxic effects of BHT metabolites on zebrafish (Danio rerio) eggs and embryos by observing behavioral patterns, cardiac toxicity, and morphological abnormalities. We selected zebrafish larvae as the test subjects owing to their ease of culture and maintenance in large quantities, rapid growth, and transparent embryos, which facilitate straightforward observations. Notary, zebrafish are widely used in toxicity studies of environmental pollutants as they reproduce freely under laboratory conditions25,26,27. The results of our study may provide valuable insights into the potential risks of environmental exposure to BHT metabolites in aquatic ecosystems.
Results and discussion
Acute toxicity test
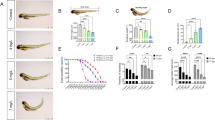
To select a sublethal concentration of BHT metabolites, we performed an acute toxicity test using zebrafish embryos and confirmed the LC50 value. The water solubility values for BHT-CHO, BHT-COOH, BHT-OH, BHT-Q, and BHT-quinol were 0.1, 100.0, 27.0, 1.3, and 19.6 mg/L, respectively. Correspondingly, the LC50 values (95% confidence interval) at 120 h were > 0.1 mg/L for BHT-CHO, 15.85 mg/L (14.15–17.74 mg/L) for BHT-COOH, 4.51 mg/L (3.70–5.49 mg/L) for BHT-OH, > 1.3 mg/L for BHT-Q, and 3.46 mg/L (2.45–4.90 mg/L) for BHT-quinol (Table 1).
Sarmah et al.28 evaluated the acute toxicity of BHT in zebrafish embryos and reported an LC50 value of 4.388 mg/L at 96 h and an effective concentration (EC50) of 1.375 mg/L. They also reported that exposure to BHT adversely affected normal organ development in zebrafish embryos, suggesting significant developmental toxicity. These findings emphasize the potential risks posed by BHT to aquatic organisms and underscore the need for careful environmental monitoring and regulation. In the present study, we compared the LC50 values of BHT to those of its metabolites, revealing distinct differences in their acute toxicity. Specifically, BHT-COOH exhibited a higher LC50 value than that of BHT, indicating a lower acute toxicity than that of BHT. Additionally, BHT-OH and BHT-quinol had LC50 values similar to that of BHT, suggesting comparable levels of toxicity with BHT. The LC50 values of BHT-CHO and BHT-Q were difficult to accurately evaluate owing to their low water solubility, thus complicating the determination of precise toxicity thresholds. These variations underscore the different toxicological profiles of BHT and its metabolites.
Morphologic abnormalities caused by BHT metabolites
To determine the morphological effects of the BHT metabolites on the zebrafish embryos, we conducted post-exposure microscopy. The hatching and survival rates of the zebrafish embryos in the control and treatment groups exposed to all BHT metabolites were greater than 80% at 120 H post-fertilization (hpf). However, BHT-CHO did not induce embryotoxicity at water-soluble concentrations. We observed no morphological abnormalities in body length or eye size in the control or 0.1 mg/L treatment groups (Fig. S1). However, we observed morphological anomalies, including tail deformities and intestinal developmental malformations, in embryos exposed to 1 mg/L. We also noted pericardial edema and intestinal and intracardiac blood coagulation in embryos exposed to 5 and 10 mg/L BHT-COOH (Fig. 1A–G). Notably, the body (Fig. 1H) and eye sizes (Fig. 1I) of the embryos decreased with increasing BHT-COOH concentrations, exhibiting a statistically significant reduction in body and eye sizes between the control and high-concentration groups. In contrast, we observed no abnormalities in body length and eye size in embryos exposed to varying BHT-OH concentrations. However, we noted uninflated swim bladders in four of nine embryos exposed to the highest (2 mg/L) BHT-OH concentration (Fig. 2). As BHT-Q did not exhibit embryotoxicity at water-soluble concentrations, we compared the control and 1.3 mg/L treatment groups. However, we observed normal body length and eye size in both groups; the only morphological abnormalities observed were uninflated swim bladders (Fig. 3).
Morphological effects of BHT-COOH exposure to zebrafish larvae at 120 hpf. (A) Control; (B) fishtail deformity (FD) after exposure to 1 mg/L BHT-COOH; (C) intestinal inflammation (IF) after exposure to 1 mg/L BHT-COOH; (D) pericardial edema (PE) after exposure to 5 mg/L BHT-COOH; (E) blood coagulation (BC) and PE after exposure to 5 mg/L BHT-COOH; (F) PE after exposure to 10 mg/L BHT-COOH; (G) PE and BC after exposure to 10 mg/L BHT-COOH. At 5 mg/L, PE was confirmed in five of nine larvae sampled; at 10 mg/L, PE was confirmed in eight of nine larvae. Effects of BHT-COOH exposure on (H) body length and (I) eye size of zebrafish larvae (*p < 0.05, **p < 0.005).
The body length of the embryos exposed to BHT-quinol remained unchanged (Fig. 4G); however, the eye size significantly differed (P < 0.05; Fig. 4H). Embryos exposed to 0.1 mg/L BHT-quinol displayed tail deformities and delayed swim bladder development. Embryos exposed to 1 mg/L BHT-quinol exhibited pericardial edema, delayed swim bladder development, and eye and jaw deformities. We also observed tail deformities in embryos exposed to 2 mg/L BHT-quinol (Fig. 4A–F). Embryos exposed to BHT-COOH exhibited morphological abnormalities, including changes in the heart, intestine, body length, and eye size. Similarly, the group exposed to BHT-quinol displayed abnormalities in the tail, heart, eye size, swim bladder, and jaw. Notably, embryos exposed to BHT-OH and BHT-Q exhibited abnormal swim bladder development.
Morphological effects of BHT-quinol exposure to zebrafish larvae at 120 hpf. (A) Control; (B) fishtail deformity (FD) and uninflated swimming bladder (USB) after exposure to 0.1 mg/L BHT-quinol; (C) USB after exposure to 0.1 mg/L BHT-quinol; (D) Pericardial edema (PE) after exposure to 1 mg/L BHT-quinol; (E) Malformed jaw and eye (JA) after exposure to 1 mg/L BHT-quinol; (F) FD after exposure to 2 mg/L BHT-quinol. (G) There was no difference in body length between the control and BHT-quinol-treated groups. (H) There was a statistically significant difference in eye size between the control and BHT-quinol-treated groups (*p < 0.05).
The inflammatory response in zebrafish intestines indicates intestinal damage induced by environmental pollutants29. Similar to the inflammatory response of zebrafish exposed to the pesticides imidacloprid30 and chlorpyrifos31, we observed intestinal redness (blood), which is indicative of an inflammatory response, in zebrafish embryos. Stress from pollutants can decrease the expression of the embryonic immune response genes interleukin 1 beta (IL1β) and immune responsive gene 1-like (IRG1L) in the intestine compared to other parts of the body, potentially leading to inflammation and intestinal abnormalities32. Therefore, stress from BHT-COOH exposure may have induced inflammation in the intestines of the zebrafish larvae, likely contributing to the occurrence of intestinal redness and damage.
In the present study, exposure to BHT-COOH and BHT-quinol resulted in decreased eye size, which is associated with developmental abnormalities and delayed eye development. Decreased eye and head sizes have been reported in zebrafish larvae exposed to various pesticides33,34. These morphological changes have been linked to disruptions in hindbrain segmentation signaling and impaired brain development35. However, the specific molecular mechanisms through which BHT-COOH and BHT-quinol affect eye development remain unknown.
As the swim bladder regulates body density and buoyancy, it plays a critical role in fish physiology; the dysfunction or underinflation of this organ may lead to erratic swimming patterns36,37. Wang et al.38 and Yang et al.39 highlighted the impact of various pollutants on swim bladder development and function. Their findings suggest that swim bladder abnormalities serve as indicators of environmental toxicity. In the present study, we observed delayed swim bladder development in embryos exposed to BHT-OH, BHT-Q, and BHT-quinol. Although the precise mechanisms underlying the development of swim bladder abnormalities remain unclear, potential pathways include altered expression of bladder-specific genes40, transcriptional changes in prolactin receptors41, and disruptions in protein interactions crucial for swim bladder distension42.
Cardiotoxicity
Cardiotoxicity refers to the dysfunction of heart electrophysiology or damage to the heart muscle. We measured the heart rate to determine cardiotoxicity resulting from exposure to BHT metabolites. Analysis of heartbeat per minute (BPM) across different concentrations of BHT metabolites revealed varied responses among the treatment groups compared to the control group. The BPM of embryos was significantly different between the control and BHT-CHO treated groups (P < 0.05). Compared to that of the control group, the BPM of embryos initially increased upon exposure to 1 mg/L BHT-COOH and subsequently decreased upon exposure to 5 and 10 mg/L BHT-COOH. We also observed a significant increase in the BPM between the control and embryos treated with 1 mg/L BHT-COOH (P < 0.05). In contrast, we observed a significant reduction in the BPM between the control and embryos treated with 10 mg/L BHT-COOH (P < 0.05). Compared to that of the control, the BPM of embryos exposed to BHT-OH was significantly decreased. Conversely, the BPM of the BHT-Q group was not significantly different from that of the control group. Moreover, exposure to 0.1 mg/L and 1 mg/L BHT-quinol did not significantly alter the BPM compared to that of the control group. However, we observed a significant difference upon exposure to 2 mg/L BHT-quinol (P < 0.05; Fig. 5). We noted significant changes upon exposure to BHT metabolites, except for BHT-Q.
The heart is the initial functional organ during organogenesis in zebrafish larval development43. Heart rate monitoring is a crucial metric for assessing cardiac function during toxicity evaluations44. Pericardial edema, correlated with reduced contractility and irregular heart development, is an indicator of cardiac dysfunction45,46. In this study, we observed significant concentration-dependent reductions in heartbeat in larvae exposed to BHT-OH and BHT-quinol. We also observed a notable reduction in the heartbeat rate in embryos exposed to elevated BHT-COOH concentrations. These findings imply that exposure to sublethal levels of BHT metabolites may disrupt normal embryonic heart development, leading to potential anomalies in cardiac function. During zebrafish development, various factors, such as oxidative stress-induced cell death, tumor promotion, and dysregulation of hormonal signaling pathways may cause alterations in the heart rate, body length, and morphological development. Therefore, further studies are needed to determine the effects of BHT metabolites on the heart, such as detailed morphological analysis of differentiated hearts using MF20/S46 Immunofluorescence and analysis of genes involved in heart differentiation.
Behavioral toxic effects of BHT metabolites
Neurotoxicity induced by various environmental chemicals, including pesticides, is closely associated with changes in the locomotor behavior of zebrafish during the early stages of development. In the present study, we observed no statistically significant differences in the total distance traveled, velocity, angular velocity, or turning angle among embryos exposed to varying BHT-CHO concentrations. However, there was a significant difference in heading (P < 0.05). Compared to the control, BHT-COOH exposure resulted in a concentration-dependent reduction in the speed of and distance traveled by the embryos (P < 0.05), whereas angular velocity, heading, and turn angles did not significantly differ between the two groups. Similarly, BHT-OH exposure resulted in a concentration-dependent reduction in the total distance traveled and velocity, with no significant effects on the angular velocity or turn angle compared to those in the control group. However, there was a significant difference (P < 0.05) in the heading upon exposure to the 1 mg/L concentration. BHT-Q did not induce observable differences in behavioral parameters compared to the control. Notably, the locomotor activities resulting from exposure to 0.1 mg/L BHT-quinol did not significantly differ from those of the control; however, exposure to elevated BHT-quinol concentrations (1 and 2 mg/L) significantly reduced both the distance traveled and speed (P < 0.05), while the angular velocity, turn angle, and heading remained unchanged (Fig. 6).
At 96 hpf, zebrafish larvae exhibit a fully developed central nervous system47, which is crucial for coordinating movement in conjunction with skeletal muscle formation. This developmental stage is pivotal for assessing developmental neurotoxicity through activity studies in zebrafish larvae48,49,50. Perturbations in neurotransmitter systems caused by external compounds induce movement disorders and anxiety-like behaviors in fish51. The impact of BHT on zebrafish larvae has been linked to altered locomotor activity and dark avoidance behavior, coinciding with the dysregulation of transcripts involved in dopamine signaling23. These behavioral changes in zebrafish larvae may stem from disturbances in dopamine signaling pathways. To identify changes in the dopamine signaling pathway, the expression of genes involved in the dopamine signaling pathway (th1, drd1, drd2a, drd2b, drd2c, drd3)23 was analyzed. We found that BHT metabolites affect the neurobehavioral outcomes of zebrafish. However, further investigation is needed to elucidate the specific mechanism, and genetic studies, such as those examining changes in the dopamine signaling pathway, should be conducted.
Conclusions
Despite the heightened detection of BHT and its metabolites in the environment due to increased BHT use, research on its environmental effects is lacking. In this study, we determined the toxic effects of BHT metabolites on zebrafish larvae. BHT-CHO exposure induced an increase in the heart rate but no other symptoms. BHT-COOH induced morphological abnormalities, including changes in eye size and growth, while BHT-quinol affected morphological abnormalities and eye size. Exposure to BHT-OH and BHT-Q induced delayed swim bladder development. Moreover, exposure to BHT metabolites also induced behavioral changes. The findings of this study provide a more comprehensive toxicological profile of BHT metabolites than currently available in the literature. However, further studies are required to explore changes in gene and protein expression that affect larval development. Such research would enhance our understanding of the mechanisms underlying the effects of BHT metabolites on the development, morphology, and behavior of aquatic organisms.
Materials and methods
Chemicals and reagents
BHT-OH, BHT-CHO, BHT-COOH, BHT-Q, and BHT-quinol were purchased from Sigma-Aldrich (St. Louis, MO, USA), Tokyo Chemical Industry (Tokyo, Japan), and Santa Cruz Biotechnology (Dallas, TX, USA) (Table S1). Tap water was passed through membrane filters (pore size: 5, 1, and 0.2 μm) to remove particulate matter and then through a high-grade activated carbon filter to remove organic contaminants. Filtered tap water was used as dilution water for the experiments and was aerated for at least 24 h before the experiments. Oxygen saturation was maintained above 80%, and pH was maintained at 7.0–8.0 throughout the experiments. The quality of the dilution water was periodically analyzed based on the water quality standards of the United States Environmental Protection Agency (EPA) at the Korea Testing & Research Institute (Gyeonggi-do, Republic of Korea).
Zebrafish husbandry
The animal ethics of this study were conducted in accordance with the IACUC (Institutional Animal Care and Use Committee) of the KIT (Korea Institute of Toxicology) in the Republic of Korea and the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guideline. Mature zebrafish (wild AB strain, 8–12 months) were reared at 23–25 °C (water temperature) in the fish culture room of the Environmental Safety and Risk Assessment Center, Gyeongnam Branch Institute, KIT. A 16 h/8 h light/dark photoperiod, with a 30-min dawn and dusk transition period, was maintained. The zebrafish individuals were sourced from the Bentley aqua (Goseong, Gyeongsangnam-do, Republic of Korea) and were fed Gemma® micro ZF300 (Skretting, Tooele, UT, USA) at 5% of their body weight twice in the morning and afternoon. Eggs produced through random mating of mature individuals were obtained, with fertilization rates above 70% in all experiments. The fertilized and unfertilized eggs were removed after 2, 6, and 24 h.
Acute toxicity test for zebrafish embryo
Before the acute toxicity test, the water solubility of each BHT metabolite was measured to determine the appropriate exposure concentrations52. Approximately 100 mg of each BHT metabolite was stirred in 1 L filtered tap water for 48 h and then filtered through an Omnipore™ filter paper (polytetrafluoroethylene membrane with 0.45 µm pore size; Merck Millipore, Burlington, MA, USA). The filtrate was subjected to high-performance liquid chromatography (HPLC) and liquid chromatography-tandem mass spectrometry (LC–MS/MS). Subsequently, the exposure concentrations for each BHT metabolite were established at five nominal concentrations, which were below the maximum solubility in water (Table 2).
The acute toxicity test was conducted in compliance with the Organisation for Economic Co-operation and Development Test Guideline No. 236. (No, 2013)53. Each well of a 24-well plate contained 2 mL of an exposure concentration of BHT metabolites; an embryo was placed in each well and incubated for 96 h. 3,4-Dichloroaniline (4.0 mg/L) was used as the positive control to ensure a minimum mortality of 30% at the end of the 96-h incubation period. Twenty embryos per BHT metabolite concentration were assigned to each of the control and treatment groups. Each 24-well plate was covered with a gas-permeable lid and incubated at 27 ± 1 °C with a 14/10 h light/dark photoperiod was maintained. Embryo mortality and hatching rates were recorded daily. CETIS software ver. 1.8.7.15 (Tidepool Scientific Software; Palo Alto, CA, USA) was used to calculate the lethal concentration (LC) values of the BHT metabolites using mortality as the endpoint.
Instrument conditions
An LC–MS/MS apparatus (6460 Triple Quad; Agilent Technologies, Santa Clara, CA, USA) was used to determine the concentrations of BHT-CHO, BHT-COOH, BHT-Q, and BHT-quinol in water. BHT-OH was analyzed using an HPLC–DAD (Agilent Technologies). Details of the LC–MS/MS and HPLC procedures used in this study are presented in Tables S2–6.
Exposure experiment for toxicological effects
The exposure concentrations of the BHT metabolites were determined based on the results of the acute toxicity test. The exposure concentrations of BHT-CHO and BHT-Q were 0.1 and 1.3 mg/L, respectively. The exposure concentrations of BHT-COOH were 1, 5, and 10 mg/L, and those of BHT-OH and BHT-quinol were 0.1, 1, and 2 mg/L. All experiments were conducted in triplicate. Fifty zebrafish embryos were exposed to 100 mL of the test solution (2 mL per embryo) in a 180 mL crystallizing dish for 120 h. The dishes were covered with a glass cover to minimize evaporation during the testing period. The crystallizing dishes were incubated at 27 ± 1 °C in a chamber (JSSI-300CL, JSR, Republic of Korea) with a 14/10 h light/dark photoperiod. Larval development, behavior, and toxicological parameters such as heartbeat, morphology, and locomotor activity were assessed at 120 hpf.
Heartbeat assay
To evaluate cardiac toxicity, nine larvae (three larvae were randomly selected from each of the three replicates) were randomly selected from each treatment group. The larvae were mounted on slides using 3.5% methylcellulose. The embryos were observed at 10.5 × magnification under a Leica M60 microscope (Leica, Wetzlar, Germany), and videos of the embryos were recorded for 1 min. The heartbeat observed through the videos was automatically counted using DanioScope v1.2 software (Noldus Information Technology, Wageningen, Netherlands).
Morphological observation
Nine larvae (three larvae from each of the three replicates) were randomly selected from each treatment group. The larvae were mounted onto glass slides using 3.5% methylcellulose and then observed under a Leica M60 microscope (Leica). Images of the larvae were captured and analyzed using DanioScope v1.2 software (Noldus Information Technology) to quantify the total body length, eye size, and swim bladder size of the larvae.
Locomotor activity test
Behavioral observations of the larvae were performed in a DanioVision observation chamber (Noldus Information Technology, maintained at 27 ± 1 °C using a DanioVision temperature control unit. A total of 12 larvae per treatment (4 larvae per replicate) were randomly selected from the 24-well plates. The selected larvae were acclimatized in 24-well plates (in the dark) for 10 min before the behavioral tests. The behavioral tests lasted for 20 min: two cycles of 5 min of darkness, 5 min of light, 5 min of darkness, and 5 min of light. All behavioral tests were performed between 13:00 and 16:00 to maintain the stable basal metabolic activity of the larvae54. The recorded videos of the larvae were analyzed using EthoVision XT 15 software (Noldus Information Technology) to assess the swimming distance, velocity, angular velocity, and angular turning of the larvae.
Statistical analysis
Experimental data were statistically analyzed using Jamovi version 2.6.13 (Jamovi Project, Sydney, Australia). The behavior, morphology, and heart rate of the larvae exposed to BHT metabolites were analyzed using T-tests and one-way analysis of variance, followed by Tukey’s post hoc tests. The homogeneity of variances (Levene) and normality (Shapiro–Wilk) were confirmed prior to the analysis. The differences between the treatment groups were considered statistically significant at P < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Carocho, M. & Ferreira, I. C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 51, 15–25 (2013).
Karavalakis, G., Hilari, D., Givalou, L., Karonis, D. & Stournas, S. Storage stability and ageing effect of biodiesel blends treated with different antioxidants. Energy 36, 369–374 (2011).
Porter, W., Levasseur, L. & Henick, A. Evaluation of some natural and synthetic phenolic antioxidants in linoleic acid monolayers on silica. J. Food Sci. 42, 1533–1535 (1977).
Saad, B. et al. Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chem. 105, 389–394 (2007).
Shahidi, F., Janitha, P. & Wanasundara, P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 32, 67–103 (1992).
Fernandez-Alvarez, M. et al. Photo-solid-phase microextraction of selected indoor air pollutants from office buildings. Identification of their photolysis intermediates. J. Chromatogr. A 1216, 8969–8978 (2009).
Matsuo, M., Mihara, K., Okuno, M., Ohkawa, H. & Miyamoto, J. Comparative metabolism of 3, 5-di-tert-butyl-4-hydroxytoluene (BHT) in mice and rats. Food Chem. Toxicol. 22, 345–354 (1984).
Wang, W. et al. Synthetic phenolic antioxidants and their metabolites in indoor dust from homes and microenvironments. Environ. Sci. Technol. 50, 428–434 (2016).
Liu, R. & Mabury, S. A. Synthetic phenolic antioxidants and transformation products in dust from different indoor environments in Toronto, Canada. Sci. Total Environ. 672, 23–29 (2019).
Fries, E. & Püttmann, W. Monitoring of the antioxidant BHT and its metabolite BHT-CHO in German river water and ground water. Sci. Total Environ. 319, 269–282 (2004).
Zhang, R., Li, C., Li, Y., Cui, X. & Ma, L. Q. Determination of 2, 6-di-tert-butyl-hydroxytoluene and its transformation products in indoor dust and sediment by gas chromatography–mass spectrometry coupled with precolumn derivatization. Sci. Total Environ. 619, 552–558 (2018).
Liu, R., Lin, Y., Ruan, T. & Jiang, G. Occurrence of synthetic phenolic antioxidants and transformation products in urban and rural indoor dust. Environ. Pollut. 221, 227–233 (2017).
Liu, R., Song, S., Lin, Y., Ruan, T. & Jiang, G. Occurrence of synthetic phenolic antioxidants and major metabolites in municipal sewage sludge in China. Environ. Sci. Technol. 49, 2073–2080 (2015).
Wang, X. et al. Synthetic phenolic antioxidants and their metabolites in mollusks from the Chinese Bohai Sea: Occurrence, temporal trend, and human exposure. Environ. Sci. Technol. 52, 10124–10133 (2018).
Nieva-Echevarría, B., Manzanos, M. J., Goicoechea, E. & Guillén, M. D. 2, 6-Di-tert-butyl-hydroxytoluene and its metabolites in foods. Compr. Rev. Food Sci. Food Saf. 14, 67–80 (2015).
Le Gal, K. et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 7, 308308–308308 (2015).
Nakagawa, Y. & Tayama, K. Nephrotoxicity of butylated hydroxytoluene in phenobarbital-pretreated male rats. Arch. Toxicol. 61, 359–365 (1988).
Nakagawa, Y., Tayama, K., Nakao, T. & Hiraga, K. On the mechanism of butylated hydroxytoluene-induced hepatic toxicity in rats. Biochem. Pharmacol. 33, 2669–2674 (1984).
Alofe, O. et al. Determining the endocrine disruption potential of industrial chemicals using an integrative approach: Public databases, in vitro exposure, and modeling receptor interactions. Environ. Int. 131, 104969 (2019).
Witschi, H. Enhanced tumour development by butylated hydroxytoluene (BHT) in the liver, lung and gastro-intestinal tract. Food Chem. Toxicol. 24, 1127–1130 (1986).
Oikawa, S. et al. Oxidative DNA damage and apoptosis induced by metabolites of butylated hydroxytoluene. Biochem. Pharmacol. 56, 361–370 (1998).
Nagai, F., Ushiyama, K. & Kano, I. DNA cleavage by metabolites of butylated hydroxytoluene. Arch. Toxicol. 67, 552–557 (1993).
Liang, X. et al. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. 257, 113624 (2020).
Yang, X. et al. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 643, 559–568 (2018).
Lammer, E. et al. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test?. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 149, 196–209 (2009).
McCollum, C. W., Ducharme, N. A., Bondesson, M. & Gustafsson, J. A. Developmental toxicity screening in zebrafish. Birth Defects Res. C Embryo Today Rev. 93, 67–114 (2011).
Voelker, D. et al. Differential gene expression as a toxicant-sensitive endpoint in zebrafish embryos and larvae. Aquat. Toxicol. 81, 355–364 (2007).
Sarmah, R. et al. Toxicity of a synthetic phenolic antioxidant, butyl hydroxytoluene (BHT), in vertebrate model zebrafish embryo (Danio rerio). Aquacult. Res. 51, 3839–3846 (2020).
Xie, S. et al. Benzo [a] pyrene induces microbiome dysbiosis and inflammation in the intestinal tracts of western mosquitofish (Gambusia affinis) and zebrafish (Danio rerio). Fish Shellfish Immunol. 105, 24–34 (2020).
Luo, T., Wang, X. & Jin, Y. Low concentrations of imidacloprid exposure induced gut toxicity in adult zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 241, 108972 (2021).
Wang, X., Shen, M., Zhou, J. & Jin, Y. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 216, 19–28 (2019).
Brun, N. R. et al. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ. Sci. Nano 5, 904–916 (2018).
Cook, L. W., Paradise, C. J. & Lom, B. The pesticide malathion reduces survival and growth in developing zebrafish. Environ. Toxicol. Chem. Int. J. 24, 1745–1750 (2005).
Jablonski, C. A. et al. Acute toxicity of methomyl commercial formulation induces morphological and behavioral changes in larval zebrafish (Danio rerio). Neurotoxicol. Teratol. 89, 107058 (2022).
Liu, X. et al. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 199, 16–25 (2018).
Lindsey, B. W., Smith, F. M. & Croll, R. P. From inflation to flotation: contribution of the swimbladder to whole-body density and swimming depth during development of the zebrafish (Danio rerio). Zebrafish 7, 85–96 (2010).
Robertson, G. N., Lindsey, B. W., Dumbarton, T. C., Croll, R. P. & Smith, F. M. The contribution of the swimbladder to buoyancy in the adult zebrafish (Danio rerio): A morphometric analysis. J. Morphol. 269, 666–673 (2008).
Wang, J. et al. Perfluoropolyether carboxylic acids (novel alternatives to PFOA) impair zebrafish posterior swim bladder development via thyroid hormone disruption. Environ. Int. 134, 105317 (2020).
Yang, L., Ivantsova, E., Souders, C. L. II. & Martyniuk, C. J. The agrochemical S-metolachlor disrupts molecular mediators and morphology of the swim bladder: Implications for locomotor activity in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 208, 111641 (2021).
Li, X. et al. Exposure to water-accommodated fractions of two different crude oils alters morphology, cardiac function and swim bladder development in early-life stages of zebrafish. Chemosphere 235, 423–433 (2019).
Peng, W., Liu, S., Guo, Y., Yang, L. & Zhou, B. Embryonic exposure to pentabromobenzene inhibited the inflation of posterior swim bladder in zebrafish larvae. Environ. Pollut. 259, 113923 (2020).
Yang, L. et al. Evaluation and comparison of the mitochondrial and developmental toxicity of three strobilurins in zebrafish embryo/larvae. Environ. Pollut. 270, 116277 (2021).
Stainier, D. Y., Lee, R. K. & Fishman, M. C. Cardiovascular development in the zebrafish I. Myocardial fate map and heart tube formation. Development 119, 31–40 (1993).
Li, H. et al. Developmental toxicity and potential mechanisms of pyraoxystrobin to zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 151, 1–9 (2018).
Antkiewicz, D. S., Burns, C. G., Carney, S. A., Peterson, R. E. & Heideman, W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 84, 368–377 (2005).
Chen, J. Impaired cardiovascular function caused by different stressors elicits a common pathological and transcriptional response in zebrafish embryos. Zebrafish 10, 389–400 (2013).
Legradi, J., El Abdellaoui, N., Van Pomeren, M. & Legler, J. Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity. Environ. Sci. Pollut. Res. 22, 16277–16289 (2015).
Granato, M. et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399–413 (1996).
Selderslaghs, I. W., Hooyberghs, J., De Coen, W. & Witters, H. E. Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotoxicol. Teratol. 32, 460–471 (2010).
Shi, Z., Liang, X., Zhao, Y., Liu, W. & Martyniuk, C. J. Neurotoxic effects of synthetic phenolic antioxidants on dopaminergic, serotoninergic, and GABAergic signaling in larval zebrafish (Danio rerio). Sci. Total Environ. 830, 154688 (2022).
Ogungbemi, A., Leuthold, D., Scholz, S. & Küster, E. Hypo-or hyperactivity of zebrafish embryos provoked by neuroactive substances: a review on how experimental parameters impact the predictability of behavior changes. Environ. Sci. Europe 31, 1–26 (2019).
OECD. Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures. Series on Testing and Assessment No. 23. Organ. Econ. Co-operation Dev. 23, 1–81 https://doi.org/10.1787/0ed2f88e-en. (2019).
OECD, T. N. 236: Fish embryo acute toxicity (FET) test. OECD Guidelines for the Testing of Chemicals, Section 2, 1–22 https://doi.org/10.1787/9789264203709-en. (2013).
Chiffre, A. et al. Psychotropic drugs in mixture alter swimming behaviour of Japanese medaka (Oryzias latipes) larvae above environmental concentrations. Environ. Sci. Pollut. Res. 23, 4964–4977 (2016).
Funding
This work was funded by Korea Institute of Toxicology, 2710008768.
Author information
Authors and Affiliations
Contributions
Won Noh: Writing- Original Draft Preparation, Methodology, Data Curation and Editing; Yeong-Jin Kim and Sung-Gil Choi: Validation and Analysis; Jin-Woo Park and Ji-Young An: Resources; Jong-Hwan Kim and Jong-Su Seo: Supervision, Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Noh, W., Kim, YJ., Choi, SG. et al. Developmental and toxicological effects of butylated hydroxytoluene metabolites on zebrafish larvae. Sci Rep 15, 7911 (2025). https://doi.org/10.1038/s41598-025-91409-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91409-x
Keywords
This article is cited by
-
Butylated hydroxytoluene (BHT) induces zebrafish spinal cord defects and scoliosis by inhibiting the hedgehog pathway
Scientific Reports (2025)
-
A Review of Toxicological and Exposure Data on Polymeric Antioxidant By-products
Reviews of Environmental Contamination and Toxicology (2025)