Abstract
The breast cancer specific survival (BCSS) benefits of Neoadjuvant therapy (NeoAT) for triple-negative metaplastic breast cancer (TNMpBC) was uncertain. This study aimed to develop a prediction model for assessing the BCSS for TNMpBC patients with NeoAT. The primary cohort of 1163 patients with TNMpBC, from which a nomogram was established based on the results of a LASSO regression analysis, was derived from multi-centers data in China and the SEER database. This model was further validated by an independent cohort of 155 TNMpBC patients with NeoAT, with discrimination and calibration assessed. Totally 155 (13.3%) TNMpBC patients received NeoAT, with 45 (29.0%) cases demonstrating pathologic complete response (pCR), were enrolled. Subjects acquired pCR had superior BCSS. Four variables significantly associated with BCSS were incorporated in the establishment of model: age at diagnosis, T stage, N stage, and response to NeoAT. This model was well validated, with a C-index of 0.82, and area under the curves of 0.838, 0.866 in training cohort, respectively, for 3- years and 5-years BCSS. Based on the cutoff scores from the TNMpBC-NeoBCSS model and calculated by X-tile analysis, patients in high risk group had a inferior BCSS (HR = 6.77, P < 0.0001) when compared with those in low-risk group. TNMpBC-NeoBCSS model provides a favorable tool for assessing the BCSS for the TNMpBC patients with NeoAT and may help doctors and TNMpBC patients optimally make decision on the necessity of neoadjuvant therapy on the basis of individual BCSS.
Similar content being viewed by others
Introduction
Neoadjuvant therapy (NeoAT) in breast cancer meant one of the delivering of multimodal therapeutic approaches prior to surgery1. The administration of NeoAT was recommended as an indispensable and standard options for breast cancer patients by National Comprehensive Cancer Network (NCCN) guidelines2, due to the particular advantages distinguished from adjuvant therapy (AT), such as the downgrading of tumor, reduction in the extent of surgery3, increase in the rate of breast conserving surgery4, prevention in the potential axillary lymph nodes metastasis5, participation of the vivo drug-sensitivity testing and avoidance of the side effect from ineffective treatment6. However, due to the rarity and heterogeneity, nearly all the large, randomized controlled clinical studies concerning NeoAT were conducted in invasive ductal carcinoma rather than metaplastic breast cancer (MpBC), which generally presents with triple negative phenotype breast cancer7,8,9.
Metaplastic breast cancer (MpBC), first described in 1973 and accounted for about 0.2-5% of breast cancer, was recognized by World Health Organization (WHO) as a distinct breast cancer subtype in 200010,11. Patients with MpBC always suffered from the larger tumor12, triple-negative breast cancer (TNBC)13 and a higher risk in distant metastasis11,14,15,16. Several small size studies reported that patients with MpBC after NeoAT always performed a lower pathologic complete response (pCR) rate of approximately 10-23% and little survival benefits compared to those with TNBC9,17,18,19,20,21,22,23,24,25,26,27,28. Therefore, there were currently no standard treatment strategies for TNMpBC, owing to the controversy in survivorship efficacy of NeoAT for the TNMpBC, and the preferred therapeutic options for TNMpBC patients were lack of clinical evidence7.
Consequently, a large cohort of women with primary TNMpBC from multi-centers and Surveillance, Epidemiology and End Results database (SEER) and Chinese multi-centers data were conducted imminently in order to further explore and ascertain the benefits of NeoAT in patients with TNMpBC, identifying the prognostic variables of breast cancer specific survival (BCSS) for related population and develop a TNMpBC survival prediction model for assessing the BCSS for TNMpBC patients with NeoAT.
Method
Data source
The multi-centers data was collected from the first affiliated hospital of Xi’an Jiaotong University, the affiliated Cancer Hospital of Xinjiang Medical University and GanSu Cancer Hospital, written informed consent was not needed due to the retrospective study. In this study, all of the procedures complied with approved guidelines, and the details of ethics for this study were examined and approved by the first affiliated hospital of Xi’an Jiaotong University.
The SEER data was obtained from the SEER database, incidence-SEER Research data, 17 Registries, Nov 2023 Sub (2000–2021). The case listing was generated by SEER*Stat Software (Version: 8.4.3, https://seer.cancer.gov/seerstat/ r.gov/seerstat/, Information Management Service, Inc. Calverton, MD, USA, User ID : 16863-Nov2021). The related private and identity information were erased by the SEER database, thus the formalities for inform consent from patients could be omitted.
Patient cohort
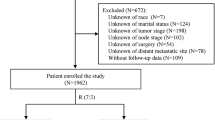
Totally, from multi-center hospitals, the clinical information of 182 patients, who were pathologically confirmed primary female TNMpBC in 2010–2023,were collected in the current study. The diagnosis criterion of MpBC was according to the 5th edition of WHO classification of tumours of the breast29. All specimens were diagnosed and verified by pathologists (Yina Jiang and Ran Zhang). Patients were excluded based on the following criteria with: (1) non -TNBC MpBC; (2) no NeoAT or AT following NeoAT; (3) distant metastasis cases; (4) absence of surgery or chemotherapy; 5)loss of follow-up.
Moreover, the inclusion criteria were as following: (1) primary female TNMpBC8 (ICD-0-3 morphology codes: 8032, 8035, 8052, 8070–8075, 8560, 8562, 8570–8575, 8980–8982); (2) non metastatic cases; (3) received NeoAT (systemic therapy before surgery) or AT (systemic therapy after surgery). The exclusion criteria were following, with: (1) no surgery or chemotherapy; (2) absence of T stage or N stage; (3) unknown or indefinite pathological response status to NeoAT. Data with missing values in the aforementioned were deleted.
A total of 1163 participants (alive or death caused by TNMpBC) were selected as the original cohort. One patient’s age at diagnosis had a recordation with 90 + was regarded as 90 years old. The NeoAT group was divided precisely into pCR (pathologic complete response) cohort, PR (partial response) and NR (no response) subgroups according to the stated pathologic response. Randomized group was performed to assign patients in NeoAT cohort to training and validation data set with a ratio 1 to 1 to construct and validate the predictive model for the forward research. The flowchart of selection could be seen in Supplement Fig. 1.
End points
The follow-up end time of multi-centers cohort was set at Dec 31, 2023, and the end time of SEER cohort was Dec 31, 2021. The principal outcome was the construction of TNMpBC-NeoBCSS model for assessing the BCSS, defined as the duration from the date of diagnosis to the date of death from TNMpBC30. One-half a month of follow up time was added to those patients with 0 recorded survival months.
Race and ethnicity, and age variables
Based on information from the item of “Race and origin recode” offered by SEER database, the racial and ethnic categories were divided into the following group: White, Black, and other race groups, patients in the multi-centers data were grouped in the other group. Age variables were divided into young adults (younger than 40 years old), middle aged (40–64 years old) and elder (65 years and older) segments according to the young age group previously reported31,32.
Statistics analysis
R package “Matching” was applied to implement propensity score match (PSM) to balance the confounding factors and basic characteristics between the NeoAT and AT groups. Nearest neighbor algorithm with a 1:1 matching ratio was chose and a caliper value set at 0.2. Pearson’s χ2 test was adopted to test the independence of patient demographics and treatment-related variables in different groups. The “tableone” package in R studio was utilized to export basic characteristics table among cohorts.
Kaplan - Meier (KM) analysis was administrated to assess the differences of survival outcome whereby we undertook the estimation of BCSS between NeoAT group and AT group. Schoenfeld residuals were tested and plotted to verify the applicability of proportional hazard assumption. Univariate and multivariate Cox regression models were conducted to assess the impact of the administration of NeoAT on survival. The least absolute shrinkage and selection operator (LASSO) regression was employed to screen out the valuable variables in NeoAT cohort to generate the predictive model. Sequentially, the nomogram of TNMpBC-NeoBCSS model constructed by R packages “rms” was established to predict BCSS probability, based on the selected variables. The points of the nomogram model were extracted on the basis of R package “nomogramFormula”. Online dynamic nomogram website built by ‘DynNom’ package was designed to provide a more intuitive visualization of the model. Harrell’s concordance-index (C-index) and receiver operating characteristics (ROC) curves were performed to evaluate the performance of the model. The calibration curve and decision curve analysis(DCA) were also plotted to assess the consistency between the observed and reality and usefulness of this predictive model. Finally, X-tile programmed by Yale University School of Medicine was preformed to calculated the optimum point and stratify the risk group for TNMpBC patients who received NeoAT. R software (version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ ) was used for calculations. A two-sided P value < 0.05 was considered statistically significant.
Results
Patient characteristics
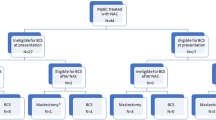
Among the 1163 patients diagnosed with primary TNMpBC enrolled (Table 1), totally 155 patients (13.3%) patients received NeoAT (NeoAT group), with 29.0% (45/155) of them acquired pathologic complete response (pCR), while 1008 (86.7%) of them underwent AT (AT group). In comparison with those in the AT group people in the NeoAT group tended to be younger age, higher proportion of advanced tumor invasion, metastatic lymph nodes, breast mastectomy and radiation therapy (Table 1). And, the majority of subjects in the pCR cohort had a tendency of earlier stage tumour and higher proportion of breast conserving surgery, when compared with those in PR and NR cohorts (Supplement Table 1).
Due to the quantity gap between the two groups, PSM analyses were employed to eliminate potential confounding factors. Totally 306 cases were enrolled in the PSM cohort, with 153 ones in each group. The discrepancies of interference variables between the two groups were mitigated, no significant differences were noted (Table 1). Moreover, the baseline characteristics estimation of our data were presented in Supplement Table 2. Additionally, comparison was conducted between those deleted because of uncertain pCR status data and those included, demographic and tumor variables were similar (Supplement Table 4).
Survival outcomes in matched and unmatched cohorts
Primarily, a total of 38 deaths (24.5%, 38/155) occurred in the NeoAT group and 162 (16.1%, 162/1008) ones in the AT group in the original cohort, respectively. Survival analysis indicated that patients approached pCR had a delightful BCSS ( 5-year survival, 96%), followed by subjects in the AT group and PR subgroup, and subjects in the NR subgroup had lowest survival rate (5-year survival: PR vs. NR, 67.6% vs. 48.4%) (P < 0.001, Fig. 1a). Approximate tendency persisted in the matched cohort (P < 0.001, Fig. 1b).
Assessment of the factors
Based on the results of univariate and multivariate Cox regression analyses affecting BCSS, T stage, N stage and treatment methods, were independent prognostic factors of BCSS for patients with TNMpBC (Tables 2 and 3). Patients in the PR subgroup and NR subgroup had nearly 5 times (HR,5.44,95% CI:0.70 to 42.49) and 10 times (HR,10.25,95% CI:1.34 to 78.68) (Table 3), respectively, risk of breast cancer specific death than those in the pCR subgroup. Therefore, we renewed the interest to construct a nomogram model to predict the BCSS for patients with TNMpBC with regard to the response to NeoAT (named as TNMpBC-NeoBCSS model).
The selection process of variables and cohorts Building
LASSO regression on the factors affecting BCSS was employed to screen the variables with regard to the TNMpBC-NeoBCSS model to predict the BCSS for patients with TNMpBC. The diagram of the LASSO regression (Supplement Fig. 2a & Supplement Fig. 2b) demonstrated that the only three predictive variables (T stage, N stage and response to NeoAT) with nonzero coefficients were identified. Age at diagnosis were included as predictive variables according to the joint discussion of authors.Then, a total of 77 participants were randomly assigned to the training cohort and 78 ones were randomly assigned to the validation cohort, with no significant differences in the clinicopathologic features observed between the two cohorts (P > 0.05).
The construction and validation of the predictive model
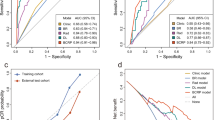
The TNMpBC-NeoBCSS model was established based on the identified variables affecting BCSS in the NeoAT cohort. The predictive model demonstrated that T stage had the greatest impact on BCSS, followed by age at diagnosis, N stage, and response to NeoAT. The C- index of the model was as high as 0.82. The 3- and 5-year BCSS probabilities could also be calculated easily by the nomogram tool (Fig. 2), with the AUC values for 3- and 5-year of 0.838, 0.866 in the training cohort, respectively, and 0.922, 0.868 in the validation cohort, respectively (Supplement Fig. 3a and d). And a delightful consistency was between the prediction and the reality (Supplement Fig. 4a and d) and the model had favorable accuracy (Supplement Fig. 4e).To visualize and apply more conveniently, an online nomogram was built (Supplement Fig. 5, https://xjtufreshnp.shinyapps.io/MpBCandNeo/).
Risk stratification
Based on the cutoff points of 150.0 extracted from the TNMpBC-NeoBCSS model and determined by X- tile software (Supplement Fig. 6), a total of 120 (77.4%, 155) ones were distributed into low risk breast cancer specific mortality group (low risk group, total points ≤ 150.0) while 35 (22.6%, total points ≥ 151.0) cases belonged to high risk breast cancer specific mortality group ( high risk group). Furthermore, as shown in Supplement Table 3, patients in the low risk group had more favorable clinicopathological featured and a pCR rate of 37.5%, in comparison with those in high risk group, with a pCR rate of 0%. In summary, patients in high risk group had highly inferior survivorship in comparison with those with low risk (3-year survival probability: 88% vs. 32%; 5-year survival probability: 81% vs. 25%) (Fig. 3, HR = 6.77, P < 0.0001).
Discussion
In the current study, a predictive model named TNMpBC-NeoBCSS was built based on clinicopathologic features, in a cohort from multi-centers data in China and the SEER database, for evaluating the 3- years and 5-years BCSS for patients with TNMpBC who had received NeoAT. The model of TNMpBC-NeoBCSS was established by combining the information of age at diagnosis, T stage, N stage and response to NeoAT. So far as we know, our model of TNMpBC-NeoBCSS is the first established to provide the predictive basis for the 3- years and 5-years BCSS for patients with TNMpBC who have received NeoAT and help the decision-making for clinicians and TNMpBC patients with NeoAT.
It was well documented that MpBC patients usually presented with larger tumor size and higher proportion of positive lymph nodes compared with those with invasive ductal carcinoma11,12,33. These patients were likely to have a dismal survival outcome with an average survival even less than 1 year26,34. And subjects with MpBC tended to have worse clinical outcome and higher risk to develop distant metastasis in comparison with ones with TNBC11. Therefore, the effects of NeoAT in downgrading stage and preventing potential local metastasis3,5 were supposed to exert its predominance. However, due to the lower chemosensitivity in preoperative chemotherapy, and the chemotherapy- refractory characteristics, there weren’t sufficient evidences to demonstrated the benefits of NeoAT for patients with triple negative MpBC (TNMpBC)7,17,18,19,20,21,22. In the current study, the total pathologic complete response (pCR) rate was 29.0%, which was higher than that of 10-23% reported previouly9,22, and lower that of 33-61% in TNBC35,36,37. Meanwhile, among the 6 eligible NeoAT cases in our multi-centers data, as high as 33% (2/6) approached pCR. In addition, median age of patients in our data (46 years old) significantly younger than the reported recordation (50 years old)12. The main reason probably lied in the differences in the criteria of exclusion and inclusion. However, the precise prognosis value of response to NeoAT for TNMpBC patients remained unknowable.
As a powerful prognostic indicator for prediction of long-term clinical benefit in TNBC, the achievement of pCR after NeoAT meant improved survival35,36,37. In the current study, patients in the pCR subgroup had the most favorable BCSS than those in the other group. Such a result suggested that the administration of NeoAT had positive effect on improving BCSS for certain types of patients with MpBC, and reinforced that patients who attained pCR had improved survival. Therefore, it was necessary to forecast the breast cancer-specific survivorship benefit for the TNMpBC patients after NeoAT to help do the treatment decision-making in clinical works, based on the factors affecting BCSS.
Through PSM analysis, univariate and multivariate Cox regression analysis, we observed patients in PR and NR subgroups had nearly 10 times, 20 times higher risk in death from breast cancer, when compared to those acquired pCR. In addition, patients in T4 tumor had also stunning breast cancer death risk (HR = 30.17 & HR = 19.40, Tables 2 and 3), which indicated the remarkable survival effect caused by tumor stage, while the lymph nodes status also influenced the breast cancer death significantly. On the basis of multivariate Cox regression analysis, we noticed that age at diagnosis, T stage, N stage and response to NeoAT manifested significantly statistical association with BCSS. Such results were in accordance with the previous works23,27,38,39. Additionally, one research that published recently indicated that patients in no-response group exposed higher risk in death from breast cancer9.
Based on selected variables identified by the LASSO regression and reported previously, the predictive model named TNMpBC-NeoBCSS model was constructed to provide more accurate evidence on BCSS estimation and therapy evaluation in patients with TNMpBC. The C-index of 0.82 in training cohort indicated the good performance of the nomogram in predicting the BCSS outcome for MpBC patients after NeoAT. The point to each factor could be attained by drawing a downward vertical line on the nomogram, and the aggregate point would be easily calculated, then the survival probabilities would be acquired. Subsequently, an online nomogram tool was designed to the clinical work conveniently. The points of all cases were extracted by specific R package further, anticipating to calculate more accurately. Based on the provided information for a 45 years old woman with PR status at T3N1 stage, the total score on the TNMpBC-NeoBCSS model would be nearly 139 scores. Meanwhile the patient was classified in low risk population with a good prognosis: the 3- and 5-year survival probabilities for this patient were 83% and 76%, could be observed clearly.
To validate the ability of the predictive model to discriminate between patients at different risk levels, a risk score system was also established based on the scores in TNMpBC-NeoBCSS model. And then patients were divided into low and high risk subgroups based on the cutoff score of 150.0 from the TNMpBC-NeoBCSS model through X- tile software. It is shocking that patients in the low risk group had as high as 37.5% pCR rate, and improved BCSS, and patients in the high risk group had a pCR rate of 0%, which indicated the low chemosensitivity and the corresponding BCSS. Notably, elder age, poorer differentiation level, higher proportion of advanced tumor stage and involved lymph node were observed for patients in high risk subgroup. Survival analysis concurrently revealed that these people had the poorest prognosis. Such results further confirmed the great significance of prediction effect in TNMpBC-NeoBCSS model and provide the important recommendations for appropriate population in the application of NeoAT among the MpBC.
The reliability of the current study lied on its sufficient sample size, which enabled us to comprehensively analyzed and constructed the model to predict the prognosis of MpBC patients with NeoAT. The robust AUC value represented the confidential prediction efficacy, enhancing the application of the model to forecast BCSS for related population. This study also had several shortcomings. First, as a retrospective research, it couldn’t avoid the existence of the selection bias. Patients who underwent AT following NeoAT and those who had uncertain pCR status were excluded, which may influence the value of pCR rate. We also didn’t conduct a survival analysis further to ascertain individuals who received adjuvant chemotherapy without pCR in comparison with those who achieved pCR. Second, some important clinicopathological variables weren’t included in the current study, such as family history, patient anxiety, histology of MpBC40, concrete regimens of NeoAT and AT, frailty, or comorbid conditions, due to the lack of these data in SEER database. Subsequent research cannot be pursued because of the aforementioned constraints. In our multi-center cohort, oral capecitabine is typically used for postoperative consolidation therapy. Additionally, we exhibit the specific Chemo regimens administered to patients in multi-centers data in Supplement Table 5 and comprehensive histological details of metaplastic carcinoma in Supplement Table 6. Thirdly, tumor grade would differentiate better after the application of NeoAT3, which may improve survival and influence the result, though differentiation level had no significant statistical disparity in this study. Lastly, on account of the inclusion criteria enrolling the TNMpBC patients only, the TNMpBC-NeoBCSS model wasn’t appropriate to the patients with positive hormone receptor or positive HER2 receptor (human epidermal growth factor receptor-2). Therefore, the result need to be verified further in a larger multi-center clinical cohort. Despite these limitations, the conclusions and the predictive model had well application value.
Conclusion
Our study demonstrated that the total pathologic complete response (pCR) was 29.0%, and the acquirement of pathologic complete response (pCR) meant the improved BCSS for patients with TNMpBC. Based on selected variables identified by the LASSO regression and reported in the previous researches, the prediction model of TNMpBC-NeoBCSS was built. And the TNMpBC-NeoBCSS model could provide a favorable tool for assessing the BCSS for TNMpBC patients with NeoAT and may help doctors and TNMpBC patients optimally make decision on the necessity of neoadjuvant therapy on the basis of individual BCSS.
Data availability
The primary data were obtained from the public open database: Surveillance, Epidemiology, and End Results database: https://seer.cancer.gov/And the multi-centers data could be available after the approval of corresponding authors.
References
Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 39 (13), 1485–1505. https://doi.org/10.1200/JCO.20.03399 (2021).
Gradishar, W. J. et al. NCCN Guidelines® insights: breast cancer, version 4.2023. J. Natl. Compr. Canc Netw. 21 (6), 594–608. https://doi.org/10.6004/jnccn.2023.0031 (2023).
Thompson, A. M. & Moulder-Thompson, S. L. Neoadjuvant treatment of breast cancer. Ann. Oncol. 23 (Suppl 10), x231–x236. https://doi.org/10.1093/annonc/mds324 (2012).
Killelea, B. K. et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer database. J. Am. Coll. Surg. 220 (6), 1063–1069. https://doi.org/10.1016/j.jamcollsurg.2015.02.011 (2015).
Colomer, R. et al. Neoadjuvant management of early breast cancer: A clinical and investigational position statement. Oncologist 24 (5), 603–611. https://doi.org/10.1634/theoncologist.2018-0228 (2019).
Heil, J. et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Ann. Oncol. 31 (1), 61–71. https://doi.org/10.1016/j.annonc.2019.10.012 (2020).
Tray, N., Taff, J. & Adams, S. Therapeutic landscape of metaplastic breast cancer. Cancer Treat. Rev. 79, 101888. https://doi.org/10.1016/j.ctrv.2019.08.004 (2019).
Ma, Y. et al. Research on the role of combined chemotherapy and radiotherapy in patients with N + Non-Metastatic metaplastic breast carcinoma: A competing risk analysis model based on the SEER database, 2000 to 2015. Front. Oncol. 10, 583488. https://doi.org/10.3389/fonc.2020.583488 (2021).
Zhang, M., Yuan, J., Wang, M., Zhang, M. & Chen, H. Chemotherapy is of prognostic significance to metaplastic breast cancer. Sci. Rep. 14 (1), 1210. https://doi.org/10.1038/s41598-024-51627-1 (2024).
Thomas, H. R. et al. Metaplastic breast cancer: A review. Crit. Rev. Oncol. Hematol. 182, 103924. https://doi.org/10.1016/j.critrevonc.2023.103924 (2023).
El Zein, D. et al. Metaplastic carcinoma of the breast is more aggressive than Triple-negative breast cancer: A study from a single institution and review of literature. Clin. Breast Cancer. 7 (5), 382–391. https://doi.org/10.1016/j.clbc.2017.04.009 (2017).
Bian, T. et al. Metaplastic carcinoma of the breast: Imaging and pathological features. Oncol. Lett. 12 (5), 3975–3980. https://doi.org/10.3892/ol.2016.5177 (2016).
Weigelt, B., Eberle, C., Cowell, C. F., Ng, C. K. & Reis-Filho, J. S. Metaplastic breast carcinoma: More than a special type. Nat. Rev. Cancer. 14 (3), 147–148. https://doi.org/10.1038/nrc3637 (2014).
Abouharb, S. & Moulder, S. Metaplastic breast cancer: Clinical overview and molecular aberrations for potential targeted therapy. Curr. Oncol. Rep. 17 (3), 431. https://doi.org/10.1007/s11912-014-0431-z (2015).
Tan, Y., Yang, B., Chen, Y. & Yan, X. Outcomes of metaplastic breast Cancer versus Triple-Negative breast cancer: A propensity score matching analysis. World J. Surg. 47 (12), 3192–3202. https://doi.org/10.1007/s00268-023-07106-1 (2023).
Tadros, A. B. et al. Survival outcomes for metaplastic breast Cancer differ by histologic subtype. Ann. Surg. Oncol. 28 (8), 4245–4253. https://doi.org/10.1245/s10434-020-09430-5 (2021).
Hennessy, B. T. et al. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann. Oncol. 17 (4), 605–613. https://doi.org/10.1093/annonc/mdl006 (2006).
Abada, E. et al. Clinicopathologic characteristics and outcome descriptors of metaplastic breast carcinoma. Arch. Pathol. Lab. Med. 146 (3), 341–350. https://doi.org/10.5858/arpa.2020-0830-OA (2022).
Wong, W. et al. Poor response to neoadjuvant chemotherapy in metaplastic breast carcinoma. NPJ Breast Cancer. 7 (1), 96. https://doi.org/10.1038/s41523-021-00302-z (2021).
Al-Hilli, Z. et al. Metaplastic breast cancer has a poor response to neoadjuvant systemic therapy. Breast Cancer Res. Treat. 176 (3), 709–716. https://doi.org/10.1007/s10549-019-05264-2 (2019).
Haque, W. et al. Neoadjuvant chemotherapy for metaplastic breast cancer: response rates, management, and outcomes. Clin. Breast Cancer. 22 (5), e691–e699. https://doi.org/10.1016/j.clbc.2022.01.006 (2022).
Yam, C. et al. Molecular characterization and prospective evaluation of pathologic response and outcomes with neoadjuvant therapy in metaplastic Triple-Negative breast Cancer. Clin. Cancer Res. 28 (13), 2878–2889. https://doi.org/10.1158/1078-0432.CCR-21-3100 (2022).
Zheng, C. et al. Clinical characteristics and overall survival prognostic nomogram for metaplastic breast cancer. Front. Oncol. 13, 1030124. https://doi.org/10.3389/fonc.2023.1030124 (2023).
Li, Y. et al. Construction and validation of prognostic nomogram for metaplastic breast cancer. Bosn J. Basic. Med. Sci. 22 (1), 131–139. https://doi.org/10.17305/bjbms.2021.5911 (2022).
Haque, W., Verma, V., Naik, N., Butler, E. B. & Teh, B. S. Metaplastic breast cancer: Practice patterns, outcomes, and the role of radiotherapy. Ann. Surg. Oncol. 25 (4), 928–936. https://doi.org/10.1245/s10434-017-6316-2 (2018).
Tang, J., Zhang, D. & Pan, X. Development and validation of competitive risk model for older women with metaplastic breast cancer. BMC Womens Health. 23 (1), 374. https://doi.org/10.1186/s12905-023-02513-x (2023).
Thériault, K. et al. A Single-Center 18-Year Series of 73 Cases of Metaplastic Carcinoma of the Breast. Breast J. ; 2024:5920505. (2024). https://doi.org/10.1155/2024/5920505
Yang, W. T. & BuH Updates in the 5(th) edition of WHO classification of tumours of the breast. Zhonghua Bing Li Xue Za Zhi. 49 (5), 400–405 (2020).
Rakha, E. A. et al. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br. J. Cancer. 112 (2), 283–289. https://doi.org/10.1038/bjc.2014.592 (2015).
Yang, Z. et al. Research on the cutoff tumor size of omitting radiotherapy for BCSS after breast conserving surgery in women aged 65 years or Oder with low-risk invasive breast carcinoma: results based on the SEER database. Breast 60, 287–294. https://doi.org/10.1016/j.breast.2021.11.015 (2021).
Jiang, D. et al. Trends in cancer mortality in China from 2004 to 2018: A nationwide longitudinal study. Cancer Commun. (Lond). 41 (10), 1024–1036 (2021).
Seldon, C. et al. Variation in management of extremity Soft-Tissue sarcoma in younger vs older adults. JAMA Netw. Open. 4 (8), e2120951 (2021).
Jung, S. Y. et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res. Treat. 120 (3), 627–637. https://doi.org/10.1007/s10549-010-0780-8 (2010).
Takala, S., Heikkilä, P., Nevanlinna, H., Blomqvist, C. & Mattson, J. Metaplastic carcinoma of the breast: prognosis and response to systemic treatment in metastatic disease. Breast J. 25 (3), 418–424. https://doi.org/10.1111/tbj.13234 (2019).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Zhang, L. et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): Results from a multicenter, randomized controlled, open-label phase II trial. Int. J. Cancer. 150 (4), 654–662. (2022).
Shepherd, J. H. et al. CALGB 40603 (Alliance): Long-Term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in Triple-Negative breast Cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.21.01506 (2022).
Zhu, K. et al. Prognostic factor analysis and model construction of Triple-Negative metaplastic breast carcinoma after surgery. Front. Oncol. 12, 924342. (2022).
Hu, J. et al. The mixed subtype has a worse prognosis than other histological subtypes: A retrospective analysis of 217 patients with metaplastic breast cancer. Breast Cancer Res. Treat. 200 (1), 23–36. https://doi.org/10.1007/s10549-023-06945-9 (2023).
McMullen, E. R., Zoumberos, N. A. & Kleer, C. G. Metaplastic breast carcinoma: Update on histopathology and molecular alterations. Arch. Pathol. Lab. Med. 143 (12), 1492–1496. https://doi.org/10.5858/arpa.2019-0396-RA (2019).
Acknowledgements
We are thankful to the support from Xi’ an Jiaotong University and SEER database. The Figures are drawn by R software (version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ ).
Author information
Authors and Affiliations
Contributions
Peng Ni: data curation, formal analysis(Figure1-3, Supplement Table 4), Project administration, original draft writing . Yu Wang: formal analysis(Tables 1, 2 and 3, Supplement 5), visualization, primary artical revision. Xiaorong Bai: formal analysis(Supplement Tables 1, 2, 3 and 6, Supplement Figs. 3, 4 and 5), Software, Investigation.Tao Wu, Chen Gao and Fan Liu: data curation in each uni-center and Project administration. Zejian Yang, Yuan Cheng and Bohui Shi: formal analysis, validation of table in main manuscript and Investigation (Supplement Figs. 1, 2 and 6, prognosis information collection and verification). Ligang Niu, Yu Yan, Yuhui Zhou, and Guanqun Ge: Methodology, Software(R studio software). Yi long Cheng and Zhishen Ge: Investigation, Resources and Software(R studio software). Yina Jiang and Ran Zhang: Validation and Resources(verification of specimens).Bo Wang: Conceptualization, Data curation, Supervision, Validation and Writing–review & editing.Yu Ren: Conceptualization, Formal analysis, Supervision and Writing review & editing. Can Zhou: Conceptualization, project administration, funding acquisition, final supervision and Writing review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures in this study was performed in accordance with approved guidelines. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiao tong University. The ethic number, No: XJTU1AF2024LSYY-168.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ni, P., Wang, Y., Bai, X. et al. TNMpBC-NeoBCSS model: a breast cancer specific survival prediction model for triple-negative metaplastic breast carcinoma patients with neoadjuvant therapy. Sci Rep 15, 8351 (2025). https://doi.org/10.1038/s41598-025-91888-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91888-y