Abstract
Associations between fluctuations in blood glucose levels and medically unexplained symptoms, such as palpitations, numbness in the extremities, dizziness, and drowsiness, have been a topic of debate for an extended period; however, consensus remains elusive. We therefore examined the associations of blood glucose and insulin levels with symptoms by performing a 5 h glucose tolerance test in patients without diabetes with complaints. A total of 139 (54 men and 85 women, mean age 35.4 years) were included in this study. Blood glucose and insulin levels were measured before and at 15, 30, 60, 90, 120, 150, 180, 240, and 300 min after a 10-h fast and 75 g glucose load. The insulin index was used to divide the participants into early, intermediate, and delayed insulin secretion groups. Hypoglycemic symptoms (neuroglycopenic: warmth, drowsiness, weakness, fainting, decreased thinking ability, and dizziness) and autonomic symptoms (neurogenic: palpitations, cold sweats, anxiety/nervousness, tremors, hunger, and tingling) were evaluated separately. Hypoglycemia (< 70 mg/dL (3.9 mmol/L)) occurred at 240–300 min in 92% of all patients, with hypoglycemic and autonomic symptoms. Hypoglycemic symptoms increased over time and were highest at 240 min, while autonomic symptoms decreased significantly for 15 min and increased at 240 and 300 min. Although hypoglycemic symptoms were not significantly different among the early, intermediate, and delayed secretion groups, the incidence rates of autonomic symptoms were higher in the early insulin secretion group than in the other groups at 30–60–90 min. The characteristics of the early insulin secretion group suggested that symptoms were associated with not only blood glucose levels but also the timing of insulin secretion. Further studies are needed to determine whether dietary interventions tailored to insulin secretion profiles can alleviate these symptoms.
Similar content being viewed by others
Introduction
Medically unexplained symptoms are symptoms that suggest the presence of a physical illness, but appropriate medical examination and testing cannot detect any causative illness1. The percentage of patients with medically unexplained symptoms, such as rapid heart palpitations, numbness, and anxiety, have been reported to be 15%–19%2 and 40.2%–49%3, and treatment is often difficult4,5. Additionally, the cause of these symptoms is unknown, resulting in repeated examinations and high medical costs6.
In 1924, the first case of postprandial hypoglycemia in a patient without diabetes was reported by Harris indicating that hypoglycemic symptoms can occur even in patients without diabetes who do not inject insulin7. Postprandial hypoglycemia and hypoglycemic symptoms have been suggested to occur more frequently than expected in patients without diabetes who have never been diagnosed with diabetes or other abnormalities in blood glucose regulation8,9,10.
Furthermore, there has been a long-standing debate about whether fluctuations in blood glucose levels are associated with medically unexplained symptoms; however, no definitive consensus has been reached. Hypoglycemia cannot be detected in some cases despite the presence of symptoms suspicious of hypoglycemia11,12,13,14. Therefore, the symptoms of suspected hypoglycemia cannot be explained only by physiological changes due to hypoglycemia itself, but may be related to fluctuations in glucose and insulin levels and autonomic nervous system function8,15. Furthermore, post-load hypoglycemia has been suggested to be associated with high insulin levels in 4 or 5 h oral glucose tolerance tests (OGTT) in subjects without diabetes16, and high insulin levels have been reported to be associated with subsequent psychiatric symptoms, such as depression17,18,19,20. However, few studies have examined the associations between insulin levels and the timing of insulin secretion symptoms.
Therefore, this study aimed to examine the associations of blood glucose and insulin levels with symptoms by performing a 5 h OGTT in patients without diabetes with symptoms. Additionally, since it has been noted that insulin secretion can be divided into early and delayed secretion16, this study aimed to examine the association between different patterns of insulin secretion and symptoms. We hypothesized that the various symptoms observed after glucose loading may be associated with not only transient hypoglycemia but also fluctuations in blood glucose and insulin levels and insulin fluctuation patterns. If an association is observed between fluctuations in glucose and insulin levels and symptoms, dietary guidance to control rapid increases in insulin levels and rapid decreases in blood glucose levels and other lifestyle changes can be provided.
Results
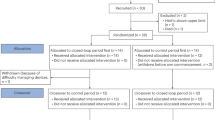
Of the 169 patients enrolled in this study, 139 (54 males and 85 females, mean age 35.4 years) were included in the analysis (Fig. 1). Table 1 shows the characteristics of the study subjects. Of the 139 patients, 88 were in the delayed secretion group, 40 in the intermediate secretion group, and 11 in the early secretion group. Significant differences in insulin and glycoalbumin levels were observed between the three groups before glucose loading, and no significant differences in mean age, BMI, blood glucose, 1.5 AG, HbA1c, body temperature, blood pressure, or other items were observed between the three groups.
Flow chart for the participant selection in this study. *Patients requested the 5 h oral glucose tolerance test (OGTT) mainly because of symptoms such as postprandial drowsiness (44%), lethargy (22%), dizziness (15%), and palpitations (10%). **Medications, including antiallergic, antianxiety, or antipsychotic drugs or sleeping pills.
The early secretion group showed the highest insulin area under the curve (AUC) (insulin secretion volume) during the examination (7125 cm2) compared with the delayed secretion group (4405 cm2), a difference of 2720 cm2 (p < 0.01). Furthermore, the maximum insulin level for each subject was the highest in the early secretion group, averaging 72.0 μU/mL, almost twice that of the delayed secretion group (36.6 μU/mL) (p < 0.01). The mean maximum blood glucose level was the highest in the delayed secretion group (166.5 mg/dL (9.25 mmol/L)) and the lowest in the early secretion group (134.1 mg/dL (7.45 mmol/L)) (p < 0.01).
The frequency of occurrence of the criteria defined as hypoglycemia (70 mg/dL(3.9 mmol/L) or less) and the time after glucose loading were checked. Of the 139 patients, 35 (25%) had hypoglycemia (50 mg/dL (2.8 mmol/L)) and 128 (92%) had hypoglycemia (70 mg/dL (3.9 mmol/L)) during the examination. Most occurred at 240 min (90 patients) or 300 min (98 patients) after glucose loading. Of the 128 participants who had a blood glucose level below 50 mg/dL (2.8 mmol/L) at least once during the test, 14 participants (11%) had no change in symptoms when their blood glucose level fell below 70 mg/dl (3.9 mmol/L) (data not shown).
Changes in glucose and insulin levels and symptoms during the 5-h OGTT were checked. The mean blood glucose levels were significantly lower at 240 min (70.5 mg/dL (3.9 mmol/L)) and 300 min (65.7 mg/dL (3.7 mmol/L)) after glucose loading than before (78.3 mg/dL (4.3 mmol/L)). Insulin and blood glucose followed monophasic patterns, with insulin peaking at 30 min (37.4 μU/ml) and falling gradually as blood glucose levels dropped. Hypoglycemic symptoms increased over time, with the highest score in 240 min (11.6 points). Autonomic symptoms decreased significantly with increasing blood glucose levels starting at 15 min after loading and gradually increased with decreasing blood glucose levels, with significant increases at 240 (6.0 points) and 300 min (6.8 points) (Fig. 2).
Regarding the three insulin groups, in the delayed and intermediate insulin secretion groups, hypoglycemic symptoms increased over time, peaking at 240 min and worsening, whereas symptom scores decreased from 240 to 300 min. Autonomic symptoms decreased significantly from 15 min after loading and increased significantly at 240 and 300 min. Insulin levels showed a monophasic pattern, peaking at 90 min (26.2 μU/ml) in the delayed secretion group and at 60 min (35.3 μU/ml) in the intermediate secretion group. The delayed secretion group showed the highest blood glucose level at 60 min (159 mg/dL (8.8 mmol/L)), whereas the intermediate secretion group showed the highest level at 30 min (137.8 mg/dL (7.6 mmol/L)). Then, blood glucose levels gradually decreased in a staircase-like pattern, reaching the lowest levels 240 and 300 min after loading (Fig. 3).
Changes in glucose and insulin levels and symptoms during the 5 h OGTT over time by insulin secretion pattern. OGTT : oral glucose tolerance test. Significant differences for each time point before and after glucose loading were compared. †,★,▲,*p < .05, ††,★★,▲▲,**p < .01, and †††,★★★,▲▲▲,***p < .001.
In the early secretion group, hypoglycemic and autonomic symptom scores remained almost the same from the start, In the early secretion group, there was no decrease in autonomic symptom scores from fasting and an increase in symptom scores was observed from 240 to 300 min. Additionally, mean insulin levels showed a biphasic pattern with a significant increase after 15 min, peaking at 30 min (60.5 μU/ml) and again at 150 min (31.6 μU/ml). Blood glucose levels were highest at 30 min (123.4 mg/dl (6.9 mmol/L)), decreased at 60 min, and increased at 90 min. However, the blood glucose level at 300 min was lower than that before glucose loading (62.8 mg/dl (3.5 mmol/L) vs. 77.0 mg/dl (4.3 mmol/L)) (Fig. 3).
Regarding the shape of the classical glycemic curve used to diagnose hypoglycemia, the delayed and intermediate insulin secretion groups tended to be “monophasic,” and the early insulin secretion group tended to be “biphasic” or “polyphasic.” In the delayed insulin secretion group, 67 (77%) patients were monophasic. In the intermediate secretion group, 20 (50%) patients were monophasic and 20 (50%) patients were biphasic or polyphasic. In the early secretion group, 10 patients (91%) were biphasic or polyphasic.
Table 2 shows the mean symptom scores among the three groups at each time. No statistically significant difference in the occurrence of hypoglycemic symptoms was observed between the three groups at each time. However, the early insulin secretion group showed higher autonomic symptom scores than the other groups at 30, 60, and 90 min. The early insulin secretion group had a score of 4.8 at 30 min (vs. 2.2 and 2.7 in the delayed and intermediate insulin secretion groups, respectively), 4.8 at 60 min (vs. 2.2 and 2.5 in the delayed and intermediate insulin secretion groups, respectively), and 4.7 at 90 min (vs. 2.4 and 2.7 in the delayed and intermediate insulin secretion groups, respectively), with a statistically significant difference (p < 0.05) (Table 2).
Discussion
In the present study, the 5 h OGTT was performed in subjects without diabetes with medically unexplained symptoms. Among them, 92% had hypoglycemia below 70 mg/dL (3.9 mmol/L) 4–5 h after glucose loading, and their symptoms worsened during that time period. This finding suggested that the complaints included many symptoms associated with hypoglycemia. Additionally, the analysis based on the pattern of insulin secretion revealed that autonomic symptoms persisted even after glucose loading in the early insulin secretion group, indicating that the symptoms may be related not only to hypoglycemia but also to the timing and amount of insulin secretion. Therefore, monitoring blood glucose and insulin fluctuations in patients with medically unexplained symptoms could be useful.
Furthermore, the delayed and intermediate insulin secretion groups had more neuroglycopenic symptoms during the 5-h OGTT and a greater change from peak glucose to glucose nadir. Moreover, the peak of symptoms was observed in both groups at 240 min after glucose loading; however, besides the low blood glucose level at that time, a large fluctuation from the value at 180 min after glucose loading was observed. Although the blood glucose levels at 300 min after glucose loading were low, the symptoms were less severe than those at 240 min; therefore, it was thought that the large fluctuations in blood glucose levels, in addition to hypoglycemia, may be related to the symptoms.
In our study, the 5 h OGTT showed that 92% of patients had blood glucose levels below 70 mg/dl (3.9 mmol/L) 4–5 h after sugar loading, even among those who had no problems with fasting blood glucose or HbA1c, and many showed hypoglycemic and autonomic symptoms. Of the 139 patients, 35 (25%) developed hypoglycemia below 50 mg/dl (2.8 mmol/L). These findings are consistent with those of previous studies, in which the 5 h OGTT was performed in 52 patients with postprandial complaints, showing that 21 of 52 patients (40.4%) developed hypoglycemia [blood glucose < 55 mg/dl (3.1 mmol/L))21. Another study reported that, of 40 hospitalized patients with complaints, 12 (30%) developed hypoglycemia (blood glucose < 55 mg/dl (3.1 mmol/L))22. Based on these findings, the complaints may include symptoms associated with hypoglycemia.
Meanwhile, since the relationship between blood glucose and symptoms is unclear, some studies have examined the diagnostic utility of the 5 h OGTT11,12,13,14. Charles et al. reported that 23%–25% of those who underwent the 5 h OGTT developed hypoglycemia during the test, with or without hypoglycemic symptoms. Furthermore, 78% of those who did not experience hypoglycemia experienced hypoglycemia-like symptoms13. Thus, they concluded that hypoglycemia was not related to symptoms but to other factors. Our study results showed a similar frequency of post-load hypoglycemia and occurrence of symptoms consistent with hypoglycemia. However, hypoglycemia alone could not explain all symptoms associated with blood glucose fluctuations. Therefore, other factors involved in blood glucose regulation, such as insulin, may be influential.
The analysis of insulin secretion patterns revealed that the early secretion group had higher insulin secretion and maximal insulin levels than the other groups, and no improvement in fasting autonomic symptoms was observed with glucose intake. It is possible that higher insulin levels lowered blood glucose levels and sustained autonomic symptoms; however, in this study, blood glucose levels during that period were within the normal range. Therefore, it is possible that the sympathomimetic effects of insulin itself sustained autonomic symptoms. Hyperinsulinemia increases heart rate and venous blood noradrenaline concentrations and is associated with increased vasoconstrictive nerve activity in the skeletal muscle, keeping sympathetic nerves above baseline23, although the underlying mechanism is unclear, and further research is needed.
Accumulated evidence has demonstrated that insulin passes through the brain and regulates central nervous system functions such as feeding, depression, and cognitive behavior24. Furthermore, high insulin levels have often been reported to be associated with subsequent depression and other psychiatric symptoms17,18,19,20. Studies have shown that high insulin levels in early to mid adulthood may be an important predictor of long-term risk for metabolic, psychiatric, and neurobehavioral dysfunctions20 and that depressive symptoms are associated with high insulin levels in older people19. Furthermore, severe depressive symptoms have been reported to positively correlate with insulin levels, especially in young men17. These findings indicate that insulin hypersecretion may be responsible for some complaints.
Our study showed an association between early insulin secretion and autonomic symptoms. Since it is not usually possible to self-measure postprandial insulin levels, it is important to understand that insulin secretion may be involved in the symptoms even when blood glucose levels are normal at the onset of symptoms associated with blood glucose fluctuations. Furthermore, providing appropriate dietary guidance may be necessary to avoid foods that cause a rapid rise in blood glucose, insulin secretion, and subsequent hypoglycemia. Portis reported an association between hyperinsulinemia and significant clinical improvement in extreme weakness, tremors, sweating, and dizziness with a diet low in free glucose25. In other words, it may be recommended to eat protein, fat, and fiber first and carbohydrates in small amounts last and to use low glycemic index carbohydrates that raise blood glucose levels slowly26,27,28,29,30. In addition, future interventional studies should be conducted to confirm the effect of such dietary guidance in the early insulin secretion group.
The strength of the study is that the 5 h OGTT revealed that hypoglycemia and hypoglycemia-related symptoms often occur 4–5 h after glucose loading, even in patients without diabetes or hypoglycemia on general testing. The changes in blood glucose levels and symptoms beyond 120 min after glucose loading could only be detected by the 5 h OGTT. Additionally, observation of symptoms according to the pattern of insulin secretion showed that not only hypoglycemia but also early hypersecretion of insulin may be associated with symptoms.
This study has several limitations. First, although postprandial hypotension may present with symptoms similar to those of postprandial hyperglycemia, we did not measure blood pressure levels during OGTT loading. Second, although insulin autoimmune syndrome (e.g., Hirata’s disease), which may cause reactive hypoglycemia, may be present in the target population, insulin autoantibodies were not measured in this study. This should be considered in future studies. Third, because gastric emptying was well correlated with the 1-h post-OGTT plasma glucose level, gastric emptying may confound the results of this study. In fact, individuals who have undergone metabolic surgery experience very rapid gastric emptying and a relatively high prevalence of postprandial hypoglycemia31. Therefore, the difference in gastric emptying between the groups may have influenced the differences in insulin and glucose levels between the groups. Because the gastric emptying rate was not measured in this study, further studies are necessary to clarify the underlying mechanism. Fourth, our study suggests that differences in the timing of insulin secretion affect the occurrence of autonomic symptoms; however, it was also thought that blood glucose levels may also affect the occurrence of autonomic symptoms. It was difficult to consider the extent to which insulin and blood glucose levels affected symptoms separately. Also, this study was a single-center study and included only patients who already had symptoms. This may limit the generalizability of the results. In addition, the causal relationship between insulin and the occurrence of symptoms is unclear due to the cross-sectional nature of the study without a control group, because it was unethical to perform a 5-h glucose tolerance test on individuals without symptoms. Finally, 14 participants (11%) experienced no change in symptoms when their blood glucose levels fell < 70 mg/dL (3.9 mmol/L). This may have been due to the use of glucose rather than a mixed meals, which resulted in asymptomatic hypoglycemia. Therefore, further studies are needed to confirm whether a diet that suppresses insulin secretion improves symptoms.
In conclusion, we suggest that symptoms that cannot be explained by blood glucose levels alone can be explained by classifying the symptoms according to large fluctuations in blood glucose levels and the timing of insulin secretion. The 5 h OGTT in patients without diabetes with complaints revealed that postprandial hypoglycemia occurred 4–5 h after glucose loading in 92% of the subjects, and many experienced hypoglycemic and autonomic symptoms during this period. Autonomic symptoms persisted even after glucose loading in the early insulin secretion group. The study results suggest that blood glucose and insulin fluctuations are associated with previously unexplained complaints. Further studies are needed to determine whether dietary interventions for different types of insulin secretion can improve hypoglycemic and autonomic symptoms.
Materials and methods
Participants and study design
This was a cross-sectional study. A total of 169 patients with medically unexplained symptoms who visited the Shinjuku Mizoguchi Clinic, Tokyo, Japan, between February 2015 and April 2016 for a 5 h 75 g OGTT were enrolled in this study. The Shinjuku Mizoguchi Clinic is a nutritional therapy clinic that offers a 5 h OGTT to those who wish to undergo the test. We defined “medically unexplained symptoms” as symptoms that cannot be medically explained when organic disease has been ruled out by blood tests, CT scans, and ultrasound examinations, among others, at a university hospital before presentation to our clinic. After physical measurements and blood tests were performed on these participants, a 75 g OGTT was performed. Before the test, a questionnaire was administered to determine lifestyle habits, including exercise, smoking, alcohol consumption, and regular medication, and symptoms usually experienced. Furthermore, participants were asked to complete a symptom score questionnaire during the loading test. The inclusion criteria included patients aged 20–60 years and those who completed the symptom score questionnaire. The exclusion criteria included patients with diabetes mellitus, those with a fasting blood glucose level of 110, those taking antiallergic, antianxiety, or antipsychotic drugs, sleeping pills, or other medications that could affect blood glucose fluctuations, and those with incomplete (blank) entries in the symptom score questionnaire.
This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol was approved by the Ethics Committee of Fukushima Medical University (approval number IPPAN 2020–065). Written consent forms were obtained from all subjects.
5 h OGTT
After fasting for at least 10 h, the 5 h OGTT was performed at 9:30 in the morning. Participants had their blood drawn at 15, 30, 60, 90, 120, 150, 180, 240, and 300 min before and after ingestion of 75 g glucose load to measure glucose and insulin levels. On the day of the test, the participants were instructed to bring supplementary meals and eat their meals after the test. Furthermore, they were observed at the clinic until their symptoms improved to the same level as before the examination, after which they were allowed to return home.
All test items were measured in the same laboratory (LSI Medience Corporation, Itabashi, Tokyo, Japan). Glucose was measured by an enzymatic method using an automatic analyzer (JCA-BM9130, JEOL Ltd.) with an Iatro LQ GLU kit (Nipro Corporation). Insulin was measured by a chemiluminescence immunoassay method using an Architect i2000 SR analyzer (Abbott Japan, Chiba, Japan) with an Architect Insulin kit (Abbott Japan). Before the OGTT, HbA1c was measured by an enzymatic method using an automatic analyzer (JCA-BM9130, JEOL Ltd.) with a Cinque HbA1c kit (Arkray Factory Inc.) and a Lucica GA-L kit (Asahi Kasei Corporation), and 1,5-AG was measured by an enzymatic method using an automatic analyzer (JCA-BM8060, JEOL Ltd) with Determiner-L 1,5AG assay (Kyowa Medix Corporation).
Symptom score
Various symptoms that are considered to occur during hypoglycemia were evaluated separately for hypoglycemic and autonomic nervous system symptoms. Autonomic symptoms (neurogenic symptoms) are the result of sympathetic and parasympathetic activation, whereas hypoglycemic symptoms (neuroglycopenic symptoms) are secondary to decreased brain glucose utilization32. On the symptom score questionnaire, participants recorded a symptom score for each blood draw and the symptoms and duration of any other symptoms.
The authors developed the symptom questionnaire based on previous studies32,33,34,35,36,37, and it requires no special permission for use or publication. The scale assesses 12 symptoms that are known to occur during hypoglycemia, including hypoglycemic symptoms (neuroglycopenic symptoms: feeling warm, drowsiness, weakness, feeling faint, decreased thinking ability, and dizziness) and autonomic symptoms (neurogenic symptoms: palpitations, cold sweats, anxiety/nervousness, tremors, hunger, and tingling). The scale includes 12 items under 2 subscales (6 each)32,33,34,35,36,37. Each symptom was scored from 0 (none) to 6 (very strong) at 15, 30, 60, 90, 120, 150, 180, 240, and 300 min of blood collection before and after glucose loading, respectively, as subjective symptom intensity.
Other laboratory items
Blood pressure was measured at baseline in the sitting position. Body temperature was measured at baseline and at 15, 30, 60, 90, 120, 150, 180, 240, and 300 min of blood collection in the sitting position by the patients and recorded in the symptom score questionnaire. Some data on blood pressure, smoking, alcohol consumption, and body temperature could not be collected from all patients (Table 1).
Comorbidities were recorded using a medical questionnaire. In terms of comorbidities, binge eating disorders were observed in 6%, insomnia in 5%, allergic disease in 5%, premenstrual syndrome in 2%, and Graves’ disease in 0.7%. Mental disorders included depression (5%), panic disorder (2%), adaptive adjustment disorder (1.4%), bipolar disorder (0.7%), dysmorphophobia (0.7%), and schizophrenia (0.7%). The shape of the glycemic curve38 used for diagnosing hypoglycemia was not used in this study; however, for reference, the proportion of monophasic and polyphasic patients in the three groups of insulin secretion timing was counted.
Statistical analysis
Insulin secretion varies between individuals and is divided into early and delayed secretion17. Therefore, this study focused on the characteristics of insulin secretion after glucose intake to evaluate the symptoms associated with hypoglycemia and those associated with insulin secretion and used the insulin index to divide the participants into early, intermediate, and delayed insulin secretion groups. Then, the association between symptom onset and blood glucose and insulin levels was investigated.
The insulinogenic index was used to divide the study subjects into three groups. It was calculated as the ratio of the change in insulin to the change in glucose from 0 to 30 min as follows: insulinogenic index = (Insulin30 [μU/mL] − Insulin0 [μU/mL])/(Glucose30 [mg/dL] − Glucose0 [mg/dL]) According to the conventional definition of the insulin index, < 0.4 indicates diabetic, and > 0.4 indicates healthy. However, based on the data on the nondiabetic control group from previous studies in Asia39,40,41,42,43,44, we defined delayed insulin secretion in this study as < 0.4, intermediate insulin secretion as 0.4–0.8 (More than 0.4 but less than 0.8), and early insulin secretion as > 0.8. Subject characteristics among the three groups were compared using statistical analysis.
The mean and standard deviation of each parameter at baseline were calculated to examine the differences among the delayed, intermediate, and early secretion groups in terms of each test item and symptom using analysis of variance (ANOVA). When significant differences were found among the three groups, post hoc multiple comparisons were performed using the Tukey test to determine where the differences occurred among the three groups. Then, for comparisons of insulin, glucose, hypoglycemic symptoms, and autonomic symptoms at 15, 30, 60, 90, 120, 150, 180, 240, and 300 min, the means were calculated for each time period and differences from baseline were analyzed using the paired t-test. Furthermore, to compare the values for each time period (15, 30, 60, 90, 120, 150, 180, 240, and 300 min) for the three groups, the means for each time period were calculated for insulin, glucose, hypoglycemic symptoms, and autonomic symptoms to be examined using ANOVA, and multiple comparisons using the Tukey test. In addition, these analyses were performed stratified by the three secretion groups. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses. Statistical significance was set at p < 0.05 with a two-tailed test.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to o ethical considerations, but are available from the corresponding author on reasonable request.
References
Hatcher, S. & Arroll, B. Assessment and management of medically unexplained symptoms. BMJ 336, 1124–1128 (2008).
Burton, C. Beyond somatization: A review of the understanding and treatment of medically unexplained physical symptoms (MUPS). Br. J. Gen. Pract. 53, 231–239 (2003).
Haller, H., Cramer, H., Lauche, R. & Dobos, G. Somatoform disorders and medically unexplained symptoms in primary care. Dtsch. Ärztebl. Int. 112, 279–287. https://doi.org/10.3238/arztebl.2015.0279 (2015).
Nimnuan, C., Hotopf, M. & Wessely, S. Medically unexplained symptoms: An epidemiological study in seven specialities. J. Psychosom. Res. 51, 361–367. https://doi.org/10.1016/s0022-3999(01)00223-9 (2001).
Scott, M. J., Crawford, J. S., Geraghty, K. J. & Marks, D. F. The ‘medically unexplained symptoms’ syndrome concept and the cognitive-behavioral treatment model. J. Health Psychol. 27, 3–8. https://doi.org/10.1177/13591053211038042 (2022).
Husain, M. & Chalder, T. Medically unexplained symptoms: Assessment and management. Clin. Med. 21, 13–18. https://doi.org/10.7861/clinmed.2020-0947 (2021).
Harris, S. Hyperinsulinism and dysinsulinism. JAMA 83, 729–733. https://doi.org/10.1001/jama.1924.02660100003002 (1924).
Posel, M. M. A case of dysinsulinism. S.A. Med. Dec. 9, 794–795. https://doi.org/10.10520/AJA20785135_6888 (1933).
Musha, C. & Ohta, H. Relationship between unidentified complaints and hypoglycemia in the female. Jpn. Soc. Psychosom. Obstet. Gynecol. 14, 254–261 (2010) ((in Japanese)).
Osame, H. Analysis of the 5-h glucose tolerance test in 26 healthy subjects. Practice 29, 109–111 (2012) ((in Japanese)).
Cahill, G. F. Jr. & Soeldner, J. S. A noneditorial on nonhypoglycemia. N. Engl. J. Med. 291, 905–906. https://doi.org/10.1056/NEJM197410242911712 (1974).
Yager, J. & Young, R. T. Non-hypoglycemia is an epidemic condition. N. Engl. J. Med. 291, 907–908. https://doi.org/10.1056/NEJM197410242911713 (1974).
Charles, M. A. et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes 30, 465–470. https://doi.org/10.2337/diab.30.6.465 (1981).
Nelson, R. L. Hypoglycemia: Fact or fiction?. Mayo Clin. Proc. 60, 844–850. https://doi.org/10.1016/S0025-6196(12)64790-9 (1985).
Ohira, T., Jinnouchi, Y., Aoyama, N. & Mizoguchi, T. Trends in domestic and international research on blood glucose variability and psychiatric symptoms [in Japanese with English abstract]. Compr. Med. 20, 25–30. https://doi.org/10.32183/ifcm.20.1_25 (2021).
Altuntaş, Y. Postprandial reactive hypoglycemia. Med. Bull. Sisli Hosp. 53, 215–220. https://doi.org/10.14744/semb.2019.59455 (2019).
Timonen, M. et al. Depressive symptoms and insulin resistance in young adult males: results from the Northern Finland 1966 birth cohort. Mol. Psychiatry 11, 929–933. https://doi.org/10.1038/sj.mp.4001838 (2006).
Akbaraly, T. N. et al. Glycemia, insulin resistance, insulin secretion, and risk of depressive symptoms in middle age. Diabetes Care 36, 928–934. https://doi.org/10.2337/dc12-0239 (2013).
Diniz, B. S., Fisher-Hoch, S. & Mccormick, J. The association between insulin resistance, metabolic variables, and depressive symptoms in Mexican–American elderly: A population-based study. Int. J. Geriatr. Psychiatry 33, e294–e299. https://doi.org/10.1002/gps.4792 (2018).
Abbasi, F. et al. Insulin resistance and accelerated cognitive aging. Psychoneuroendocrinology 147, 105944. https://doi.org/10.1016/j.psyneuen.2022.105944 (2023).
Pant, V., Mathema, S., Jha, S., Paudel, S. D. & Baral, S. The detection of postprandial hypoglycemia with 5-hour oral glucose tolerance test. EJIFCC 32, 451–457 (2021).
Hall, M., Walicka, M., Panczyk, M. & Traczyk, I. Metabolic parameters in patients with suspected reactive hypoglycemia. J. Pers. Med. 11, 276. https://doi.org/10.3390/jpm11040276 (2021).
Berne, C., Fagius, J., Pollare, T. & Hjemdahl, P. Sympathetic response to euglycemic hyperinsulinemia: Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35, 873–879. https://doi.org/10.1007/BF00399935 (1992).
Agrawal, R. et al. Insulin action in the brain regulates both central and peripheral functions. Am. J. Physiol. Endocrinol. Metab. 321, E156–E163. https://doi.org/10.1152/ajpendo.00642.2020 (2021).
Portis, S. A. Life situations, emotions and hyperinsulinism. Res. Publ. Assoc. Res. Nerv. meant. Dis. 29, 390–405. https://doi.org/10.1001/jama.1950.02910340027008 (1949).
Wolever, T. M., Jenkins, D. J., Jenkins, A. L. & Josse, R. G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 54, 846–854. https://doi.org/10.1093/ajcn/54.5.846 (1991).
Jenkins, D. J. et al. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 76, 266S-S273. https://doi.org/10.1093/ajcn/76/1.266S (2002).
Esfahani, A. et al. The glycemic index: Physiological significance. J. Am. Coll. Nutr. 28, 439S-445S. https://doi.org/10.1080/07315724.2009.10718109 (2009).
Feinman, R. D. et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 31, 1–13. https://doi.org/10.1016/j.nut.2014.06.011 (2015).
Musha, C. Treatment of hypoglycemic attacks (Q&A). Available online at https://www.jmedj.co.jp/journal/paper/detail.php?id=3807 (2015).
D’Hoedt, A. & Vanuytsel, T. Dumping syndrome after bariatric surgery: prevalence, pathophysiology and role in weight reduction—A systematic review. Sci. Rep. 13, 11–476. https://doi.org/10.51821/86.3.11476 (2023).
Towler, D. A., Havlin, C. E., Craft, S. & Cryer, P. Mechanism of awareness of hypoglycaemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 42, 1791–1798. https://doi.org/10.2337/diab.42.12.1791 (1993).
Heller, S. R. & Cryer, P. E. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40, 223–226. https://doi.org/10.2337/diab.40.2.223 (1991).
Cryer, P. E. Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. A vicious cycle. Diabetes 41, 255–260. https://doi.org/10.2337/diab.41.3.255 (1992).
Cryer, P. E. Symptoms of hypoglycemia, thresholds for their occurrence, and hypoglycemia unawareness. Endocrinol. Metab. Clin. N. Am. 28, 495–vi. https://doi.org/10.1016/s0889-8529(05)70084-0 (1999).
Bremer, J. P., Jauch-Chara, K., Hallschmid, M., Schmid, S. & Schultes, B. Hypoglycemia unawareness in older compared with middle-aged patients with Type 2 diabetes. Diabetes Care 32, 1513–1517. https://doi.org/10.2337/dc09-0114 (2009).
Olsen, S. E. et al. Hypoglycemia symptoms and impaired awareness of hypoglycemia in adults with Type 1 diabetes: The association with diabetes duration. Diabetes Med. 31, 1210–1217. https://doi.org/10.1111/dme.12496 (2014).
Tschritter, O. et al. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 26, 1026–1033. https://doi.org/10.2337/diacare.26.4.1026 (2003).
Dagogo-Jack, S., Askari, H. & Tykodi, G. Glucoregulatory physiology in subjects with low-normal, high-normal, or impaired fasting glucose. J. Clin. Endocrinol. Metab. 94, 2031–2036. https://doi.org/10.1210/jc.2008-1348 (2009).
Nagai, E. et al. Incretin responses tothe orall glucose load in Japanese nonobese healthy subjects. Diabetes Ther. 2, 20–28. https://doi.org/10.1007/s13300-010-0017-1 (2011).
Iwahashi, H. et al. Insulin-secretion capacity in normal glucose tolerance, impaired glucose tolerance, and diabetes in obese and nonobese Japanese patients. J. Diabetes Investig. 3, 271–275. https://doi.org/10.1111/j.2040-1124.2011.00180.x (2012).
Aono, D. et al. Insulin secretion and risk for future diabetes in subjects with a nonpositive insulinogenic index. J. Diabetes Res. 2018, 5107589. https://doi.org/10.1155/2018/5107589 (2018).
Shimada, H. et al. Comparison of glucose tolerance between kidney transplant recipients and healthy controls. J. Clin. Med. 8, 920. https://doi.org/10.3390/jcm8070920 (2019).
Sato, M. et al. Characteristics associated with elevated 1-h plasma glucose levels during a 75-g oral glucose tolerance test in nonobese Japanese men. Diabetes Investig. 11, 1520–1523. https://doi.org/10.1111/jdi.13245 (2020).
Acknowledgements
The authors thank Enago (www.enago.jp) for the manuscript review and editing support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by Toru Mizoguchi, Naoki Aoyama, Yoh Jinnouchi, and Mikiko Inoue. Analysis was performed by Yoh Jinnouchi, Mikiko Inoue, Eri Ecuhi, and Tetsuya Ohira. The first draft of the manuscript was written by Toru Mizoguchi, Naoki Aoyama, Yoh Jinnouchi,and Mikiko Inoue and all authors critical reviewed on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mizoguchi, T., Aoyama, N., Jinnouchi, Y. et al. Associations of fluctuations in blood glucose and insulin with hypoglycemic symptoms. Sci Rep 15, 11579 (2025). https://doi.org/10.1038/s41598-025-91544-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91544-5