Abstract
Buzz pollination is essential for plants which have poricidal anther dehiscence, such as tomato. Due to the lack of microscopic level observations and precise analyses of the buzz pollination process, the mechanism of tomato pollen ejection during buzz pollination is unclear. In this study, Micro-CT imaging was used to measure the internal structure and size of tomato flowers. Then, a discrete element simulation 3D model of a standardized tomato flower was constructed. Finally, the process of collision and adhesion between pollen grains during buzz pollination was directly solved by numerical calculations. By comparing and analyzing with existing research results, a discrete element simulation of buzz pollination was developed. This study provides an intuitive, feasible, simple yet efficient research method for buzz pollination, which is helpful to understand and explore the mechanism of pollen ejection from pored anthers during mechanical or natural bee-induced vibrations.
Similar content being viewed by others
Introduction
As a plant with poricidally dehiscent anthers, tomato (Solanum lycopersicum L., Solanaceae) relies principally upon buzz pollination in nature1,2. During floral sonication, the thorax (mesosoma + propodeum) and wings (slightly) of bees vibrate, causing pollen grains to be forcefully ejected from the anther sacs (locules), moving and interacting continuously within the anthers3. Pollen is ejected through small apical anther pores. Pollination occurs when the pollen grains are deposited on a floral stigma during a subsequent bee visit4. Vibrations transmitted to the air create a “buzzing” sound, which is where the term buzz pollination comes from. However, the airborne buzzing sound waves do not aid in pollination5.
Scholars have conducted extensive research to reveal the nature and importance of bee-induced vibrations on anthers and flowers during buzz pollination6. In an early biophysical model of buzz pollination7, the rate of anther release pollen was proportional to the vibration velocity perpendicular to the length of the anther. Harder & Barclay8 found a strong relationship between pollen release and vibration frequency. However, De Luca et al.9 and Tayal et al.10 believed that the impact of vibration frequency had no significant effect on pollen release, but rather the amplitude and duration of vibration had a greater impact on pollen release. Rosi‐Denadai et al.11 found a positive relationship between vibration amplitude and tomato pollen release, whereas the relationship between pollen release and frequency depended upon whether amplitude was positively or negatively correlated as a covariate. In addition to these mechanical effects12, electrostatic interactions between pollen grains and anther walls might also play an important role in pollen release13. Due to the lack of direct microscopic level observations and vibrational analyses of tomato pollination, although buzz pollination was discovered at least a century ago, our detailed understanding of tomato pollination is still relatively limited14.
Existing research is influenced by two factors. Firstly, due to the closed and complex internal structure of tomato anther, it is extremely difficult to observe the movement of hundreds of thousands of micro-scale pollen grains inside the anther locules during buzz pollination; on and secondly, due to limitations in experimental conditions, it is difficult to freely set the relevant vibration parameters during buzz pollination. Therefore, in this paper, Micro-CT methods were used to scan freshly picked tomato flowers. Then, the discrete element method15,16 was used to directly solve the forces and trajectories of pollen grains during tomato buzz pollination. We quantitatively analyzed the impact of vibration parameters on tomato pollination. Finally, the simulation results were compared with the research findings of Rosi Denadai et al.11 to verify the feasibility of the discrete element method. The verification experiment demonstrates that the integration of Micro-CT and the discrete element method enables the visualization and precise calculation of the buzzing pollination process. This approach is anticipated to emerge as a potent methodology for investigating buzz pollination.
Materials and methods
The flowchart of the current study is depicted in Fig. 1.
3D model building
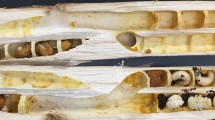
The tomato (Solanum lycopersicum L., Solanaceae, variety Shengnvmaliya from Taiyuan Kesaike Seed Industry Co., Ltd) which were about to bloom were selected and sampled when the pollen had matured. After removing their sepals and petals, the flowers were fixed for 3 days using FAA fixative (70% ethanol, Nanjing Senbeijia Biotechnology Co., Ltd., Nanjing, China) containing 1% phosphotungstic acid. Then the flowers were placed in a critical point dryer (Autosamdri-815A, Tousimis, USA) to dry and prepare samples. In contrast to traditional anatomical methods, Micro-CT facilitates a more efficient assessment of the internal architecture and measurements of anthers, thereby enabling the development of more accurate three-dimensional models17,18. We used a Micro-CT (NanoVoxel 3000, Sanying Company, Tianjin, China) instrument to perform three-dimensional microscopic imaging of the internal structure of tomato flowers. The prepared critically point dried flower samples were affixed to a high-precision 360° rotating sample platform, and Micro-CT was used for horizontal and vertical plane scanning. The scanning voltage was 60.0 kV and the current was 60.0 μA. The exposure time was 0.12 s. The scanning time was 1.5 h with a resolution of 4.76 μm. Finally, micron-scale plane images of tomato flowers were obtained, as shown in Fig. 2. The anther exhibited a length of 10.5 mm and a maximum diameter of 4.02 mm. The inner diameter of the aperture at the anther’s apex was measured to be 0.8 mm. The sac (= anther locule) diameter was determined to be 0.2 mm. The style possessed a length of 8.6 mm and a diameter of 0.5 mm. The stigma featured a diameter of 0.7 mm and a length of 0.4 mm.
In order to reduce the modeling difficulty and improve the calculation efficiency, SolidWorks 2020 (SolidWorks Corporation, Massachusetts, USA) was used to reconstruct the 3D model of the tomato flower anther cone. The sepals, petals, and ovary were removed, and the anther pores, slits and gaps were preserved. The villi and folds on the inner and outer surfaces of anthers, and the granular protrusions and grooves on the exine surface of pollen grains, were also were ignored. The simplified 3D model is shown in Fig. 3.
The petals and sepals were removed and the anthers were dried in an oven at 30 ℃ for 8 h. The anthers were then gently crushed with a glass stirring rod and the released pollen was screened with sieves of 100, 200, 300, and 400 micron mesh, and finally frozen at -18 ℃ for later use. After the pollen had been fixed with a 4% glutaraldehyde fixative solution, the pollen was dehydrated using a 50%, 60%, and 70% ethanol gradient and subsequently dried in the critical point dryer (Autosamdri-815A, Tousimis, Rockville, Maryland, USA) for sample preparation. In order to circumvent the charging effect and enhance the signal-to-noise ratio, the samples were surface coated with gold atoms for 1–1.5 min using an ion-coating instrument. The polar axis length (P) and equatorial axis length (E) of tomato pollen grains were measured using a field emission scanning electron microscope (SU8020, Hitachi, Tokyo, Japan). In total, 30 pollen grains were measured, and their representative equatorial, polar, and exine surface decorative patterns were selected for photos, as shown in Fig. 4a. The measurement results indicate that the P/E ratio of tomato pollen is close to 1, with an average diameter of 20 μm, and the grains are approximately spherical. Ignoring the microscopic changes on the surface of pollen grains, the three-dimensional model is simplified to represent a standard spherical shape. The 3D model of pollen is shown in Fig. 4b.
As shown in Fig. 2a, a tomato flower has 6 sacs (= anther locules), each pollen sac (locule) is divided into two parts by the locules wall, shaped like a butterfly in cross section. A tomato flower contains approximately 300,000 pollen grains19. In the simplified 3D model, pollen grains are evenly distributed within the hollow tubular anther locules along the bottom ¾ of the hollow tubes. After the 3D model was imported into the EDEM 2021 software (Altair, Michigan, USA), 50,000 pollen grains were generated inside one anther locule, as shown in Fig. 5.
Calculation model selection
For grains at the micrometer scale, the van der Waals force typically exceeds gravitational forces by three orders of magnitude or more. Tomato pollen grains exhibit intricate adhesion and contact properties during the process of pollination. Utilizing the Johnson-Kendall-Roberts contact model, various forces such as van der Waals force, liquid bridge force, and electrostatic force are categorized as adhesion forces. These adhesion forces are incorporated as an additional parameter in the contact deformation equation to analyze the contact collision characteristics of grains influenced by adhesion forces. The normal force acting between the grains is represented in formula (1)20.
where \({F}_{JKR}\) is the normal force between grains, \(\gamma\) is surface energy of grains, \({E}_{E}\) is the equivalent modulus of elasticity,\(\alpha\) is the interaction parameter between grains, \({R}_{E}\) is the equivalent curvature radius of the contact area, \({\delta }_{n}\) is the normal overlap between grains. Utilizing the discrete element method to address Eq. (1) allows for the acquisition of data regarding the position, velocity, and force characteristics of pollen grains.
In order to ascertain the discrete element parameters of Solanum pollen, the authors conducted a parameter calibration experiment20. Experimental measurements were utilized to derive constitutive parameters, including pollen morphology, diameter, and density. Subsequently, the contact and collision dynamics of the pollen were examined using an atomic force microscope, which facilitated the calculation of Young’s modulus, surface energy, and recovery coefficient of the pollen through the application of the Johnson-Kendall-Roberts contact model. A Plackett–Burman test was implemented to identify significant factors, employing discrete element method simulations to evaluate the Young’s modulus of pollen grains, the static friction coefficient between pollen grains, and the static friction coefficient between pollen and polypropylene on a plastic surface. The parameter range was subsequently refined through a steepest ascent method. Finally, a regression equation was established based on the outcomes of the Box-Behnken response surface analysis.Based upon research conducted and existing research results21, the parameters of the simulation are given in Table 1.
When pollen grains move in the air, due to the small mass of the pollen and air resistance, they decelerate rapidly. Therefore, we adopted the classical Schiller and Naumann drag model22 to solve the drag force of the pollen air system.
Simulation test method
As shown in Fig. 6a, when a buff-tailed bumblebee sonicates a tomato flower, it bites the anther cones with its mandibles and tightly clamps the anther with its front and rear leg tarsal claws, while curling its body around the anthers5. During pollination, the bee’s thoracic indirect flight muscles vibrate rapidly (~ 100-500 Hz), and the resulting vibration is transmitted to the anther through mandibles or head, thorax, and abdomen, causing the flower to vibrate in the Y–Z plane23,24. According to the spatial posture and vibration characteristics of tomato flowers during the vibrational period, the coordinate system is defined as shown in Fig. 6.
Only 446 species of bees are known to buzz flowers to collect pollen (in 83 genera from all 7 bee families)16. These include large carpenter bees as well as minute sweat bees3. There are significant differences in the vibration characteristics of different bee species during buzz pollination. Bumblebees (specifically Bombus terrestris in Europe and Bombus impatiens in the United States) are now the most popular choice of tomato pollinators in commercial hydroponic greenhouses25. In bumblebee buzz pollination, the average buzz duration was 1.08 ± 0.07 s (range 0.21–3.26 s) and with a peak frequency of 324 ± 3.67 Hz (range 264–386 Hz); average peak amplitude was 19.13 ± 1.5 mm s−1 (range 1.29–84.08 mm s−1)26.
The vibration parameters of our discrete element simulation is as follows: the vibration type used was sinusoidal vibration, the vibration frequency was 324 Hz,a vibration amplitude was 9.4 μm, and the vibration direction was Y–Z diagonal. The corresponding peak amplitude and acceleration were 19.13 mm s−1 and 77.91 m s−2, respectively. The time integration was Euler method27,28, the time step automatic, and the total time was 1.08 s, target save interval was 0.01 s (save calculation data every 0.01 s), cell size was 0.025 mm (approximate number of Cells was 89,600,000). The calculation domain size is 10 × 10 × 14 mm. Generated initial particles using the particle factory function in EDEM software, as shown Fig. 6b.
Upon finalizing the aforementioned parameter configurations, the simulation computations may commence.
Results and discussion
When the anther begins to vibrate, the pollen grains leave the anther through the pollen sacs (anther locules) slits and the gaps of anther pore and move toward the anther pores under the synergistic effect of gravity and the vibration force. At the moment of simulation, at 0.03 s, most pollen grains were located in the front part of the anther, as shown in Fig. 7. At this time, it can be observed that a small number of pollen grains have already left through the anther pores. At the moment of simulation (0.07 s), nearly a hundred of aggregated pollen grains were ejected into the air in the form of a small pollen cloud. This indicates that the vibrations delivered during buzz pollination have a high efficiency in agitating pollen within the anther locules and causing them to emerge from the anther pores. At this moment, the majority of pollen particles inside the anther had collected near the anther pores, with only a small amount of pollen remaining inside the anther locules under the action of mutual adhesion.
As the vibration continues, more and more pollen grains are ejected from the anther pores. Until the end of the simulation, the pollen grains were still being continuously released. Counts of the number of pollen grains leaving the computational domain at an interval of 0.01 s, are shown in Fig. 8. The computational domain was larger than the volume of the tomato anther. After pollen grains left the anther, it continued to move in the computational domain for another 0.03 s. Therefore, the number of pollen grains in the computational domain began to significantly decrease after the initial moment of simulation at 0.1 s.
From Fig. 8, it can be seen that the rate of pollen release remains relatively constant during vibrational excitation. The amount of pollen released is linearly positively correlated with the vibration duration. Therefore, in the actual process of buzz pollination, bees usually collect more pollen by prolonging the floral buzzing (vibration) time or increasing their number of repeated flower visits. According to a previous study, an individual Solanum flower is visited by bees an average of 3 times (up to 11 times)29,30 until it moves to another likely more rewarding flower.
Rosi-Denadai et al.11 found that there was a relationship between pollen released (z, μg) and vibration frequency (x, Hz) and acceleration (y, m s−2), fitting formula as follows: z = 128.89 − 0.08 x + 1.18 y. To verify the accuracy of simulation calculations, our calculation time was extended to 2 s and two additional simulation variables were added, and calculated, as shown in Table 2. Based on pollen density and volume, the mass of a single tomato pollen was calculated to be approximately 5.42 × 10−12 g. According to the fitting formula, the amount of pollen released under different vibration conditions is shown in the “fitting results” column of Table 2.
Comparing the simulation values with previously published reference values, the relative errors are 6.59%, 9.72%, and 14.65%, respectively. The simulation results are close to the fitting results.
Conclusions
In summary, DEM simulation calculation of buzz pollination based on a discrete element method is a likely explanation for pollen grain explusion in Solanum lycopersicum. By using simulation methods, the vibration frequency, amplitude, and direction could be varied freely and their effects examined in relation to the model. In our simulation of buzz pollination, the process of pollen particles collision, adhesion, and movement were directly observed. Therefore, we have provided a new method for scientists studying the vibrational natue of buzz pollination. Yet, there are still certain shortcomings invovled this study, including the relatively simplified 3D model of the tomato anthers within the anther cone compared to the natural tomato blossom. Also, the the dynamical characteristics of the porose anther and filament tissues were not completed simulated or studies. Therefore, the accuracy of our simulations and calculations can be improved with future studies that more accurately reflect the anatomical nuances of the tomato anther cone, and especially the complex interactions among the tens of thousands of pollen grains inside the anther locules and the adhesion properties between pollen grains and anther walls.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
29 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-00089-0
References
Kleiman, B. & Koptur, S. Weeds enhance insect diversity and abundance and may improve soil conditions in mango cultivation of south Florida. Insects 14(1), 65. https://doi.org/10.3390/insects14010065 (2023).
Bashir, M. A. et al. Role of pollination in yield and physicochemical properties of tomatoes (Lycopersicon esculentum). Saudi J. Biol. Sci. 25(7), 1291–1297. https://doi.org/10.1016/j.sjbs.2017.10.006 (2018).
Pritchard, D. J. & Vallejo-Marín, M. Buzz pollination. Curr. Biol. 30(15), R858–R860. https://doi.org/10.1016/j.cub.2020.05.087 (2020).
Vallejo-Marin, M. & Russell, A. L. Harvesting pollen with vibrations: Towards an integrative understanding of the proximate and ultimate reasons for buzz pollination. Ann. Bot. 133(3), 379–398. https://doi.org/10.1093/aob/mcad189 (2024).
Vallejo-Marín, M. Buzz pollination: Studying bee vibrations on flowers. New Phytol. 224(3), 1068–1074. https://doi.org/10.1111/nph.15666 (2019).
King, M. J. & Buchmann, S. L. Sonication dispensing of pollen from Solanum laciniatum flowers. Funct. Ecol. 449–456. https://doi.org/10.2307/2389937 (1996).
Buchmann, S. L. & Hurley, J. P. A biophysical model for buzz pollination in angiosperms. J. Theor. Biol. 72(4), 639–657. https://doi.org/10.1016/0022-5193(78)90277-1 (1978).
Harder, L. D. & Barclay, R. M. R. The functional significance of poricidal anthers and buzz pollination: Controlled pollen removal from Dodecatheon. Funct. Ecol. 509–517. https://doi.org/10.2307/2390076 (1994).
De Luca, P. A., Giebink, N., Mason, A. C., Papaj, D. & Buchmann, S. L. How well do acoustic recordings characterize properties of bee (Anthophila) floral sonication vibrations?. Bioacoustics 29(1), 1–14. https://doi.org/10.1007/s00442-012-2535-1 (2020).
Tayal, M., Chavana, J. & Kariyat, R. R. Efficiency of using electric toothbrush as an alternative to a tuning fork for artificial buzz pollination is independent of instrument buzzing frequency. BMC Ecol. 20(1), 1–7. https://doi.org/10.1186/s12898-020-00278-7 (2020).
Rosi-Denadai, C. A., Araújo, P. C. S., Campos, L. A. D. O., Cosme, L. Jr. & Guedes, R. N. C. Buzz-pollination in Neotropical bees: Genus-dependent frequencies and lack of optimal frequency for pollen release. Insect Sci. 27(1), 133–142. https://doi.org/10.1111/1744-7917.12602 (2020).
King, M. J. & Buchmann, S. L. Floral sonication by bees: mesosomal vibration by Bombus and Xylocopa, but not Apis (Hymenoptera: Apidae), ejects pollen from poricidal anthers. J. Kansas Entomol. Soc. 295–305 (2003).
Corbet, S. A. & Huang, S. Q. Buzz pollination in eight bumblebee-pollinated Pedicularis species: Does it involve vibration-induced triboelectric charging of pollen grains?. Ann. Bot. 114(8), 1665–1674. https://doi.org/10.1093/aob/mcu195 (2014).
Arroyo-Correa, B., Beattie, C. & Vallejo-Marín, M. Bee and floral traits affect the characteristics of the vibrations experienced by flowers during buzz pollination. J. Exp. Biol. 222(4), 198176. https://doi.org/10.1242/jeb.198176 (2019).
Liu, X., Mao, J., Zhao, L., Shao, L. & Li, T. The distance potential function-based finite-discrete element method. Comput. Mech. 66, 1477–1495. https://doi.org/10.1007/s00466-020-01913-2 (2020).
De, T., Das, A. & Kumar, J. On the prediction of particle collision behavior in coarse-grained and resolved systems. Part. Sci. Technol. 41(8), 1170–1184. https://doi.org/10.1080/02726351.2023.2188333 (2023).
Begot, L. et al. Precision phenotyping of nectar-related traits using x-ray micro computed tomography. Cells 11(21), 3452. https://doi.org/10.3390/cells11213452 (2022).
Xiao, Z. et al. 3D visualisation of voids in grapevine flowers and berries using X-ray micro computed tomography. Aust. J. Grape Wine Res. 27(2), 141–148. https://doi.org/10.1111/ajgw.12480 (2021).
Hansen, M., Lanes, G. C., Brito, V. L. & Leonel, E. D. Investigation of pollen release by poricidal anthers using mathematical billiards. Phys. Rev. E 104(3), 034409. https://doi.org/10.1103/PhysRevE.104.034409 (2021).
Shi, Q., Wang, B., Mao, H. & Liu, Y. Calibration and measurement of micrometre-scale pollen particles for discrete element method parameters based on the Johnson-Kendal-Roberts model. Biosyst. Eng. 237, 83–91. https://doi.org/10.1016/j.biosystemseng.2023.11.013 (2024).
Jankauski, M., Ferguson, R., Russell, A. & Buchmann, S. Structural dynamics of real and modelled Solanum stamens: implications for pollen ejection by buzzing bees. J. R. Soc. Interface 19(188), 20220040. https://doi.org/10.1098/rsif.2022.0040 (2022).
Liu, W., He, Y., Li, M., Huang, C. & Liu, Y. Effect of drag models on hydrodynamic behaviors of slurry flows in horizontal pipes. Phys. Fluids https://doi.org/10.1063/5.0107952 (2022).
Buchmann, S. L. What a Bee Knows: Exploring the Thoughts, Memories, and Personalities of Bees (Island Press, 2023).
Byrne, D. N., Buchmann, S. L. & Spangler, H. G. Relationship between wing loading, wingbeat frequency and body mass in homopterous insects. J. Exp. Biol. 135(1), 9–23. https://doi.org/10.1242/jeb.135.1.9 (1988).
Cooley, H. & Vallejo-Marín, M. Buzz-pollinated crops: A global review and meta-analysis of the effects of supplemental bee pollination in tomato. J. Econ. Entomol. 114(2), 505–519. https://doi.org/10.1093/jee/toab009 (2021).
De Luca, P. A. et al. Variability in bumblebee pollination buzzes affects the quantity of pollen released from flowers. Oecologia 172, 805–816. https://doi.org/10.1007/s00442-012-2535-1 (2013).
Schilling, M., Schütz, S. & Piesche, M. Numerical simulation of the transport and deposition behaviour of particles on filter fibres using Euler‐Lagrange Method and coupling of CFD and DEM. In AIP Conference Proceedings Vol. 1207, 789–795 (American Institute of Physics, 2010). https://doi.org/10.1063/1.3366464.
Zhong, X. C., Chen, J. Y. & Fan, Z. Y. A particle swarm optimization-based method for numerically solving ordinary differential equations. Math. Probl. Eng. 2019(1), 9071236. https://doi.org/10.1155/2019/9071236 (2019).
Kawai, Y. & Kudo, G. Effectiveness of buzz pollination in Pedicularis chamissonis: significance of multiple visits by bumblebees. Ecol. Res. 24, 215–223. https://doi.org/10.1007/s11284-008-0500-6 (2009).
Shelly, T. E., Villalobos, E., Bell, L., Burden, A., Fox, M., Granoff, I., Gunawardene, N., Holman, M., Hornor, A., MacLeod, J., Michalek, J. & Watson, O. Buzzing bees (Hymenoptera: Apidae, Halictidae) on Solanum (Solanaceae): floral choice and handling time track pollen availability. Florida Entomol. 180–180. https://doi.org/10.2307/3496153 (2000).
Funding
This work was supported by the National Natural Science Foundation of China, under Grant [Nos. 32201686 and 32071905].
Author information
Authors and Affiliations
Contributions
Conceptualization, Q.S. and H.M.; methodology, Q.S. and Y.L.; software, Q.S.; validation, Q.S., B.W. and Y.L.; formal analysis, Q.S. and Y.L.; investigation, Q.S. and Y.L.; resources, Q.S. and Y.L.; data curation, Q.S. and Y.L.; writing—original draft preparation, Q.S. and Y.L.; writing—review and editing, Q.S., Y.L., B.W., Y.W., X.D., Y.Z., H.M. and X.Y.; visualization, Q.S.; supervision, Q.S. and Y.L.; project administration, Q.S. and B.W.; funding acquisition, Q.S. and H.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Funding section in the original version of this Article was omitted. Full information regarding the correction made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, Q., Liu, Y., Wang, B. et al. Discrete element simulation of buzz pollination in tomato. Sci Rep 15, 6710 (2025). https://doi.org/10.1038/s41598-025-91490-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91490-2