Abstract
Heat-related illnesses cause multiple organ injuries, including acute kidney injury (AKI). Recent studies have reported that heat-induced AKI can progress to chronic kidney disease (CKD). We previously reported that urinary levels of liver fatty acid-binding protein (L-FABP) are elevated in patients with severe heat-related illness. In this study, we prospectively examined the detection ability of L-FABP rapid assay kit (L-FABP Point-of-Care [POC] kit) for heat-induced organ damage in prehospital settings. After applying the exclusion criteria, 65 Japanese male military personnel who intended to carry out training in a hot environment were analyzed. The L-FABP POC kit enabled the detection of heat-induced kidney and/or liver damage after heat exposure (defined as serum creatinine [Cr] ≥ 1.2 mg/dL and total bilirubin ≥ 1.2 mg/dL) with a high negative predictive value (95.7%). L-FABP-positive participants showed higher serum Cr and total bilirubin levels than L-FABP-negative participants. Moreover, L-FABP-positive participants showed higher acyl-to-free carnitine ratios, indicating carnitine insufficiency which leads to impaired fatty acid oxidation, as well as high and rapid elevation of their core temperature in comparison to L-FABP-negative participants. In conclusion, the L-FABP POC kit may be useful as a screening tool for detecting heat-induced organ damage, which would prevent prolonged organ dysfunction.
Similar content being viewed by others
Introduction
The recent increase in global warming has become a global concern. The United Nations intergovernmental panel on climate change (IPCC) predicted that average temperatures will rise 2.4–4.4 °C by the end of this century1,2. This climate change could increase the occurrence of heat-related illnesses. In Japan, the number of emergency medical calls due to heat-related illness exceeded 70,000 per year in 20223 and will continue to increase in the future4.
Heat-related illness is mainly diagnosed by clinical symptoms, based primarily on the triad of hyperthermia, neurologic abnormalities, and recent exposure to hot weather or physical exertion5. The Japanese Association of Acute Medicine (JAAM) divides heat-related illness into three grades: grade I, first aid and observation (heat cramps and heat syncope); grade II, should be taken to a medical institution (heat exhaustion); and grade III, inpatient hospital care (heatstroke)6. However, because the classification relies on symptoms of heat-related illness, the determination of its severity can vary widely among evaluators. Over 60% of patients with heat-related illnesses who were transported by ambulance were categorized as mild3, which may overburden emergency medical care during the hot season. On the other hand, as we previously reported, even mild-to-moderate heat-related illnesses can cause organ damage, such as acute kidney injury (AKI)7.
Recent studies have shown that heat-induced AKI, even with mild and transient elevations of serum creatinine (sCr), could result in chronic kidney disease (CKD), which is called “heat stress nephropathy (HSN)”8,9,10. The incidence of HSN appears to increase as global warming progresses10, although it is still underestimated because HSN is generally regarded as a CKD of unknown etiology when heat-induced AKI is not evident. Therefore, there is an urgent need to develop methods for the early and on-site identification of heat-induced organ damage, which could motivate patients to visit a hospital to prevent further organ dysfunction.
We recently reported that urinary liver fatty acid-binding protein (L-FABP), an established AKI biomarker, was elevated in patients with severe heat-related illness and in patients with kidney injury due to heat stress7. However, the assay of urinary L-FABP takes more time than multiorgan dysfunction may appear after heat stress. Currently, the L-FABP rapid semiquantitative assay kit has been developed, and several studies have reported its usefulness11,12,13,14,15. However, none of these studies focused on heat-related illness.
We also recently reported that carnitine insufficiency, which inhibits fatty acid oxidation (FAO) and decreases ATP production in tubular cells, is associated with heat-induced kidney injury16. As L-FABP transports fatty acids, which are the primary source of energy in the renal proximal tubules, we hypothesized that a rapid L-FABP detection kit could detect heat-induced organ damage and the elevated urinary L-FABP levels are associated with carnitine insufficiency.
To translate our recent findings into actual clinical practice for heat-related illnesses, we conducted a prospective observational study focusing on the prehospital detection of heat-induced organ damage using the L-FABP semiquantitative assay kit. We also examined the relationship between urinary L-FABP elevation and body temperature and/or carnitine insufficiency, which may underlie heat stress-induced organ damage.
Results
Characteristics of participants
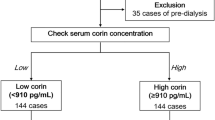
A total of 81 individuals provided their informed consent and underwent a situational training exercise for heat load (Fig. 1a). After excluding 16 individuals based on the exclusion criteria, 65 participants were included in the analysis (Fig. 1b). All the participants were male. The median (IQRs) age and body mass index (BMI) were 34 (26–42) and 23.4 (21.9–25.6), respectively. Eight (12.2%) participants received medication: two (3.0%) had hypertension, two (3.0%) had dyslipidemia, one (1.5%) had diabetes, and three (4.6) had other diseases (Table 1). None of the participants had chronic kidney disease. On the days of the situational training exercise, the weather was sunny with average WBGTs > 29 °C (Supplemental Table S1 on line), which increased to > 40 °C with protective gear correction (+ 11 °C).
Study design. (a) Study protocol. First, participants answered the questionnaire and initial blood and urine samples were collected. Within one week afterward, participants carried out a situational training exercise while in chemical protective gear, which included (1) carrying patients on a stretcher for 50 m, (2) ascending and descending stairs, (3) running on an obstacle course, and (4) simulated area chemical decontamination. During the situational training exercise, 20 participants had their rectal temperature measured. After finishing the training circuit (2–16 h later), participants were asked about their symptoms by questionnaire and blood and urine samples were collected. Urinary L-FABP was assessed by the L-FABP POC kit immediately on-site. (b) Flow chart of participant selection. Eighty-one male participants who were well-trained gave their informed consent and enrolled in the study. Of the 81 participants, 5 were excluded because they performed the situational training exercise on a day with a relatively low wet bulb globe temperature (WBGT) of < 29 °C. One participant had a poor physical condition prior to heat stress and 10 did not provide post-heat stress samples. We excluded these participants. Sixty-five participants were ultimately included in the final analysis.
Association between detection of L-FABP by POC kit and incidence of heat-related illness
Nineteen of the 65 participants (29.2%) were L-FABP-positive according to the L-FABP POC kit after exposure to heat stress. There were no differences in age, BMI, or medical history between the L-FABP-positive and -negative groups (Table 1). Table 1 also shows the number of participants who had heat-related illness symptoms listed in the JAAM classification and heat-related organ damage as defined by the JAAM-HS-WG criteria. Eight (12.3%) participants had symptoms of heat-related illnesses, and 8 (12.3%) had heat-related organ damage. In this study, no participants had central nervous system (CNS) dysfunction or coagulopathy. The rate of heat-related illness symptoms did not differ between the L-FABP-positive and -negative groups. However, the rate of heat-related organ damage in the L-FABP-positive group was significantly higher than that in the L-FABP-negative group (31.6 vs. 4.3%, p = 0.006, Table 1).
We also investigated whether the L-FABP POC kit could detect heat-induced organ damage. Heat-induced organ damage was not detected using the heat-related illness symptoms listed in the JAAM classification (positive predictive value [PPV], 12.5%; negative predictive value [NPV] 87.7%; Risk ratio [RR] 1.02 (95% confidential interval [CI] 0.14–7.23); p = 1.00). However, we were able to detect heat-related illness using the L-FABP POC kit (PPV, 31.6%; NPV, 95.7%; RR, 7.26 [95% CI 1.61–32.8], p = 0.006). The L-FABP POC kit testing demonstrated a positive likelihood ratio of 3.29 and a negative likelihood ratio of 0.32 in detecting heat-induced organ damage.
Association between L-FABP detection by POC kit and the changes in kidney and liver injury markers
We investigated the relationship between L-FABP POC kit-positive cases and markers of kidney and liver injury. Although the level of blood urea nitrogen (BUN) was similar (Fig. 2a), the L-FABP-positive group showed significantly increased serum Cr levels after heat exposure (pre-stress vs. post-stress, p < 0.01) and showed significantly higher serum Cr levels at post-stress than those of the L-FABP-negative group (p < 0.01, Fig. 2b). Similarly, the L-FABP-positive group showed a significantly decreased eGFR (p < 0.01) after heat exposure and showed a significantly lower eGFR at post-stress in comparison to the L-FABP-negative group (p < 0.01, Fig. 2c). The L-FABP-positive group also showed significantly higher serum T.bil levels at post-stress in comparison to the L-FABP-negative group (p < 0.05, Fig. 2d), although the serum T.bil levels before and after heat exposure in the L-FABP-positive group did not differ to a statistically significant extent (Fig. 2d). The levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) did not differ between the L-FABP groups (Fig. 2e,f). Although no difference was observed between the results of L-FABP in the post-stress measurement, there was a significant increase in serum sodium (Na) and a significant decrease in serum potassium (K) due to heat exposure (Table 2).
Changes in kidney and liver injury markers according to the results of the L-FABP POC kit. (a) Blood urea nitrogen (BUN), (b) serum creatinine, (c) estimated glomerular filtration rate (eGFR), (d) total bilirubin (T.bil), (e) serum aspartate aminotransferase (AST), and (f) serum alanine aminotransferase (ALT). Dots are presented for each individual and straight lines from pre-stress to post-stress connect the median values. Bars show the interquartile range (IQR). Data were analyzed by a mixed effects analysis with age and body mass index (BMI) as confounding variables. L-FABP, liver fatty acid-binding protein. *p < 0.05, **p < 0.01 vs. pre-stress. †p < 0.05, ††p < 0.01 vs. L-FABP-negative.
Correlation between L-FABP and changes in sCr and T.bil
We also investigated the correlation between urinary L-FABP and changes in serum Cr (ΔsCr) and T.bil (ΔT.bil), which were calculated as the level after heat exposure minus the level before heat exposure. Urinary L-FABP levels were evaluated by measuring the test line intensity of the L-FABP POC kit. We confirmed that urinary L-FABP levels were significantly higher in the L-FABP-positive group than in the L-FABP-negative group (Supplementary Fig. S1 on line). We found that urinary L-FABP levels were positively correlated with ΔsCr (ρ = 0.30, p = 0.01, Fig. 3a), but not with ΔT.bil (Fig. 3b).
Correlation between levels of urinary L-FABP and changes in serum creatinine and total bilirubin. (a,b) Correlation between the levels of urinary L-FABP and changes in (a) serum creatinine (ΔsCr), (b) total bilirubin (ΔT.bil). Data were analyzed using Pearson’s correlation coefficient. (c) A flow chart of subgroup analysis. Participants were divided into 3 subgroups according to the time of sample collection: 2, 6, and 16 h after heat stress had finished. (d,e) Urinary L-FABP levels (d) and changes in serum creatinine levels (e) according to the time of sample collection. Dots represent individual participants. Box-and-whisker plots: within each box, horizontal lines denote the median values; boxes extend from the 25th to the 75th percentile of each group’s distribution of values; vertical extending lines denote adjacent values (i.e., the most extreme values within the 1.5 interquartile range [IQR] of the 25 and 75th percentile of each group). Data were analyzed using the Mann–Whitney U-test. **p < 0.01; ***p < 0.001. (f–h) Correlation between the levels of urinary L-FABP and changes in serum creatinine at 2 (f), 6 (g), and 16 h (h) after heat stress. L-FABP showed a significant positive correlation with ΔsCr, especially at 6 h after heat stress. Each dot represents an individual participant. Data were analyzed using Spearman’s correlation coefficient.
We performed subclass analyses when samples were collected (at 2, 6, and 16 h, Fig. 3c). The baseline characteristics of the participants are presented in (Table 3). There were three (14.3%), three (27.3%), and 13 (39.4%) L-FABP-positive participants at 2, 6, and 16 h, respectively. The urinary L-FABP levels did not differ between the 2- and 6-h groups after heat exposure while the level of the 16-h group was significantly lower than the levels in the 2- and 6-h groups (Fig. 3d). In contrast, ΔsCr values after heat exposure were significantly higher in the 6-h group than in the 2- and 16-h groups (Fig. 3e). Although correlations between urinary L-FABP and ΔsCr were observed in the 2- and 16-h groups (Fig. 3f,h), urinary L-FABP only showed a positive correlation with ΔsCr in the 6-h group (ρ = 0.63, p = 0.04, Fig. 3g).
Association between the detection of L-FABP by POC kit and carnitine insufficiency
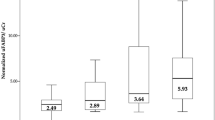
We recently showed that carnitine insufficiency was associated with heat-induced kidney and/or liver damage and demonstrated that carnitine supplementation prevented heat-induced kidney injury by preserving the FAO pathway and enhancing ATP production16. As L-FABP is a cell membrane transporter of fatty acids, we examined whether the expression of L-FABP is associated with carnitine insufficiency. Although serum total and free carnitine levels did not differ between the L-FABP-positive and-negative groups (Fig. 4a,b), the serum acylcarnitine levels in the L-FABP-positive group were significantly higher than those in the L-FABP-negative group (p < 0.05, Fig. 4c). The acyl-to-free carnitine (AC/FC) ratio was significantly higher in the L-FABP-positive group, which indicated carnitine insufficiency (p < 0.05, Fig. 4d). The difference remained significant after adjusting for age, BMI, baseline serum Cr, and T.bil (p = 0.008).
Levels of carnitine according to the results of the L-FABP POC kit. Levels of (a) total carnitine, (b) free carnitine, (c) acyl carnitine, and (d) AC/FC ratio according to the results of the L-FABP POC kit. The levels of acyl carnitine and AC/FC ratio were significantly higher in the L-FABP-positive group than in the L-FABP-negative group. Each dot represents an individual participant. Box-and-Whisker plots, within each box, horizontal lines denote the median values; boxes extend from the 25 to the 75th percentile of each group’s distribution of values; vertical extending lines denote adjacent values (i.e., the most extreme values within the 1.5 interquartile range [IQR] of the 25 and 75th percentile of each group). Data were analyzed using the Mann–Whitney U-test. *p < 0.05.
Association between the detection of L-FABP by POC kit and changes in core temperature
Finally, we investigated whether L-FABP POC kit-positive cases were associated with changes in core temperature during heat exposure. Of the 65 participants, 20 gave their consent for the measurement of their rectal temperature (Fig. 5a). Their characteristics are presented in Supplemental Table S2 on line. Of the 20 participants, 12 (60%) were L-FABP-negative and eight (40%) were L-FABP-positive. As shown in Fig. 5b, changes in core temperature from baseline (ΔTc) in the L-FABP-positive group increased significantly and quickly in comparison to the L-FABP-negative group (p = 0.001).
Changes in core temperature (ΔTc) during heat stress according to the results of the L-FABP POC kit. (a) Flow chart of participant selection. (b) Time course of ΔTc during heat stress. In the L-FABP-positive group, the Tc value increased higher and faster than in the L-FABP-negative group. Data were presented as mean ± standard error (SE). Data were analyzed using a mixed effects model with adjustment for age, BMI, and pre-stress core temperature.
Discussion
The principal finding of this study was that the L-FABP POC kit enabled the detection of heat-induced organ damage more reliably than heat-related symptoms, which are generally used for field identification of heat-related illness severity. Detecting elevated L-FABP in the prehospital setting was able to identify mild and asymptomatic organ damage due to heat stress, thereby helping prevent severe and/or chronic organ damage by allowing for early therapeutic intervention. Furthermore, we showed that positive cases of the L-FABP POC kit are involved in elevating core temperature and carnitine insufficiency, which may be the underlying mechanisms of heat-induced organ damage.
Previous studies have reported that even mild organ damage due to heat stress can cause prolonged organ dysfunction10. One reason could be that, as shown in this study, patients with mild heat-induced organ damage have no or only mild symptoms and thus may not seek medical attention, which results in prolonged organ dysfunction due to lack of appropriate treatment. We demonstrated that the L-FABP POC kit could detect HRI-induced organ damage with a positive likelihood ratio of 3.29 and a negative likelihood ratio of 0.32. Given that the pre-test probability of heat-related illness-induced organ damage is estimated to be 30–40% in exertional heat-related illness5,7,17, when a patient who feels unwell in a hot environment undergoes L-FABP POC testing and the result is positive, the post-test probability is elevated to 59–69%, whereas for a negative result, the post-test probability decreased to 12–17%. Consequently, on-site screening with this kit would enable medical providers to make more informed decisions for athletes, laborers, and military personnel who feel somewhat unwell in hot environments, encouraging them to visit medical facilities for further examination and early treatment, which could prevent prolonged organ dysfunction.
High body temperature leads to direct cytotoxic effects and inflammatory responses that cause multiple organ dysfunctions5,10. We, as well as other investigators, have demonstrated that heat stress can cause mitochondrial injury in tubular cells16,18. Mitochondrial injury was shown to lead a decrease in ATP production that exacerbates tubular damage, worsens the inflammatory response, and inhibits repair, resulting in kidney fibrosis in an AKI experimental model19. We also recently reported the association between heat-related organ damage and carnitine insufficiency, which inhibits fatty acid oxidation, which is the main energy source in tubular cells16. The L-FABP-positive group showed a high AC/FC ratio (Fig. 4d), suggesting that an increase in urinary L-FABP results from an increase in the demand for fatty acids to enhance ATP production in tubular cells, because L-FABP is a transporter of fatty acids in tubular cells. Further clinical studies are needed to investigate whether carnitine supplementation can positively affect the levels of urinary L-FABP and heat stress resilience.
The L-FABP-positive group also exhibited a high core temperature (Fig. 5). To our knowledge, this is the first report to show an association between L-FABP expression and high body temperature. We believe that there are two possible mechanisms through which hyperthermia leads to an increase in urinary L-FABP levels. One reason is that the expression of L-FABP may increase due to energy imbalance caused by hyperthermia-induced mitochondrial damage and high energy demands during high-intensity exercise, as mentioned above. Second, L-FABP may be increased to scavenge reactive oxygen species (ROS) induced by hyperthermia20. Further studies should be conducted to test these hypotheses.
Urinary L-FABP levels were positively correlated with ΔsCr after heat stress, especially at 6 h after heat stress (Fig. 3). Urinary L-FABP increases as early as 4 h and decreases at 12 h after cardiac surgery21. In line with previous study, we observed that urinary L-FABP levels were significantly lower in the 16 h group than in the 2- or 6 h groups (Fig. 3d). Given that ΔsCr was highest in the 6 h group (Fig. 3e), urinary L-FABP at an early time (2–4 h) after heat exposure might be useful for not only detection but also prediction of heat-induced AKI. The predictive ability of urinary L-FABP for heat-induced AKI should be examined in further studies.
Several limitations associated with the present study warrant mention. First, our findings have resulted from some artifacts of relative homogeneity; the participants were all male, relatively young, and only Japanese. One report showed that there was no marked difference in urinary L-FABP concentrations between males and females22. Further research should focus on more diverse groups, such as women, the elderly, children, and other ethnic groups. Vulnerable populations, such as children, older adults, pregnant women, and those with chronic health conditions, are all at risk of heat-related illness23, and studies focusing on these populations would contribute to public health initiatives aimed at preventing heat-induced organ damage. Second, the sample size was small and may not have provided sufficient statistical power. In particular, only two participants experienced kidney damage after heat stress. A large prospective study is required to reach a definitive conclusion. Third, we defined kidney and liver injuries as serum Cr ≥ 1.2 mg/dL and serum T.bil ≥ 1.2 mg/dL, respectively, in accordance with the JAAM-HS-WG criteria. In the current study, we could not find any participants who met the Kidney disease improving global outcomes (KDIGO) criteria for AKI24 or the American Association for the Study of Liver Diseases (AASLD) criteria for acute liver injury25. However, we should focus on detecting mild organ damage caused by heat stress to prevent future organ dysfunction. Therefore, we used the JAAM-HS-WG criteria to detect mild organ damage. Fourth, although we demonstrated the associations between elevated urinary L-FABP levels and heat-induced organ damage (Fig. 3) and elevated core body temperature (Fig. 5), we were unable to directly demonstrate the performance of the L-FABP POC kit for distinguishing the severity or stage of heat-induced organ damage, partly because of the lack of patients with severe heat-related illness. Further studies should be planned to include more diverse severity patients with heat-related illness to evaluate the correlation between L-FABP POC kit results and the severity of heat-induced organ damage.
In conclusion, we demonstrated that the L-FABP POC kit can detect kidney and liver damage due to heat-related illness. The underlying mechanisms of L-FABP elevation may involve high core temperature and carnitine insufficiency due to heat exposure. We envision using the L-FABP POC kit as a screening test to make heat-induced organ damage “visible” in prehospital settings. This may be useful for decision-making in relation to further medical examinations and early definitive care for preventing prolonged organ dysfunction, such as HSN.
Materials and methods
Study design
This prospective observational study focused on the utility of the L-FABP rapid assay kit in the identification of heat-induced organ damage. Eighty-one well-trained Japan ground self-defense force (JGSDF) personnel were enrolled in this study. These personnel perform high-intensity training during the summer months with protective gear, which adds + 11 °C to wet-bulb globe temperature (WBGT). This study was approved by the Research Ethics Committee of JSDF Central Hospital (approval# 04–008) and the National Defense Medical College (approval# 4817) and conducted in compliance with the Declaration of Helsinki.
After obtaining their informed consent in written format, all participants underwent a pre-stress medical check and initial sample collection. Within one week, participants carried out a situational training exercise while in chemical protective gear, which involved (1) carrying a patient on a stretcher for 50 m, (2) ascending and descending stairs, (3) running on an obstacle course, and (4) simulated area chemical decontamination. The participants took approximately 15 min to complete the exercise. It was planned that participants would visit us for medical checks and sample collection 2–16 h later. Urinary L-FABP levels were also assessed on-site using the L-FABP point-of-care (POC) kit (Timewell Medical, Tokyo, Japan). (Fig. 1a).
Five participants who performed the situational training exercise on a day with a relatively low WBGT (≤ 29 °C) were excluded so that the WBGT would exceed 40 °C with protective gear correction (+ 11 °C). Eleven participants were excluded from the analysis due to a poor physical condition on the exercise day (n = 1) or the lack of blood and/or urine samples (n = 10). We ultimately analyzed data from 65 participants for comparison of heat-induced organ damage between L-FABP-positive and L-FABP-negative participants (Fig. 1b).
In this study, we also divided the patients into three subgroups to identify changes in the correlation between the L-FABP concentration and heat-induced organ damage at each time point of sample collection. The 3 subgroups were as follows: at 2, 6 and 16 h after heat stress had finished (Fig. 3c).
Furthermore, the core temperature during heat exposure was measured using a continuous rectal thermometer in participants who agreed to the measurement (n = 20, Fig. 5a). We compared the time course of the core temperature during heat stress between L-FABP-positive and L-FABP-negative groups.
Sample measurement and data collection
Complete blood counts were evaluated within three hours of sample collection at the laboratory of self-defense force (SDF) Central Hospital. Serum and urinary samples collected were stored at -80 °C in the Laboratory of GSDF Chemical School, thawed, and analyzed at the Laboratory of JSDF Central Hospital the following week. The detail methods are presented in Supplementary Table S3 on line. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese equation for eGFR for sCr26. Serum carnitine levels were measured using the enzyme cycling method (SRL, Tokyo, Japan). Past history (including past heat-related illness events), medications, exercise habits, and amounts of water consumed were assessed using a questionnaire. The core temperature was measured every minute during the situational training exercise using a continuous rectal thermometer (LT-8A, Gram Corp., Saitama, Japan).
L-FABP semiquantitative assay kit
L-FABP POC kits assess the levels of urinary L-FABP semi-quantitatively using a lateral flow immunoassay. After conjugating urine samples with pretreatment agents, the specimens were dropped onto the kit and the test lines were assessed 15 min later. The results were defined using the test line as follows: negative, no test line appeared (corresponding L-FABP concentration less than 12.5 ng/mL); low-positive, the test line appeared weakly (corresponding L-FABP concentration between 12.5 ng/mL and 100 ng/mL); high-positive, the test line appeared strongly (corresponding L-FABP concentration is greater than 100 ng/mL). In this study, we described low and high positivity as positive. We also quantified the levels of urinary L-FABP using a CHR-631 Rapid Test Reader (Kaiwood Technology, Taiwan City, Taiwan), which can measure the test line intensity in relative light units (RLU). Analytical validity was previously reported to be significantly correlated with ELISA values (R2 = 0.9985)11,15.
Definition of heat-induced symptoms (JAAM classification)
The JAAM was used to classify heat-related illness severity using symptoms after heat exposure as follows: grade I, dizziness, faintness, slight yawing, or muscle cramps; grade II, headache, vomiting, fatigue, or sinking feeling; and grade III, impaired orientation6. We asked the participants about the above symptoms using a questionnaire when they visited us after experiencing heat stress.
Definition of heat-induced organ damage
Heat-induced organ damage was defined using the JAAM Heatstroke Working Group (JAAM-HS-WG) criteria, which were recently established by analyzing data from a nationwide registry for heat-related illness in Japan6. Heat-related illness was defined by the presence of at least one of the following abnormalities during and/or after exposure to heat stress, when other obvious causes were excluded: (1) Glasgow Coma Scale (GCS) ≤ 14, (2) sCr ≥ 1.2 mg/dL, (3) serum total bilirubin (T.bil) ≥ 1.2 mg/dL, and JAAM disseminated intravascular coagulation (DIC) score27 ≥ 4. In line with these criteria, we defined kidney and liver injuries due to heat stress as sCr ≥ 1.2 mg/dL and serum T.bil ≥ 1.2 mg/dL, respectively.
Statistical analyses
The normality of data was tested using the Shapiro–Wilk test in the visual inspection of quantile–quantile (QQ) plots. Continuous data were presented as median and interquartile range (IQR) or mean ± standard deviation (SD). Pairwise group comparisons were performed using the Mann–Whitney U test or one-way analysis of variance (ANOVA) according to the normality of data. Multiple comparisons were performed using the Kruskal–Wallis test. Categorical data were analyzed using Fisher’s exact test. Comparisons of data with repeated measurements (e.g., comparisons between pre- and post-stress) were performed using a mixed effects analysis with Tukey’s post hoc test. Age and body mass index (BMI) were included as confounding variables in the mixed-effects analysis. Changes in core temperature were compared using mixed-effects analysis with adjustment for age, BMI, and pre-stress core temperature. A correlation analysis was performed using Spearman’s correlation analysis. The correlation coefficients are presented as ρ values. Statistical significance was set at P < 0.05. All statistical analyses were performed using the JMP software program (version 15; SAS Institute, Cary, NC, USA).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Masson-Delmotte, V. et al. Climate Change 2021: The Physical Science Basis Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2021).
Desai, Y., Khraishah, H. & Alahmad, B. Heat and the heart. Yale J Biol Med. 96 (2), 197–203 (2023).
Fire and Disaster Management Agency in Japan. Heat illness information. https://www.fdma.go.jp/disaster/heatstroke/items/r4/heatstroke_geppou_202205-09.pdf (2022)
Oka, K., Honda, Y., Phung, V. L. H. & Hijioka, Y. Prediction of climate change impacts on heatstroke cases in Japan’s 47 prefectures with the effect of long-term heat adaptation. Environ. Res. 232, 116390 (2023).
Epstein, Y. & Yanovich, R. Heatstroke. N. Engl. J. Med. 380 (25), 2449–2459 (2019).
Hifumi, T., Kondo, Y., Shimizu, K. & Miyake, Y. Heat stroke. J. Intensive Care 6, 30 (2018).
Goto, H. et al. Early biomarkers for kidney injury in heat-related illness patients: a prospective observational study at Japanese self-defense force Fuji Hospital. Nephrol. Dial. Transplant. 38 (3), 644–654 (2023).
Kupferman, J. et al. Acute kidney injury in sugarcane workers at risk for mesoamerican nephropathy. Am. J. Kidney Dis. 72 (4), 475–482 (2018).
Tseng, M. F. et al. Risk of chronic kidney disease in patients with heat injury: A nationwide longitudinal cohort study in Taiwan. PLoS One 15 (9), e0238826 (2020).
Glaser, J. et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin. J. Am. Soc. Nephrol. 11 (8), 1472–1483 (2016).
Katagiri, D. et al. Urinary L-type fatty acid-binding protein predicts oxygen demand of COVID-19 in initially mild cases. Crit. Care Explor. 5 (3), e0873 (2023).
Okuda, H., Obata, Y., Kamijo-Ikemori, A. & Inoue, S. Quantitative and qualitative analyses of urinary L-FABP for predicting acute kidney injury after emergency laparotomy. J. Anesth. 36 (1), 38–45 (2022).
Suzuki, G. et al. Clinical significance of urinary L-FABP in the emergency department. Int. J. Emerg. Med. 12 (1), 24 (2019).
Sato, R. et al. A newly developed kit for the measurement of urinary liver-type fatty acid-binding protein as a biomarker for acute kidney injury in patients with critical care. J. Infect. Chemother. 21 (3), 165–169 (2015).
Usman, A. et al. Metformin use in patients hospitalized with COVID-19: lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. J. Thromb. Thrombolysis 53 (2), 363–371 (2022).
Goto, H. et al. L-carnitine pretreatment ameliorates heat stress-induced acute kidney injury by restoring mitochondrial function of tubular cells. Am. J. Physiol. Renal Physiol. 326 (3), F338–F351 (2024).
Abriat, A., Brosset, C., Brégigeon, M. & Sagui, E. Report of 182 cases of exertional heatstroke in the French Armed forces. Mil. Med. 179 (3), 309–314 (2014).
Sato, Y. et al. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am. J. Physiol. Renal Physiol. 317 (5), F1111–F1121 (2019).
Miguel, V. et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. 131 (5), e140695 (2021).
Yamamoto, T. et al. Renal L-type fatty acid–binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 18 (11), 2894–2902 (2007).
Portilla, D. et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 73 (4), 465–472 (2008).
Kamijo, A. et al. Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J. Lab. Clin. Med. 145 (3), 125–133 (2005).
Rublee, C., Dresser, C., Giudice, C., Lemery, J. & Sorensen, C. Evidence-based heatstroke management in the emergency department. West. J. Emerg. Med. 22 (2), 186–195 (2021).
Kellum, J. A. et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2 (1), 1–138 (2012).
Polson, J. & Lee, W. M. American association for the study of liver disease. AASLD position paper: the management of acute liver failure. Hepatology 41 (5), 1179–1197 (2005).
Matsuo, S. et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53 (6), 982–992 (2009).
Gando, S. et al. Japanese association for acute medicine disseminated intravascular coagulation (JAAM DIC) study group. A multicenter prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit. Care Med. 34 (3), 625–631 (2006).
Acknowledgements
We thank the members of the Department of Research at JGSDF Chemical School for their training supervision. We also thank the Department of Research at SDF Central Hospital for their administrative assistance in this research and support for sample collection and laboratory testing. We thank Keiko Komoda and Tomoko Morita for their invaluable administrative assistance. Portion of Fig. 1 were created under a license from H.G. using BioRender.com.
Disclaimer
The views expressed in this presentation are those of the authors and do not necessarily reflect the official policies of the Department of the Army, Department of Defense, or the U.S. Government.
Funding
This study was supported by internal grants from the Ministry of Defense.
Author information
Authors and Affiliations
Contributions
H.G., T.I., and K.O. designed the study; H.G., T.I., R.A., and H.S. collected the samples and assessed the results of the L-FABP POC kit; H.G., K.M., and H.N. performed the experiments under the supervision of M.K. and N.O.; T.S. assisted with the use of the L-FABP assay kit and reader; H.G., B. M. K., H.N., M.K., and N.O. edited and revised the manuscript. The final version was approved by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goto, H., Ishikiriyama, T., Oe, K. et al. Liver fatty acid-binding protein point-of-care testing detects heat-induced organ damage: a pilot study in Japanese male self-defense force personnel. Sci Rep 15, 7197 (2025). https://doi.org/10.1038/s41598-025-91685-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91685-7