Abstract
Cocaine-use disorder (CUD) affects both structure and function of the brain. A triple network model of large-scale brain networks has been useful for identifying aberrant resting-state functional connectivity (rsFC) associated with mental health disorders including addiction. The present study investigated differences between people with CUD vs. controls (CONs) and whether putative differences were associated with drug-use outcomes. Participants with CUD (n = 38) and CONs (n = 34) completed a resting functional magnetic resonance imaging (fMRI) scan. Participants with CUD completed several mental health measures and participated in an 8-week, drug-use outcomes phase. A classification framework based on the triple network model was built, and triple networks (salience [SN], executive control [ECN], default mode [DMN]) and subcortical (striatum [ST], hippocampus/amygdala) regions were identified with the algorithm of group-information-guided independent components analysis (GIG-ICA) and subsequent support-vector machines. This classifier achieved 77.1% accuracy, 73.8% sensitivity, and 80.0% specificity, with an area under the curve of 0.87 for distinguishing CUD vs. CON. The two groups differed in SN-anterior DMN (aDMN) and ECN-aDMN rsFC, with the CUD group exhibiting stronger rsFC compared to CONs. They also differed in rsFC between several subcortical and triple networks, with CUD generally showing a lack of rsFC. Within the CUD group, ST-aDMN and ST-rECN rsFC were associated with differential drug-use outcomes. Exploratory results suggested SN-aDMN rsFC was associated with anxiety symptoms. These results add to the growing literature showing aberrant triple network and subcortical rsFC associated with substance use disorders. They suggest the aDMN specifically may underlie important differences between people with CUD and CONs and may be a potential target for intervention.
Similar content being viewed by others
Introduction
Cocaine use remains a critical public health issue in the United States of America. The most recent National Survey on Drug Use and Health1 indicates that in 2022, 5.3 million used cocaine, 509,000 initiated cocaine use, and 1.4 million experienced cocaine-use disorder (CUD). The number of deaths associated with cocaine before 2016 was relatively low; however, as synthetic opioids (e.g., fentanyl) infiltrated the cocaine supply, cocaine-related deaths rose by ~ 260% to over 24,000 in 20222.
CUD affects both the structure and function of the brain. Structurally, CUD is associated with decreases in gray matter volume3, white matter volume4, and cortical thickness5. Functionally, CUD is associated with prefrontal and subcortical dysfunction that is linked to cognitive deficits6,7, reward dysfunction in dopaminergic pathways8, and hyper-activity to craving-inducing drug cues9. Resting-state functional connectivity (rsFC) studies have revealed CUD is associated with aberrant rsFC within and between neural networks10,11,12. The direction of rsFC may be either positive or negative depending on whether the networks modulate in the same direction (i.e., activate together and deactivate together) or opposite directions (i.e., one activates as the other deactivates), respectively13. Both positive and negative type rsFC are considered fundamental aspects of brain organization that support cognitive processes14.
With widespread effects in the brain linked to cocaine use and CUD, an investigation into the interactions between large-scale networks of functionally coherent neural activity may facilitate a systems-level understanding of a brain chronically exposed to cocaine15. Large-scale networks refer to neural systems that contribute to a common set of functions but are distributed across the brain, which contrasts with focus on functionally isolated single regions. Disruptions to large-scale networks have been associated with psychiatric and neurological disorders, including bipolar, schizophrenia, depression, and obsessive-compulsive disorders16,17. Three well-established cortical networks include the default-mode network (DMN), consisting of medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC); the executive control network (ECN), made up of dorsolateral prefrontal (dlPFC) and parietal cortices; and the salience network (SN), including insula and anterior cingulate cortex, which have been summarized as a unifying triple network in psychopathology18. This ‘triple network’ model proposes that differential interactions (and imbalance) between SN, DMN, and ECN underlie several psychiatric and neurological disorders. Essentially, weak connections between SN and ECN result in stronger connections between SN and DMN, and is associated with reward, cognitive, and emotional dysregulation19.
The triple network model has been applied to substance-use disorders, including nicotine19, alcohol20, opioids21, and cocaine22,23,24,25. Since addiction is also characterized by dysfunction in the subcortical reward network, addiction studies that employ the triple network model have also incorporated subcortical brain regions26, including the striatum (ST) and medial temporal lobe (MTL) composed of amygdala, hippocampus, and parahippocampus. These previous studies have found network differences between people with addiction and control populations, particularly demonstrating an imbalance of SN-DMN and SN-ECN rsFC21,22,23,24,25,27,28. Connectivity of SN-DMN rsFC in those with addiction governs internal negative emotional states, like craving; in contrast, SN-ECN connectivity is linked to executive control, which tends to be diminished in those with addiction19. Compared to controls, people with CUD have exhibited aberrant SN-DMN, SN-MTL, and DMN-ECN rsFC22,23,24. Pertaining to clinical outcomes, data is emerging on the utility of large-scale network connectivity in predicting clinical outcomes22,29,30,31. For example, one study showed increased SN-ECN rsFC was associated with better outcomes30, while another showed that increased SN-DMN rsFC was associated with worse outcomes31. One of the important aspects of this research related to differential rsFC in those with addictions is that these networks may be targetable with interventions, such as transcranial magnetic stimulation, psychedelics, mindfulness, or more conventional methods (e.g., behavioral therapy).
Less of an emphasis has been placed on the role of anterior (aDMN) vs. posterior DMN (pDMN) in these large-scale networks, and much of the data on CUD has been collected from individuals who are not inpatient and not treatment-seeking (but see references26,30). In addition, data on the importance of large-scale rsFC as it relates to outcomes is still emerging. The primary aim of the study was to investigate differences in large-scale network rsFC between inpatient treatment-seeking individuals with CUD vs. controls (CONs). Secondarily, we then sought to examine possible differences in rsFC in relation to drug use outcomes and mental health symptoms. Based on prior data, we expected to find altered connectivity in CUD (vs. CONs) that would reflect reduced executive control (reduced SN-ECN rsFC); more internal rumination (increased SN-DMN rsFC; reduced DMN-ECN rsFC); and aberrant reward connectivity (e.g., reduced ST-ECN rsFC). To test these hypotheses, we built a classification framework based on the triple network model. The algorithm of group information guided independent components analysis (GIG-ICA)32 was used to identify the triple network components (aDMN and pDMN, left [lECN] and right [rECN] ECN, and SN) as well as two subcortical areas: ST and MTL. We then compared rsFC within and between these networks in CUD vs. CON groups, investigated rsFC associations with clinical outcomes, and explored rsFC relationships with mental health symptoms.
Results
Demographic information
Participants (CUD [n = 34], CONs [n = 38]) were matched on age (CUD: mean = 45.5 [standard error of the mean = ± 6.1]; CONs: 43.8 [± 3.8], p = 0.16), sex (CUD: 34 males, no females; CONs: 38 males, no females, p = 1.0], and head motion (CUD: 0.21 [± 0.12]; CONs: 0.17 [± 0.08], p = 0.07).
CUD participants also provided additional data, including years of cocaine (17 [± 8.69]) and alcohol (16.5 [± 13.19]) use; history of cannabis (48.5%) and heroin (3%) use; history of prior emotional, physical, and/or sexual abuse (54.5%); and mental health symptoms (Beck’s Depression Index - II = 9.33 [± 8.23]; Beck’s Anxiety Index = 6 [± 6.19]). The Mini-International Neuropsychiatric Interview (using DSM-IV criteria) found that 26% (8/34) and 16% (5/34) of CUD individuals also had alcohol and/or marijuana “dependence”, respectively.
Classification based on DMN/SN/ECN
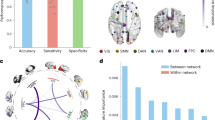
Using the GIG-ICA method, the brain was divided into 25 functional networks (FNs). The DMN, the SN, and the ECN were identified with both visual inspection and automatic template matching with group ICA maps (Fig. 1). Among the 25 FNs, two aligned with aDMN and pDMN. The aDMN includes primarily the mPFC, and the pDMN includes primarily the posterior cingulate cortex. One FN aligned with the SN, which includes the anterior cingulate cortex and insula. Two FNs aligned with the rECN and lECN, which include primarily dlPFC and dorsal lateral parietal cortex. The support vector machine (SVM) classification model was based on the triple network to classify CUD vs. CON groups. This classifier with 10-fold cross-validation (repeated 10 times) achieved 77.1%± standard deviation = 1.7% accuracy, 73.8%±1.7% sensitivity, and 80.0%±2.2% specificity. The area under the curve (AUC) was 0.87 ± 0.01 (Fig. 2).
Seven of the twenty-five spatial maps of function networks obtained by GIG-ICA. Spatial maps were obtained via one-sample t test of independent components (z-score maps) of all participants (p < 0.05, FWE corrected). FN functional network, ECN executive control network, DMN default mode network, SN salience network, ST striatal regions, MTL medial temporal lobe.
Group differences (CONs vs. CUD) in RsFC among triple network FNs
CUD and CON groups differed in rsFC between two sets of triple networks (see Fig. 3; Table 1 for full stats). The groups differed in SN-aDMN rsFC, with the CUD group having stronger (and significantly positive) rsFC (t (68) = − 3.05, p = 0.003; Hedges g = 0.72) compared to the (non-significant) rsFC in CONs. They also differed in rECN-aDMN rsFC, with the CUD group having stronger (and significantly positive) rsFC (t (68) = − 2.81, p = 0.005; Hedges g = 0.67) compared to the (significantly positive) rsFC in the CONs. Both differences survived FDR correction. Full stats for one-sample t-tests (showing positive or negative triple network rsFC) are shown in Supplemental Tables S1-S2.
Resting State functional connectivity (rsFC) within the triple network, including the large-scale functional salience network (SN), default-mode network (anterior [aDMN] and posterior [pDMN]), and executive control network (left [lECN] and right [rECN]) between individuals with cocaine-use disorder (CUD) vs. controls (CONs). The CUD group showed significantly stronger positive rsFC among two pairs of networks: SN-aDMN and aDMN-rECN. *Significant difference between CON vs. CUD groups.
Group differences (CON vs. CUD) in RsFC among subcortical regions and triple networks
The ST and MTL were identified with both visual inspection and automatic template matching with group ICA maps (Fig. 1). The CUD and CON groups differed in four sets of subcortical regions and triple networks (see Fig. 4; Table 2). The groups differed in MTL-aDMN rsFC, with the CON group having stronger (and significantly positive) rsFC compared to the (non-significant) CUD rsFC (t (68) = 2.75, p = 0.009; Hedges g = 0.65). They differed in ST-aDMN rsFC, with the CON group having stronger (and significantly negative) rsFC compared to the (non-significant) CUD rsFC (t (68) = − 2.23, p = 0.036; Hedges g = 0.53). They differed in ST-lECN rsFC, with the CON group having stronger (and significantly negative) rsFC compared to the (non-significant) CUD rsFC (t (68) = − 2.61, p = 0.007; Hedges g = 0.62). Finally, they differed in ST-rECN rsFC, with the CON group having stronger (and significantly negative) rsFC compared to the (non-significant) CUD rsFC (t (52.3) = 2.42, p = 0.021; Hedges g = − 0.58). Results involving the MTL-aDMN and ST-lECN survived FDR correction, while the ST-aDMN and ST-rECN results did not. Full stats for one-sample t-tests (showing positive or negative rsFC between subcortical regions and triple networks) are shown in Supplemental Tables S3-S4.
Resting state functional connectivity (rsFC) between subcortical networks and triple networks, including striatum (ST), amygdala/hippocampal complex (i.e., medial temporal lobe [MTL]), as well as the large-scale functional networks: salience (SN), default-mode (anterior [aDMN] and posterior [pDMN]), and executive control (left [lECN] and right [rECN]) networks. These were compared between individuals with cocaine-use disorder (CUD) vs. controls (CONs). The CUD group showed significantly different rsFC among the following pairs of regions/networks: ST-aDMN, ST-lECN, ST-rECN, and MTL-aDMN. *Significant difference between CON vs. CUD groups.
Secondary: association with drug-use outcomes
Between network rsFC differences between the CON vs. CUD groups were tested for associations with clinical outcomes. Subgroups within the CUD group that had differing levels of drug-use outcomes were classified via hierarchical clustering, with three outcome groups identified (see methods, High, Low, and Intermediate drug-use outcomes). One-way ANOVA showed significant differences between CON and drug-use groups (total = 4 groups: Control, Low, Intermediate, and High) in aDMN-rECN rsFC (F(3,66) = 2.85, p = 0.044, η2 = 0.12, CI95 [0 0.24]), SN-aDMN rsFC (F(3,66) = 4.49, p = 0.006, η2 = 0.17, CI95 [0.02 0.30]), ST-aDMN (F(3,66) = 3.61, p = 0.018), and ST-rECN rsFC (F(3,66) = 4.31, p = 0.008, η2 = 0.17, CI95 [0.01 0.30]). SN-aDMN, ST-aDMN, and ST-rECN survived FDR correction. Post-hoc tests, Tukey corrected, revealed the “High” group had significantly stronger rsFC compared to CONs for SN-aDMN (CONs vs. High, p = 0.01), ST-aDMN (CONs vs. High, p = 0.009), and ST-rECN rsFC (CONs vs. High, p = 0.004) (Fig. 5).
Associating resting state functional connectivity (rsFC) with clinical outcomes comparing controls (CONs) and subgroups within the cocaine use disorder group (CUD) based on drug use (High, Low, Intermediate). The group with “High” drug-use outcomes showed significantly difference rsFC (compared to CONs) among the following pairs of networks/regions: SN-aDMN, ST-aDMN, and ST-rECN. ST striatum, a/pDMN anterior/posterior default mode network, MTL medial temporal lobe, SN salience network, l/rECN left/right executive control network. #Significant main effect of group. *Significance difference between CUD subgroup and the CON group.
Exploratory: correlation of RsFC with other drug use outcomes and mental health variables
Between-network differences between groups were explored for relationships with prior years of drug use, depression symptoms, and anxiety symptoms (Supplementary Table S5). SN-aDMN rsFC significantly correlated with the Beck Anxiety Inventory (BAI) in the CUD group (r (33) = 0.39, p = 0.024), a result that did not survive FDR correction (Supplementary Fig. S1).
Discussion
The present study found differential rsFC between people with CUD and CONs both within the triple network model and between the triple networks and subcortical regions (i.e., striatum, amygdala, and hippocampus) using complementary statistical analyses. The pattern classification analysis demonstrated that the networks of the triple network model provided discriminative information to distinguish CUD and CON with an AUC of 0.87, and voxel-wise comparisons highlighted specific network rsFC differences between CUD and CON groups. The primary differences in the triple network occurred between the aDMN and other networks, specifically with CUD (vs. CONs) showing medium sized effects of increased SN-aDMN and aDMN-rECN rsFC. Cohen’s U3 showed that SN-aDMN and aDMN-rECN rsFC was greater than the CON group means in approximately 75% and 76% of the CUD group, respectively. Between subcortical structures and the triple network, participants with CUD generally had a lack of rsFC and differed significantly from CONs for ST-aDMN, ST-ECN (both left and right), and MTL-aDMN rsFC. These effects were all medium in size, with Cohen’s U3 showing that 74% of the CUD group had MTL-aDMN rsFC greater than the CON group mean, while 70–73% of the CUD group had rsFC lower than the CON group mean for aDMN-ST, ST-lECN, and ST-rECN. Across types of analyses, our primary findings highlight consistent differences between the CUD and CON groups in large-scale network connectivity, especially involving the aDMN (but not pDMN). Secondary results found differential rsFC involving ST and aDMN was associated with worse drug-use outcomes. Exploratory results suggest higher SN-aDMN rsFC may correlate with more anxiety symptoms, though more data is needed.
Aberrant within and between network rsFC involving the DMN is common among many addictions. A recent review summarized findings in the SUD population and found that, generally, long-term drug-use is associated with disrupted dynamics between the DMN, SN, and ECN; and cessation of substances (and accompanying withdrawal) seems to correspond with increased rsFC between the SN and DMN but decreased rsFC within the DMN and between the ECN and DMN33. However, another study found a decrease in rsFC between the SN and DMN23, results which may have been due to differences in methodological approaches (e.g., nicotine use prior to scan) and/or population (e.g., non-treatment seeking). Using nicotine (e.g., smoking cigarettes) has been shown to shift rsFC away from SN-DMN and toward SN-ECN19. Disruptions between the SN and DMN may facilitate increased sensitivity to internal sensations, stress, and negative emotions, resulting in cravings, while disruptions between the DMN and ECN may interfere with cognitive control over internal rumination and drug craving, impeding decision-making33. The present results indicate the importance of the anterior DMN, as results showed differential rsFC involving the aDMN (but not pDMN), contrary to findings from previous studies33. Research distinguishes functionality of the aDMN and pDMN, with processes related to regulation/inhibition and interoception, respectively. In this study, discrepant results may be due to several factors, including timing of substance cessation, opportunity to use nicotine prior to the scan, and role of aDMN.
The present study suggests aberrant rsFC in CUD between subcortical regions and other triple networks (Fig. 4, Supplemental Table S3). With the exception of the MTL-aDMN rsFC, the CON group displayed primarily negative rsFC between subcortical regions and triple networks (Fig. 4, Supplemental Table S4), and the CUD lacked this inverse relationship in MTL-aDMN rsFC as well as both ST-aDMN and ST-rECN rsFC. Together, the lack of rsFC in CUD and the significant negative rsFC in CONs between subcortical and triple networks resulted in significant group differences. Results may be related to interference of reward-seeking on self-focused or executive function and impact decision-making related to substance use33,34. A recent meta-analysis in substance-use disorder found reduced rsFC between subcortical regions and both DMN and ECN regions, with results primarily driven by CUD studies26. Reduced rsFC between subcortical and cortical networks may be indicative of impaired decision-making and regulation of negative affect33,35. In contrast, one study found that CUD was associated with increased connectivity between the ST and pre-frontal cortical regions; a result driven by differential trait impulsivity36.
Our secondary results showed differential connectivity involving ST and aDMN was associated with drug-use outcomes (i.e., percent positive UDS), in that participants with CUD and “High” drug use outcomes had significantly different (stronger positive) rsFC compared to CONs in SN-aDMN, ST-aDMN, and ST-rECN. Those with “Low” drug-use outcomes generally had more negative rsFC, which was similar to CONs. To our knowledge, this is the first paper to show that a differential rsFC between subcortical regions and aDMN or rECN may be predictive of worse drug-use outcomes. Results may indicate that negative ST-aDMN rsFC protects against drug use motivation by preventing interference of ST reward activation during self-focused DMN activity. Exploratory analyses found a positive (uncorrected) correlation between increased SN-aDMN rsFC and higher concurrent anxiety. If this exploratory finding were to be confirmed by future studies, it may align with studies showing altered SN-DMN rsFC in anxiety disorders37,38, perhaps reflecting impairment when goal directed attention (driven by the SN) and self-focused attention (driven by the aDMN) interfere with each other39,40.
Present results are generally in line with previous literature showing aberrant SN and/or DMN rsFC associating with worse clinical outcomes30,31, suggesting aberrant rsFC involving the DMN may be tied to craving, relapse, and increased drug-cue reactivity33. In addition, studies have found that increased (positive) rsFC between nodes in the SN with nodes in the DMN was associated with opioid relapse21, but rsFC between these same nodes were decreased when stabilized on methadone27. Other examples in the addiction literature have demonstrated negative connectivity associated with cocaine-use outcomes, including one that reported enhanced negative connectivity between nodes in aDMN and nodes in the ECN, suggesting impairment in cognitive control networks even after four weeks of abstinence24.
Targeting regions within the aDMN (e.g., mPFC) may be a viable intervention for CUD. For example, studies have shown that stimulation (e.g., via transcranial magnetic stimulation) of the mPFC decreases cocaine use41 and affects rsFC42. Additional lines of research examining changes following cognitive behavioral therapy (CBT) have demonstrated decreased activations in mPFC and treatment related changes within DMN and SN43. As CBT approaches have evidenced efficacy for the treatment of anxiety disorders44, and findings indicate CBT modulates FC between DMN, nodes within the SN and other neural networks45,46, components of CBT may complement approaches aimed at reducing the anxiety-linked SN-aDMN rsFC suggested by the present study. Emerging evidence suggests classic psychedelic (e.g., psilocybin) interventions may alter rsFC in large-scale networks47,48, which could address the disrupted FC patterns associated with CUD25.
There are several limitations to the present study. For example, this was a secondary analysis, utilizing datasets from separate CUD and CON studies. The CUD data were collected over the span of a decade from individuals with CUD in the local region, who historically have primarily consisted of males; thus, application to other populations may be limited. The CON group was obtained from an existing dataset, and thus some information was limited. Future studies corroborating results could strengthen findings by ensuring identical, contemporaneous data-collection procedures for both groups. On average, 38% of participants were retained at the end of the 8-week outcome phase, and approximately 34% of urine samples were missed. In this paper, and in the field generally, missing UDS are conventionally scored as ‘positive’, as the reason for missed appointments is often related to drug use (e.g., see49). Additionally, CUD participants were allowed to use nicotine (e.g., smoke cigarettes) before the scan; however, we do not have additional information about which participants specifically used nicotine and exactly when. In addition, there is no clear consensus on how to interpret negative rsFC, as it may relate to factors such as activation in opposite directions50, spatial distance and lack of efficiency51, and/or methodology52. Finally, the sample size is relatively small, and therefore a 10-fold cross-validation (CV) strategy was adopted to maximize the use of available data for both training and validation, with a risk of overfitting. Larger datasets (e.g., ABCD, Human Connectome Project) may be used in future studies to test replicability of findings and evaluate the classification performance with independent test datasets.
In conclusion, this study used pattern classification of large-scale brain networks to distinguish CUD and CON groups, with the most robust differences involving the aDMN, including SN-aDMN and aDMN-rECN rsFC. In addition, individuals with CUD generally had reduced rsFC between the triple network and subcortical regions, including ST-aDMN, ST-ECN, and MTL-aDMN. Secondary results found differences between drug-use groups, with high cocaine use associating with higher (positive) ST-aDMN, ST-rECN, and SN-aDMN rsFC compared to CONs (who showed lower negative rsFC). Further, exploratory results suggest higher (positive) SN-aDMN rsFC correlates with higher anxiety scores. These results add to the growing literature showing aberrant triple network and subcortical rsFC is associated with substance use disorders and suggest aDMN rsFC as an important underlying neural correlate of CUD and potential target for intervention.
Methods
Participants
This secondary analysis utilized data from 50 with CUD who were treatment-seeking and willing to go inpatient for 7–10 days. At the end of the inpatient stay, participants underwent a functional magnetic resonance imaging (fMRI) session, followed by an eight-week outpatient phase. In addition, 52 CONs were selected from a previous dataset to be used as a comparator group53. Inclusion/exclusion criteria for CUD have been described previously54. In total, 38 CONs and 34 with CUD were included in the analysis based on the following criteria: participants had structural MRI data and resting-state fMRI data; head motion was minimal (see data preprocessing), resulting groups were matched on age, sex, and head motion; Individuals with a current DSM diagnoses (other than substance-use disorder in CUD), lifetime history of head injury with loss of consciousness for more than 3 min, contraindications for MRI, and clinically significant medical conditions were excluded. This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Pennsylvania Institutional Review Board. The participants provided informed written consent to participate in the study.
Behavioral and outcomes measures
The CUD group completed mental health questionnaires at baseline (approximately 7–10 days prior to scanning), including the Beck Depression Inventory - II (BDI-II)55 and the BAI56. In addition, participants were asked to visit the study center twice per week for eight weeks to submit a UDS. Percent positive UDS (for cocaine) was calculated by counting the number of positive or missing UDS and dividing by the total number of opportunities (n = 16) to submit a UDS.
fMRI data acquisition
A 3 Tesla SIEMENS MR scanner (Erlangen, Germany) was used to obtain the structural MRI data (T1-weighted images) and resting-state fMRI data of all participants. The resting scan for each group followed standard procedures and was approximately 5 min in duration. Participants were instructed to recline in the scanner gantry with their heads stabilized by foam padding and to close their eyes (but not fall asleep). The scanner parameters for CUD: T1-weighted images were: TR = 1620 ms; TE = 3.87 ms; FA = 15 degrees; matrix = 192 × 256; slice thickness = 1 mm; and 160 slices. The resting-state fMRI data were scanned by the following parameters: TR/TE = 2000/30 ms; FOV = 220 × 220 mm; matrix = 64 × 64; FA = 90°; slice thickness = 4.5 mm; 32 interleaved even slices; and 150 volumes. For the CONs, resting-state fMRI data were acquired using a gradient echo echo-planar imaging sequence (FOV = 220 mm, 64 × 64 × 64 matrix, slice thickness = 4 mm, TR = 2s, TE = 24ms, 150 volumes with no inter-slice gap.
Data preprocessing
All T1-weighted images were performed with bias correction and segmentation to transform resting-state fMRI data from native space to standard space. The bias correction was implemented by an improved N3 algorithm57. The segmentation of T1-weighed images was implemented by DARTEL toolbox in Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm) software. Then, we conducted the preprocessing of resting-state fMRI data using SPM 12 and AFNI (https://afni.nimh.nih.gov/). Considering the magnetization equilibration, the first 6 time points of each time series were removed. The remaining fMRI volumes underwent de-spiking, slice correction, and head realignment. In order to reduce the effect of head motion, fMRI data with excessive head motion were excluded, i.e., (1) translational or rotational motion parameters were over 2 mm or 2° and (2) root mean square of framewise displacement (RMSFD) was over 0.5 mm. The intensity of each resting-state fMRI was scaled to make the mean value of the whole brain 1,000. The nuisance covariate effects of the white matter and cerebrospinal fluid, as well as 6 rigid head motion parameters, were removed. Subsequently, temporal band-pass filtering (0.01 < f < 0.08 Hz) was performed, and the fMRI data were normalized to the Montreal Neurological Institute (MNI) space via the segmented results of T1-weighted images. The normalized fMRI data were then resampled to 3 mm × 3 mm × 3 mm voxels. The Gaussian kernel with a full-width at half-maximum (FWHM) of 6 mm was used to smooth the data.
Identification of networks and classification
GIG-ICA was applied to compute the specific independent components of each participant with correspondence across all participants32. First, the tool Melodic (default setting) in FSL was used to compute the group template with 25 independent components (referred to as functional networks [or FNs] throughout) based on the preprocessed resting-state fMRI data of both the CON and CUD groups. Then the group template was used as a reference to calculate participant specific independent components and their corresponding time series with a multi-objective optimization solver. Subsequently, 7 FNs (ST, MTL, SN, aDMN, pDMN, lECN, and rECN) were identified with both visual inspection and automatic template matching with group ICA maps (Fig. 1).
A classifier based on the triple network model was built to differentiate the CUD group from the CON group. The FNs of these three resting-state networks were used as the basis for a linear subspace and were analyzed on the Grassmann manifold to calculate Riemannian distance. Networks were mapped on the Grassmann manifold58 to construct a subspace distance metric, which implemented the whole network as the feature in the classification59. Then, the Riemannian distance was used in conjunction with a support vector machine (SVM) to build the classifier53,60,61. A 10-fold CV was used to evaluate the performance of the classifier. Specifically, all participants were randomly divided into 10 subsets with equal or almost equal size. These 10 subsets were used in 10 training-testing runs. In each run, one of the 10 subsets was used as the testing set as the other nine subsets were used as the training set. The training-testing runs were repeated until all 10 subsets had been used as the testing set. Then, the accuracy of the classification, the sensitivity, and the specificity were calculated based on the 10 training-testing runs. A receiver operating characteristic (ROC) curve and the AUC were also computed based on classification scores of all participants. In order to avoid statistical bias, the 10-fold CV was repeated 10 times.
Statistical analysis
A voxel-wise rsFC map of each FN was obtained by computing the Pearson correlation coefficient between the time series of the FN and that of each voxel in the gray matter and then transformed using Fisher’s z-transformation. Such comparisons of rsFC maps enable identification of voxel-wise differences in FNs between the CON and CUD groups, complementary to the patten classification study that compares multiple FNs jointly. Then, two-sample t tests, with percentile bootstrapping (1,000 samples) and 95% confidence intervals (CI), were conducted to compare the rsFC map of each FN between the CON and CUD groups. Statistics are reported in Tables 1 and 2 (and Supplementary Tables S1-S4), including t values, p values, mean differences, confidence intervals, and Hedges g. Significant differences between the CON and CUD groups were tested for associations with mental health and drug use outcome variables. Subgroups within the CUD group that had differing levels of drug-use outcomes were classified via hierarchical clustering, with three outcome groups identified. Those with urine drug-screen (UDS) scores that averaged < 40% positive were labeled as the relatively “Low” drug-use group (n = 9); those with average UDS scores of > 85% positive were labeled as the relatively “High” drug-use group (n = 13); and those that fell in between 40% and 85% were labeled as the “Intermediate” (Int) drug-use group (n = 12). Total sum scores for the BAI and BDI-II were calculated and used as continuous measures. Spearman’s correlation was used to test associations between rsFC and outcome variables, because the drug-use, mental health, and behavioral variables were non-normally distributed. Though our significance threshold was set at p < 0.05 and we report FDR correction results, we opted for results to be presented primarily via estimation methods both to move away from “null hypothesis significance testing” and to highlight the importance of effect sizes and confidence intervals62. Interpretation of effect sizes was aided by reporting proportion of distribution overlap between groups63.
Data availability
The data that support the findings of this study are available from the corresponding and senior authors upon reasonable request.
References
Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2022 National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, 2023).
Spencer, M., Garnett, M. & Miniño, A. Drug Overdose Deaths in the United States, 2002–2022 (National Center for Health Statistics (U.S.), 2023).
Dang, J. et al. Meta-analysis of structural and functional brain abnormalities in cocaine addiction. Front. Psychiatry 13, 1 (2022).
Narayana, P. A. et al. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 221, 220–230 (2014).
Schinz, D. et al. Lower cortical thickness and increased brain aging in adults with cocaine use disorder. Front. Psychiatry 14, 1 (2023).
Meade, C. S., Conn, N. A., Skalski, L. M. & Safren, S. A. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J. Behav. Med. 34, 128–138 (2011).
Verdejo-Garcia, A. New insights on neurocognition in cocaine use disorder. Curr. Behav. Neurosci. Rep. 5, 232–237 (2018).
Ashok, A. H., Mizuno, Y., Volkow, N. D. & Howes, O. D. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry 74, 511–519 (2017).
Childress, A. R. et al. Limbic activation during cue-induced cocaine craving. AJP 156, 11–18 (1999).
Li, S. J. et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn. Reson. Med. 43, 45–51 (2000).
Kelly, C. et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry 69, 684–692 (2011).
Ding, X. & Lee, S-W. Cocaine addiction related reproducible brain regions of abnormal default-mode network functional connectivity: A group ICA study with different model orders. Neurosci. Lett. 548, 110–114 (2013).
Uddin, L. Q., Kelly, A. M., Biswal, B. B., Castellanos, F. X. & Milham, M. P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 30, 625–637 (2009).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102, 9673–9678 (2005).
van den Heuvel, M. P. & Hulshoff Pol, H. E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534 (2010).
Banich, M. T. et al. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci. Biobehav. Rev. 33, 613–630 (2009).
Ongür, D. et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 183, 59–68 (2010).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Sutherland, M. T., McHugh, M. J., Pariyadath, V. & Stein, E. A. Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage 62, 2281–2295 (2012).
Suk, J-W., Hwang, S. & Cheong, C. Functional and structural alteration of default mode, executive control, and salience networks in alcohol use disorder. Front. Psychiatry 12 (2021).
Li, Q. et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J. Psychiatry Neurosci. 43, 48–57 (2018).
Geng, X. et al. Salience and default mode network dysregulation in chronic cocaine users predict treatment outcome. Brain 140, 1513–1524 (2017).
Liang, X. et al. Interactions between the salience and default-mode networks are disrupted in cocaine addiction. J. Neurosci. 35, 8081–8090 (2015).
Martins, D. L. N. et al. Extrinsic functional connectivity of the default mode network in crack-cocaine users. Radiol. Bras. 51, 1–7 (2018).
Zhai, T., Gu, H., Salmeron, B. J., Stein, E. A. & Yang, Y. Disrupted dynamic interactions between large-scale brain networks in cocaine users are associated with dependence severity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 8, 672–679 (2023).
Taebi, A. et al. Shared network-level functional alterations across substance use disorders: A multi-level kernel density meta-analysis of resting-state functional connectivity studies. Addict. Biol. 27, e13200 (2022).
Ma, N. et al. Addiction related alteration in resting-state brain connectivity. Neuroimage 49, 738–744 (2010).
Ma, N. et al. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS ONE 6, e16560 (2011).
Lerman, C. et al. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71, 523–530 (2014).
McHugh, M. J., Gu, H., Yang, Y., Adinoff, B. & Stein, E. A. Executive control network connectivity strength protects against relapse to cocaine use. Addict. Biol. 22, 1790–1801 (2017).
Reese, E. D. et al. Triple network resting state connectivity predicts distress tolerance and is associated with cocaine use. J. Clin. Med. 8, 2135 (2019).
Du, Y. & Fan, Y. Group information guided ICA for fMRI data analysis. Neuroimage 69, 157–197 (2013).
Zhang, R. & Volkow, N. D. Brain default-mode network dysfunction in addiction. NeuroImage 200, 313–331 (2019).
Qu, Y., Galvan, A., Fuligni, A. J., Lieberman, M. D. & Telzer, E. H. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. J. Neurosci. 35, 11308–11314 (2015).
Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93 (2011).
Hu, Y., Salmeron, B. J., Gu, H., Stein, E. A. & Yang, Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry 72, 584–592 (2015).
Fan, J. et al. Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J. Affect. Disord. 223, 106–114 (2017).
Xiong, H., Guo, R-J. & Shi, H-W. Altered default mode network and salience network functional connectivity in patients with generalized anxiety disorders: an ICA-based resting-state fMRI study. Evid. Based Complement. Altern. Med. 2020, 4048916 (2020).
Jilka, S. R. et al. Damage to the salience network and interactions with the default mode network. J. Neurosci. 34, 10798–10807 (2014).
Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574 (2008).
Martinez, D. et al. Transcranial magnetic stimulation of medial prefrontal and cingulate cortices reduces cocaine self-administration: A pilot study. Front. Psychiatry 9, 1 (2018).
Beynel, L., Powers, J. P. & Appelbaum, L. G. Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: A systematic review. NeuroImage 211, 116596 (2020).
Yuan, S. et al. Neural effects of cognitive behavioral therapy in psychiatric disorders: A systematic review and activation likelihood Estimation meta-analysis. Front. Psychol. 13, 853804 (2022).
van Dis, E. A. M. et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders: A systematic review and meta-analysis. JAMA Psychiatry 77, 265–273 (2020).
Carey, G. et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease induces functional brain changes. J. Parkinsons Dis. 13, 93–103 (2023).
Schrammen, E. et al. Functional neural changes associated with psychotherapy in anxiety disorders—A meta-analysis of longitudinal fMRI studies. Neurosci. Biobehav. Rev. 142, 104895 (2022).
Doss, M. K. et al. Models of psychedelic drug action: modulation of cortical-subcortical circuits. Brain 145, 441–456 (2022).
Vollenweider, F. X. & Preller, K. H. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 21, 611–624 (2020).
McPherson, S., Barbosa-Leiker, C., Burns, G. L., Howell, D. & Roll, J. Missing data in substance abuse treatment research: current methods and modern approaches. Exp. Clin. Psychopharmacol. 20, 243–250 (2012).
Anticevic, A. et al. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 16, 584–592 (2012).
Chen, G., Chen, G., Xie, C. & Li, S-J. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect. 1, 195–206 (2011).
Murphy, K. & Fox, M. D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173 (2017).
Wetherill, R. R. et al. Classifying and characterizing nicotine use disorder with high accuracy using machine learning and resting-state fMRI. Addict. Biol. 24, 811–821 (2019).
Regier, P. S. et al. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict. Biol. 22, 1768–1777 (2017).
Beck, A. T., Steer, R. A. & Brown, G. Beck Depression Inventory–II. https://doi.org/10.1037/t00742-000 (1996).
Beck, A. T., Epstein, N., Brown, G. & Steer, R. A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult Clin. Psychol. 56, 893–897 (1988).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Harris, J. Tangent spaces to Grassmannians. In Algebraic Geometry: A First Course (eds Harris, J.) 200–210 (Springer, 1992).
Fan, Y. et al. Discriminant analysis of functional connectivity patterns on Grassmann manifold. NeuroImage 56, 2058–2067 (2011).
Cheng, H. et al. Reversibility of impaired large-scale functional brain networks in Cushing’s disease after surgery treatment: A longitudinal study. Neuroendocrinology 114, 250–262 (2023).
Jing, R. et al. Altered large-scale functional brain networks in neurological Wilson’s disease. Brain Imaging Behav. 14, 1445–1455 (2020).
Williams, S., Carson, R. & Tóth, K. Moving beyond P values in the journal of physiology: A primer on the value of effect sizes and confidence intervals. J. Physiol. 601, 5131–5133 (2023).
Magnusson, K. A Causal Inference Perspective on Therapist Effects (2023).
Acknowledgements
We would like to thank the following people and funding agencies that helped make this manuscript possible. Jieqiong Wong calculated the group information guided independent components analysis (GIG-ICA) to identify triple networks and subcortical regions. The physicians, nurses, and research assistants at the Center for Study of Addiction helped to interface with patients and collect data. This study was supported by the Commonwealth of Pennsylvania Commonwealth Universal Research Enhancement (CURE) grant.
Author information
Authors and Affiliations
Contributions
ARC was PI on the original study. YF processed the data, and his team conducted the GIG-ICA analysis. PSR and ARC conceived of the secondary analysis. PSR conducted all second-level analyses with significant input from NH. PSR wrote the main manuscript text and prepared all figures and tables. NH provided critical review of the paper that led to substantial revisions. MG and SE wrote portions of the discussion. HA provided critical feedback on the methods section. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Regier, P.S., Hager, N.M., Gawrysiak, M. et al. Differential large-scale network functional connectivity in cocaine-use disorder associates with drug-use outcomes. Sci Rep 15, 9636 (2025). https://doi.org/10.1038/s41598-025-91465-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91465-3
Keywords
This article is cited by
-
Triple network disruption in medication overuse headache: functional signatures and clinical impact
The Journal of Headache and Pain (2025)