Abstract
By migrating, ungulates take advantage of cyclical fluctuations in resources, which allows them to persist at greater population numbers than they would in the absence of these seasonal movements. We sought to identify the drivers of spring elk (Cervus canadensis) migration and evaluate how well individuals were able to optimize access to forage prior to departure, while migrating, and upon arrival on summer range. Specifically, we investigated the timing and duration of spring migration in four Colorado elk herds to test how forage quality and snow dynamics pre-, during, and post-migration influenced elk departure from winter range and the length of time spent migrating. Our analyses revealed significant heterogeneity among and within herds. Overall, spatiotemporal dynamics in forage and snow emerged as critical drivers influencing migratory phenology, but the discrete covariates associated with forage and snow were herd-specific. We did not find marked sub-strategies wherein some herds or individuals optimized a specific component of migration, but rather found that elk varied in their ability to optimize access to forage at all times analyzed. Our findings suggest that elk exhibit a flexible response to environmental cues, adjusting migration timing and duration in accordance with local conditions. These results contribute to a deeper understanding of the mechanisms driving ungulate migration and emphasize the importance of considering dynamic environmental factors in studies of migration phenology. Further, they emphasize the importance of forage conditions not just on the migratory route, but also on winter and summer range. Additionally, they provide baseline knowledge of elk migrations in Colorado, useful information for ensuring the conservation and persistence of these migratory routes and the productivity they facilitate.
Similar content being viewed by others
Introduction
The timing and duration of migration events vary broadly as a function of species, geographic location, and environmental drivers. Determinants of migratory timing in mammalian, avian, reptilian, and invertebrate species generally relate to cyclical phenomena1,2,3,4. Diurnal change in predation risk5, access to forage6 or water7, and alleviation of extreme seasonal conditions are all potential drivers of migratory behavior, with the spatiotemporal nature of these factors influencing migratory timing8,9. The magnitude and direction in which these factors influence migration is often species- or system-specific10,11. Much of our knowledge on migratory ecology is built upon single-species and even single-system work, however (but see1,11). Studies incorporating multiple populations of a single species can be fruitful in this regard, as studying migratory behavior in differing environmental contexts can help tease apart the mechanisms driving migration.
The Forage Maturation Hypothesis (FMH) links animal movement to the spatiotemporal onset and distribution of high-quality forage. The FMH postulates that animals balance resource quality and quantity by foraging on intermediate levels of forage biomass to maximize energy intake12. For migratory species, the FMH predicts that animals should time their arrival to a seasonal range to optimize access to high-quality vegetation13. An extension of the FMH, called the green wave hypothesis (GWH), asserts that migratory individuals are driven by dynamic wave-like gradients of vegetative productivity and move to maximize access to optimal forage along the migration route14,15,16,17. This movement along the vegetation gradient is often colloquially referred to as ‘surfing’ the green wave and has shown success in explaining migratory behavior and phenology in many systems and species3,18,19. The FMH and GWH are important hypotheses for the investigation of migratory timing because they directly relate migratory movements and their motivation to temporally dynamic and measurable environmental phenomena17.
Surfing, however, is only one of the mechanisms potentially driving migration in ungulates. In some systems, the motivation for migrating from winter to summer range appears to be to prioritize access to forage on summer range. For example, both caribou (Rangifer spp.) and Norwegian red deer (Cervus elaphus) opted to ‘jump’ the green-wave (hereafter jumping) to time their arrival to match peak resource availability13,15. A comparative analysis of five ungulate species failed to find evidence of surfing in elk (Cervus canadensis) populations in the Greater Yellowstone Ecosystem or in bison (Bison bison) populations in Utah11. It appears that whether an animal surfs the green wave is highly dependent on landscape-level processes, i.e., the order, rate, and duration of green-up along the migratory route, a concept referred to as the ‘greenscape’18. Further, because high-quality forage is available at different times on winter range, along the migration route, and on summer range, individual variation in foraging decisions also likely plays a role in whether or not individuals surf.
While the FMH and GWH are the main hypotheses used to make predictions regarding migration phenology, factors other than forage can impact migration. For example, the impact of snow on migratory timing has also proven to be significant for migratory ungulates, such as elk, caribou, and red deer, potentially decoupling the relationship between movement phenology and forage dynamics3,20,21. Moreover, costs and limitations associated with parturition and nursing, or neonate safety can also shape migratory decisions. Individuals may finance reproduction using resources previously acquired and stored (e.g., as fat; “capital breeding”) or by relying on resources currently available to them in the environment (“income breeding”22). Recent work conducted on mule deer in Wyoming, demonstrated that the reliance upon capital or income resources varied with season, stage of reproduction, and individual23. Thus, ungulates are likely responding to the interplay of multiple dynamic environmental and physiological factors when timing migratory events, not just forage maturation. In the spring, snow conditions or constraints associated with calving could lead ungulates to delay migration to prioritize foraging on winter range or to jump the green wave to prioritize foraging on summer range. Understanding how ungulates are trying to optimize access to forage prior to departure, along the migration route (surfing), post-arrival (potentially jumping), or through some combination of all of these can enhance theory underlying migratory timing and the sources of its variability among populations and systems.
In addition to population-level responses, individuals within populations may also vary, but oftentimes this source of variation is not revealed during population-level investigations. This may be consequential, as interindividual variation has the potential to decrease or even cancel the magnitude of the population-level response. Variation in factors such as departure dates, migration duration, distance traveled between seasonal ranges, number of stopover sites, and speed of travel among individuals has led to the concept of diverse intrapopulation ‘migratory portfolios’24,25. Ultimately, this variation can result in differential access to peak forage quality among individuals within a population and potential differences in fitness over time26. To assist in quantifying this level of variation, we developed a measure of ‘foraging optimality’ by looking at the difference in forage quality between an individual’s current use of a location and the maximum quality of forage available at that location throughout the year15. Evaluating when this optimality is highest (prior to departure, during the migration, or upon arrival) can provide insight into the drivers of individual and population-level migration (e.g. ‘surfing’, ‘jumping’, or intermediate behaviors).
Elk migration has been studied extensively across their range in North America11,27,28,29. Interestingly, their marked plasticity and movement diversity compared to other temperate migratory ungulates suggest that the determinants and parameters of their migratory timing are more flexible and likely more complex than in some other species25. Indeed, elk exhibit high plasticity in the departure and arrival dates associated with their seasonal ranges, generally as a function of environmental conditions3,26. Previous research has also found mixed evidence regarding whether elk surf the green wave11,26. Here, we leveraged a multi-system study of elk inhabiting four markedly different environments to distinguish potential drivers of elk migration. Instead of focusing on a single potential driver of migration (e.g., whether or not elk are surfing), we tested multiple potential drivers to evaluate how conditions pre-, during, and post-migration impacted elk migration phenology. We first estimated spring migratory phenology (departure date and duration) in four migratory elk herds and related those to metrics of timing of peak forage quality and snowmelt. We also investigated inter-individual variation in forage access optimality in the pre-, during-, and post-migratory periods using a clustering algorithm to test for different sub-strategies within and across elk herds. We hypothesized that individual variation and the different environmental contexts present in each system would lead to elk prioritizing different aspects of migration (pre-, during, or post-) in each herd, resulting in different covariates best explaining migration timing and duration across herds. Building on the migration portfolio hypothesis, we also predicted that there would be sub-strategies among and within herds regarding how elk prioritize access to forage. Our results offer insight into the potential mechanistic drivers of migration phenology across broad environmental contexts, work that is crucial for conservation given that migration is declining globally30,31.
Methods
Study area
We studied elk from four Colorado elk herds, the Avalanche Creek (Colorado Parks and Wildlife Data Analysis Unit [DAU] E-15), Bear’s Ears (DAU E-2), Trinchera (DAU E-33), and Uncompahgre Plateau (DAU E-20) herds that all wintered in Colorado, USA (Fig. 1). In 2021, population estimates for each herd were: Avalanche Creek ~ 4800, Bear’s Ears ~ 18,400, Trinchera ~ 14,000, and Uncompahgre Plateau ~ 12,500. Elk from the Bear’s Ears herd infrequently crossed into Wyoming, while elk from the Trinchera herd often crossed into New Mexico. Similar to other partially migratory elk populations, migrants in these systems tend to overwinter at low elevations and venture to higher elevation ranges for the summer. Resident elk, by contrast, remain year-round on one range or regularly commute between two ranges.
Elevation in the study area ranges from ~ 1500 m at lower elevations to > 4000 m in mountainous regions. Vegetation communities are diverse and largely determined by altitude and aspect32. Low elevations (below 2000 m) harbor pinyon pine (Pinus edulis)-Utah juniper (Juniperus osteosperma) forests, plains grasslands, sagebrush steppe, high desert, desert basins, and river valleys. Moderate elevations (2000–3500 m) host montane shrubs, oak (Quercus gambelii), sagebrush (Artemisia spp.), aspen (Populus tremuloides) or mixed aspen/conifer, Engelmann spruce (Picea engelmannii), and fir (Abies spp.) communities, while high altitudes (> 3500 m) are characterized by alpine systems or talus slopes. The study area is a mosaic of public and private land ownership.
Animal capture and monitoring
We captured yearling and adult female elk on winter ranges by net-gunning and darting them from a helicopter during January-March 2017–2021. We radio-collared elk with Global Positioning System (GPS) collars programmed to acquire a location every 1, 2, or 4 h (G2110E2, G5-2D, Advanced Telemetry Systems, Isanti, MN, USA) and monitored most individuals for 13–18 months. We collared 104 elk from the Avalanche Creek herd (2019–2021; ~ 2% of the estimated population), 82 elk from the Bear’s Ears herd (2019–2021; < 1% of the estimated population), 113 elk from the Trinchera herd (2017–2021; ~ 1% of the estimated population) and 158 elk from the Uncompahgre Plateau herd (2017–2021; ~ 1% of the estimated population). Additionally, we outfitted most pregnant females with vaginal implant transmitters (VITs; M3930U, Advanced Telemetry Systems, Isanti, MN, USA) during capture. See Appendix Table A1 for annual collaring totals. In all of our analyses, each elk was only included once, i.e., each elk only contributed one elk-year of data to each analysis. This study was carried out in accordance with ARRIVE guidelines33. All capture, handling, and collaring of elk were completed in accordance with applicable guidelines and regulations and were approved by the Colorado Parks and Wildlife Animal Care and Use Committee (protocol IDs: 02–2017, 01–2019, 02–2019, 01–2020, 03–2020).
Identifying migrants and residents
We followed the methods of Bunnefeld et al. (2011) and used time-series of net-squared displacement (NSD) methods and positional elevation to classify movement strategies of individual elk34. We categorized elk as either migrants, residents, commuters, or dispersers. We classified individuals that exhibited discontinuous season-specific space use with movement events between winter range and summer range as migrants. We classified individuals that inhabited one range year-round or that exhibited regular year-round movement between proximate ranges (generally along a short-distance elevational gradient) as residents. We classified individuals that demonstrated long-distance movement away from their initial location without return as dispersers. Individuals falling outside of all established categories were considered ‘ambiguous’ (Appendix Figure B1). We determined seasonal space use of each elk using the timing of their migrations, producing estimates of departure and arrival dates by fitting movement models with the R package MigrateR35 (see Appendix A for more details).
Variable extraction
For covariate extraction purposes, we included elk-years of migratory individuals with clear spring departure and arrival dates, complete spring migrations in their data, and at least two weeks of space use on both sides of spring migration events (i.e. pre-departure period in the winter range and post-arrival period in the summer range). We selected a two-week period to characterize the spatiotemporal conditions of forage immediately prior to departing from winter range and immediately after arriving on summer range. Elk-years with incomplete tracking data were included in migration metrics calculations (Table B1) when possible but excluded from other statistical analyses. Because collaring in the Uncompahgre Plateau herd occurred after spring migration had already begun, we could not estimate departure timing in the first year of monitoring, and instead used data from the second year. We extracted spatiotemporal covariates over three periods: 1) the two weeks prior to departure from winter range, 2) the spring migration, and 3) the two weeks following arrival at summer range (hereafter pre-departure, during migration, and post-arrival respectively). We used occurrence distributions calculated from continuous-time movement models to delineate space use in each period36. We determined the distance between winter and summer ranges by calculating the distance (in kilometers) between the centroids of the two-week periods of space use on either side of the spring migration.
We calculated instantaneous rate of green-up (IRG) metrics from the MODIS09Q1 Version 6 MODIS/Terra Reflectance product (250 m, 8-day resolution) by fitting a double sigmoid curve to 8-day Normalized Difference Vegetation Index15. From these curves, we extracted both interpolated daily IRG values and year-specific estimates of the ordinal day of peak annual IRG per pixel11,15. We calculated the mean per-location IRG optimality and the mean ordinal day of peak annual IRG over the spatial area encountered during the pre-departure, migration, and post-arrival periods. We calculated the instantaneous rate of green-up optimality for a given pixel and elk location as:
where OPT is optimality, y is the year, t is the ordinal day of the year, p is the pixel containing a given relocation coordinate, and IRG is the instantaneous rate of green-up value. The index theoretically ranges from 0 to 1, with 1 indicating perfect optimality, i.e., no difference between contemporaneous forage quality and the maximum forage quality value at that location for the year. This metric better reflects the current condition of forage in comparison to its maximum potential than the commonly used Days-From-Peak metric, because locations can have identical peak days, but green-up at different rates18. Additionally, we calculated the standard deviation in peak IRG date to represent variability in date of peak green-up along the migration route as a way of characterizing the greenscape18. We also extracted snow covariates from the same MODIS product. We calculated the per-pixel snow-free DOY representing the day-of-year where the absence of snow was first detected in an 8-day resolution time series for a given pixel3.
We estimated calving dates for elk in our analysis when data were available (n = 128). We estimated calving dates primarily using the expulsion date of vaginal implant transmitters (VITs; M3930U, Advanced Telemetry Systems, Isanti, MN, USA) inserted into pregnant females during capture. Occasionally, VITs failed or were expelled prematurely. In these cases, we attempted to use GPS collar location data from pregnant females to identify parturition events. If we verified a parturition event by capturing a calf or finding a birth site at a GPS cluster, we used the timestamp of the pregnant female’s first GPS location at that cluster as the estimated parturition date. We did not have calving date estimates for all elk-years used in our analyses because we were not able to identify a calving date for every elk-year, and because we sometimes used GPS datasets from elk-years in which elk no longer had VITs. As a result, analyses involving calving date sample sizes were reduced (Avalanche Creek n = 38, Bear’s Ears n = 59, Trinchera n = 12). Uncompahgre Plateau elk were excluded because we could not relate calving date to spring migration timing in this herd.
Departure and duration models
Our two response variables were departure date from the winter range and duration of the spring migration. Departure date was defined as the day-of-year (DOY) from January 1st of the focal year, and duration was defined as the number of days between departure from winter range and arrival at summer range. We estimated departure date if net-squared displacement data was asymptotic prior to displacements indicative of a migratory event (representing seasonal home-ranging behavior) to avoid including elk collared during their migration.
To generate models investigating potential determinants of spring departure date and the duration of migration, we used eight covariates as independent variables (see section above about how they were extracted or calculated). Productivity covariates included the mean peak IRG date pre-departure, the mean peak IRG date during migration, the mean peak IRG date post-arrival, and the standard deviation of peak IRG date along the migration route. Snow metrics included the mean snow-free date pre-departure, the mean snow-free date during migration, and the mean snow-free date post-arrival. Additionally, we included the distance between seasonal ranges as a covariate.
We used multiple linear regression to fit herd-specific models. We did not use a generalized linear mixed model framework that would have pooled data across herds due to convergence issues when including multiple random slopes to estimate herd-specific responses37. Our candidate models included every linear combination of each covariate with no more than four covariates per model. Models were limited to four covariates to avoid overfitting given our herd sample sizes. In the Trinchera herd we lowered this to a maximum of two covariates per model due to a lower sample size of migratory individuals relative to other herds (n = 17 elk). In each model we excluded covariates with Pearson’s correlation coefficient ≥ 0.7 (i.e. correlated variables were not included in the same model). We selected variables within correlated pairs by comparing univariate model fits and excluding the covariate whose model resulted in significantly lower explanatory power as determined by Akaike Information Criterion for small sample sizes (AICc)38. Where univariate models were equally parsimonious (ΔAICc < 2), we ran each version of the uncorrelated candidate model (i.e. models retaining one or the other correlated variable) and included them both in model selection. We fit each candidate model and selected the top model using AICc. A null, intercept-only, model was also included in the list of candidate models of elk migration phenology. We chose as our top model the most parsimonious model where ΔAICc was within two of the top-ranking model to identify the most important potential drivers (Appendix Table B2). We removed covariates with a Variance Inflation Factor ≥ 5 and removed influential outliers from the data39. We identified potential outliers using diagnostic plots and removed outliers if they were both abnormal and influential; n = 10 elk-years removed from models (Bears Ears n = 6, Uncompahgre Plateau n = 4). For models incorporating calving date as a covariate, our fitting and selection methods were identical to those used for our primary models but excluded the Uncompahgre Plateau herd, given that we could not relate calving date estimates to spring migratory timing in this herd (Appendix C).
Variation in optimality of access to forage
To explore inter-individual variation in exposure to IRG pre-, during-, and post-migration and identify potential sub-strategies, we used Gaussian-mixture model clustering from the R package mclust40. We used the mean estimated optimalities per individual during each respective period (pre-, during, and post-migration) as input covariates. Top models and the optimal number of clusters were automatically selected via Bayesian information criterion (BIC)38. We performed a chi-squared goodness of fit test using herd and optimality cluster as categorical variables to investigate the null hypothesis that there was no association between herd and optimality sub-strategy, i.e., we compared the observed frequency of herd in each cluster to a null expectation that each herd was divided evenly between clusters.
Results
We used location data from 147 migratory elk-years for departure date and duration models (sample sizes: Avalanche Creek n = 40; Bear’s Ears n = 59 [62 in total; 3 outliers excluded from each model]; Uncompahgre Plateau n = 28; Trinchera n = 17; see Appendix A for information on excluded individuals). Collectively, this represented 86,012 GPS points, with an average of ~ 585 locations per individual (SD: 245, range: 167–1,566).
We found both intra- and inter-population variation in spring departure date, migration duration, and distance (Figs. 2, 3; Appendix Table B1). Trinchera elk had the latest spring departure dates and the shortest and quickest migration (Table B1). Bear’s Ears elk migrated the farthest distances between ranges and took the longest. Notably, migration duration, distances, and elevation shifts ranged widely among all elk-years in this analysis (Duration: 0.33—69.8 days; Distances: 5.4—137 km; Elevation shifts: 0—1473 m), with some high-elevation short-distance routes taking less than a day to traverse and some long-distance migrations taking months. Mean calving date DOY was similar among herds, despite differing migratory phenology, with more than 84% of elk calving after completing their spring migration (Appendix Figure C1; see the Analyses including calving dates section below for additional details).
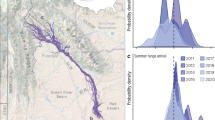
Spatial distribution of spring migratory routes in four populations of Rocky Mountain Elk in the Colorado Rocky Mountains tracked between 2017 and 2021 (generated from QGIS 3.32.3). Each contiguous line and color represent an individual and their migration route. Herds are labeled and herd-specific sample sizes used in our analyses are shown. Basemaps depict hillshade to illustrate topography. Avalanche Creek elk traveled straight-line distances of µ = 15.2 + /- 5 km (mean + /- standard deviation), Bear’s Ears elk traveled µ = 80.3 + /- 21 km, Trinchera elk traveled µ = 14.3 + /- 5.2 km, and Uncompahgre Plateau elk traveled µ = 29.5 + /- 15.1 km.
Violin plots showing herd-specific distributions of selected spring migratory statistics: spring departure date (in day-of-year or DOY), spring migration duration (in days), and spring migration distance (in kilometers). Herd acronyms are listed on the x-axis: AC Avalanche Creek (red), BE Bear’s Ears (blue), TR Trinchera (orange), UP Uncompahgre Plateau (dark yellow), Colorado, USA. The median is shown as a line in the violin plot. These values include all individuals where each respective metric was estimable (Table B1).
Departure date models
Covariates included in the top models for spring departure date varied by herd (Fig. 4, Appendix Table B2). In all models there was a positive relationship between covariates and spring departure date. The Avalanche Creek model included mean peak IRG date (peak-IRG) mid-migration (R2 = 0.65). The Bear’s Ears model included the standard deviation of peak IRG date during migration (SD peak-IRG) and the mean snow-free day of year (snowmelt) pre-departure (R2 = 0.37). The Trinchera model included peak-IRG post-arrival (R2 = 0.29). The Uncompahgre Plateau model included snowmelt mid-migration (R2 = 0.17).
Estimated response of spring departure date to covariates in the herd-specific top model for each Rocky Mountain Elk herd, Colorado, USA. The top model for every herd only had one covariate. Solid trend lines indicate statistical significance and dashed lines indicate non-significance (p ≤ 0.05). Trend line and dot colors represent herds. All independent variables are scaled. The grey shaded regions represent 95% confidence intervals for the plotted covariate.
Duration models
Covariates included in the top models for spring migration duration varied by herd (Fig. 5, Appendix Table B3). The Avalanche Creek model included peak-IRG post-arrival and snowmelt pre-departure with positive and negative trends respectively (R2 = 0.16). The Bear’s Ears model included peak-IRG post-arrival (positive), SD peak-IRG (negative), and snowmelt pre-departure (positive, non-significant; R2 = 0.46). For Trinchera, no model outperformed the null model. The Uncompahgre Plateau model included distance (positive; R2 = 0.18).
Estimated response of spring migration duration to covariates in the herd-specific top model for each Rocky Mountain Elk herd, Colorado, USA. Solid trend lines indicate statistical significance and dashed lines indicate non-significance (p ≤ 0.05). Trend line and dot colors represent herds. All independent variables are scaled. The grey shaded regions represent 95% confidence intervals for the plotted covariate.
Analyses including calving dates
In the separate analysis including calving date (Appendix Tables B4, B5), only the Avalanche Creek spring departure date model included this variable as a covariate in its top model (R2 = 0.74), with a positive relationship between calving date DOY and spring departure date (Appendix Figure C2). Notably, 9 of the 10 elk that calved on the date of their summer arrival were in Avalanche Creek (Appendix Figure C1). Calving date was not included as a covariate in the top model of any other herd.
Optimality of access to forage
The clustering algorithm produced two separate clusters (Fig. 6). Overall, the first cluster had lower optimality (i.e., how close in days to the highest vegetation quality an individual was to visiting a 250-m pixel) in all three periods compared to the second cluster. Neither cluster appeared to be predominantly defined by a single axis (Fig. 6). Cluster 1 generally comprised individuals with mid-to-high optimality in one or two dimensions, with lower values in the remaining dimensions. Cluster 2, by contrast, generally consisted of individuals with mid-to-high optimality in two or three dimensions and was more tightly clustered (Appendix Figure B2). Cluster composition varied by herd, with all herds represented in each cluster at different proportions (Appendix Figure B3; χ = 32.994, df = 7, p < 0.001). Avalanche Creek was split nearly evenly between cluster 1 (53%, n = 21) and 2 (47%, n = 19). Bear’s Ears was predominantly in Cluster 2 (Cluster 1 = 26%, n = 16; Cluster 2 = 74%, n = 46), whereas Uncompahgre was predominately in Cluster 1 (Cluster 1 = 71%, n = 20; Cluster 2 = 29%, n = 8). Trinchera was almost entirely in cluster 2 (Cluster 1 = 6%, n = 1; Cluster 2 = 94%, n = 16;). Overall, cluster 1 represented n = 58 migratory events (39%), while cluster 2 had n = 89 migratory events (61%).
Violin plots of period-specific mean optimalities (bound from 0–1) by cluster for four Rocky Mountain Elk herds in Colorado, USA. We defined optimality as the difference in forage quality between an individual’s current use of a location and the maximum quality of forage available at that location throughout the year. Cluster 1 is shown in red, while cluster 2 is shown in blue. Herds are represented by shapes. The median is shown as a line in the violin plot. Cluster-specific herd composition was variable.
Discussion
We evaluated how elk prioritized access to forage by investigating how forage and snow dynamics pre-, during, and post-migration influenced migration timing and duration. Leveraging data from four elk herds in Colorado, we found that spatiotemporal dynamics in forage and snow played an important role in influencing the phenology of spring migration. Herds varied regarding whether conditions pre-, during, or post-migration influenced their departure and duration. Overall, later green-up or snowmelt led to later migratory departure, but which aspect was most important varied by herd. Drivers of migration duration were more variable across herds.
Taken together, our models suggest that the determinants of migratory phenology in elk are multifaceted. We found that elk modulated not only departure timing and duration of migration in response to resource conditions along the migration route but also in response to resource conditions pre- and post-migration. Contrary to our expectation, we did not find sub-strategies wherein elk were optimizing a single aspect of migration (i.e. optimizing forage opportunities pre-, during, or post-migration). Instead, we identified heterogeneity in inter-individual optimality, with most individuals focused on multiple aspects of migration. Additionally, we found that some individuals were more successful at optimizing access to forage throughout the migratory process (i.e., Cluster 2). Overall, our results suggest that elk may alter multiple aspects of their migratory phenology to facilitate heightened access to forage quality and illustrate a form of migratory variation in elk.
Migration timing and duration
Much of the previously published work on ungulate migrations suggests that forage conditions encountered along the migration route are the major driver of migration timing and duration (e.g.,18). In contrast, our results revealed a more nuanced picture of the factors influencing migration phenology. None of our four herds had the same predictors of spring departure. Every herd except Bear’s Ears had only a single predictor of spring departure (Fig. 4). For both Avalanche Creek and Trinchera, departure date was influenced by peak IRG, but Avalanche Creek was influenced by peak IRG during migration, whereas Trinchera was influenced by peak IRG post-migration. The Uncompahgre Plateau herd had mean snowfree DOY during migration as the best predictor. Lastly, the Bear’s Ears herd had both snowfree day pre-departure and the variation in IRG date during migration as the best predictors. On average, herds that responded to snowmelt (Bear’s Ears and Uncompahgre Plateau) began migrating earlier, spent more time migrating, and migrated a greater distance (Fig. 3), while herds that responded to peak IRG had shorter but higher elevation migrations. Overall, our results demonstrate that there is not a single strong signal for spring departure for these elk herds. Instead, these variables likely captured local variation in green-up and snowmelt that herds responded to. Further, they provide support for prior work demonstrating that IRG and snowmelt can be influential drivers of elk migration3,29.
Drivers of migration duration appear to be highly varying across ungulate species and population, with several studies finding a decoupling between departure and arrival time41,42. Our results echo this complexity given we found different drivers of duration for most herds. Mean peak IRG date following summer range arrival had a positive effect on migration duration for two herds (Avalanche Creek and Bear’s Ears). This indicates that migrants in these herds with summer ranges peaking later take longer to perform their migration. One possibility is that elk are traveling along migration routes at a rate that minimizes phenological mismatch when arriving on summer range13. While we expected that the variation in peak IRG during the migration could increase the duration of migration due to increased potential for surfing18, we did not find a positive association between peak IRG during migration and duration in any herd. Relatedly, we also expected migration distance to be correlated with duration, but this was only a strong predictor in one herd (Uncompahgre Plateau). This herd also travelled an intermediate migration distance (Table B1). Overall, this indicates that factors determining migration duration in most of our herds are not related to traditionally considered factors such as foraging opportunities43 and distance44 but may be related to other drivers not measured here. One aspect our work did not consider is ease of movement45, which could also be shaping migration speed and duration.
Although we observed similar variation in the dates of parturition among herds, we found evidence that the timing of calving influenced the departure date of migration for only one herd (Avalanche Creek; Appendix C). Previous research has suggested that migration may be timed to match peak resource availability for parturition46,47. Depending on the duration of migration, such matching might necessitate that pregnant females anticipate when they will calve days or weeks in advance. Alternatively, if the migration route is short, migrants may be able to respond to shorter-term physiological cues that calving is imminent by quickening their pace in response to those cues. For longer-distance migrants such as individuals from the Bear’s Ears herd, such matching may not be possible, although a recent study found that only long-distance migrants gave birth shortly after arriving on summer range23. Similar to Aikens et al.23, we found that most migrants (92/109) arrived on summer range prior to calving. This suggests that a coarse-scale coupling of migration timing and parturition may exist. Future research in our system should examine the interrelationships between birth timing, maternal condition, forage availability, migration timing and duration, and potential tradeoffs made between optimal forage conditions for calving and time allocated for offspring growth prior to winter23.
Elk movement optimality
The output of our cluster analysis showed that individuals vary in optimality along a multi-dimensional gradient, with each dimension representing optimality during a particular period of the migration (pre-, during, post-). Across herds, our results indicate that elk attempted to optimize access to quality forage at every stage of the migration event–pre-departure, during migration, and upon arrival on summer range. Two clusters emerged: a high-optimality, and a lower-optimality cluster. The lower-optimality cluster had more variation than the high-optimality cluster; in this cluster individual elk were frequently less optimal on one or more axes than the others (Fig. 6). Given that ~ 61% of individuals belonged to the ‘optimal’ category, most elk in our dataset seemed to exhibit high overall optimality. Even in this optimal cluster, however, some elk had lower optimality values for one axis. This suggests that on average migratory elk displayed movements that maximized their access to high-quality forage before, during, and/or after a migratory event, but they are not necessarily able to maximize the three aspects altogether. This is reflected in when they left their winter range, how long they migrated, and when they arrived on summer range relative to the nature of the greenscape they experienced18. It also suggests that such precision lies along a continuum and that high variation exists among migrants, with varying opportunities for optimality tradeoffs available and potentially viable. This variation supports findings revealing similar adaptability in elk movement compared to some other ungulates with more predictable sub-strategies25,48. Such diversity in movement may aid elk in their ability to respond to future changes on the landscape3.
Percentages of individuals pertaining to each cluster were not equal among herds (Appendix Figure B3). Our analyses revealed stark differences in the overall optimality of different herds, with Trinchera (94.1%) and Bear’s Ears (74.2%) in the highly optimal cluster, Avalanche Creek being nearly half and half, and only a few individuals from Uncompahgre Plateau (29.6%) being part of this cluster. This implies that individuals in differing systems may be unequal in their average optimization of migratory events, likely owing to system-specific differences in forage phenology, forage predictability, and topography. The prevalence of more optimal movements in specific systems may depend upon the nature of green-up in a system18. Some green-up gradients (‘greenscapes’) experienced by specific herds or individuals are likely harder to optimize than others, leading to that variation. Additionally, individual choice in migration route and in prioritizing one aspect of forage optimization over another likely also contributes to this variation. Interestingly, the Bear’s Ears herd had the lowest mean SD IRG (16.5 days), indicating they had the least variability in timing of peak green-up along their migration routes, whereas Trinchera had the largest mean SD IRG (23.1 days). The variability in green-up for Avalanche Creek was most similar to Bear’s Ears (17.3 days) and for Uncompahgre Plateau was most similar to Trinchera (22.0 days).
According to optimal foraging theory, it can be assumed as a null hypothesis that organisms have evolved movement behaviors aimed at acquiring resources at peak quality49. This is rarely perfectly actualized, but our optimality index and clustering analysis allows for individual-level estimation of how closely optimal foraging is achieved in the context of migration events. This framework can aid our understanding of how individuals balance tradeoffs in terms of foraging as created by the structure of the greenscape pre-, during, and post-migration. A significant feature of our framework is its ability to elucidate the diversity and abundance of such sub-strategies. While not observed here, sub-strategies optimizing different components of migratory movements can be specified along this three-dimensional gradient of optimality and be used to help understand the structural drivers of migration. Linking our tri-axes optimality framework with individual-level fitness metrics could be especially promising. The degree to which forage quality alone determines migrant fitness and migratory phenology is still unresolved, however, as multiple factors are also known to influence these variables (e.g., reproductive status, predation and hunting pressure, disease dynamics, snowmelt, etc.).
An increasing number of hypotheses have been developed to explain the existence and drivers of migratory behavior12,15,30,50, yet some of these hypotheses are derivative of previous hypotheses (e.g., the green-wave surfing hypothesis builds upon the forage maturation hypothesis). Additionally, many foundational papers of ungulate migration have focused on testing the validity of a single hypothesis in a single herd to explain migratory movement (e.g.,15). Recent work, however, has looked at migration phenology and its drivers in elk across different herds inhabiting the Greater Yellowstone Ecosystem, with statistical models focused on across-herd inferences3. By comparing several populations across distinct environments, our results highlight a few key considerations. First, even when constrained by the biology (physiology, morphology, and cognition) of a species, migration phenology and its drivers are extremely nuanced. Second, in most cases, migration phenology in ungulates is likely not driven by a single variable but rather by several factors such as pre-departure forage gain, surfing during migration, and timely arrival to the summer range for foraging and/or reproduction. All of this, coupled with the low explained variation of some of our models, highlights that more work is needed to understand these systems.
Conclusions
Migration as a behavior is declining globally, and encroachment on migratory routes due to increasing habitat loss and fragmentation has resulted in population crashes of ungulates traditionally dependent on the fitness benefits of migration30,31. Mapping migratory routes can inform current conservation efforts by identifying the intersection of corridors and potentially harmful human features51, but identifying the temporal aspects of how animals engage in migratory behavior could be equally important in predicting the impact of future changes. Better understanding of the drivers and parameters of migration timing and route is therefore critical, especially considering potential shifts in vegetative phenology that are anticipated due to climate change52. Such shifts may incur negative consequences for migratory ungulates, such as increases in phenological mismatch leading to reductions in fitness13,53,54. Thus, preservation of systems and populations harboring intact migratory behaviors in the face of changing environments is a major goal in ungulate conservation55. Through this work, we developed a greater understanding of the phenological triggers and conditions signaling the timing and duration of migration, which can be used by managers to predict potential shifts in these parameters as a function of environmental factors. Though we have shown that most elk in our system optimize forage quality to a heightened degree, their ability to adjust and maintain this optimality in shifting conditions is uncertain. If elk cannot respond to changing conditions, it may have consequences for individual fitness and population viability. Further research is needed to understand the rate of vegetative phenology shifts, to quantify the ways and rates at which migrants may respond to such changes, and to evaluate how plastic or consistent differences in foraging strategy are among individuals and herds.
Data availability
All data were collected by Colorado Parks and Wildlife. Data available from the Dryad Digital Repository https://doi.org/10.5061/dryad.v41ns1s71.
References
Bastille-Rousseau, G. et al. Animal movement in the absence of predation: environmental drivers of movement strategies in a partial migration system. Oikos 126, 1004–1019 (2017).
De Robertis, A. Size-dependent visual predation risk and the timing of vertical migration: An optimization model. Limnol. Oceanogr. 47, 925–933 (2002).
Rickbeil, G. J. M. et al. Plasticity in elk migration timing is a response to changing environmental conditions. Glob. Chang. Biol. 25, 2368–2381 (2019).
Robson, D. & Barriocanal, C. Ecological conditions in wintering and passage areas as determinants of timing of spring migration in trans-Saharan migratory birds. J. Anim. Ecol. 80, 320–331 (2011).
Ringelberg, J. The photobehaviour of Daphnia spp. as a model to explain diel vertical migration in zooplankton. Biol. Rev. 74, 397–423 (1999).
Norris, D. R. & Marra, P. P. Seasonal interactions, habitat quality, and population dynamics in migratory birds. Condor 109, 535–547 (2007).
Cain, J. W., Krausman, P. R., Rosenstock, S. S. & Turner, J. C. Mechanisms of thermoregulation and water balance in desert ungulates. Wildl. Soc. Bulletin 34, 570–581 (2016).
Kovach, R. P., Ellison, S. C., Pyare, S. & Tallmon, D. A. Temporal patterns in adult salmon migration timing across southeast Alaska. Glob. Chang. Biol. 21, 1821–1833 (2015).
Thorup, K., Vardanis, Y., Tøttrup, A. P., Kristensen, M. W. & Alerstam, T. Timing of songbird migration: individual consistency within and between seasons. J. Avian Biol. 44, 486–494 (2013).
Franklin, K. A. et al. Individual repeatability of avian migration phenology: A systematic review and meta-analysis. J. Anim. Ecol. 91, 1416–1430 (2022).
Merkle, J. A. et al. Large herbivores surf waves of green-up during spring. Proc. R. Soc. B Biol. Sci. 283, 1833 (2016).
Fryxell, J. M. Forage quality and aggregation by large herbivores. Am. Nat. 138, 478–498 (1991).
Post, E. & Forchhammer, M. C. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos. Trans. R. Soc. B Biol. Sci. 363, 2369–2373 (2008).
Abrahms, B. et al. Emerging perspectives on resource tracking and animal movement ecology. Trends Ecol. Evol. 36, 1–13 (2021).
Bischof, R. et al. A migratory northern ungulate in the pursuit of spring: Jumping or surfing the green wave?. Am. Nat 180, 407–424 (2012).
Drent, R. H., Ebbinge, B. S. & Weijand, B. Balancing the energy budgets of arctic-breeding geese throughout the annual cycle: a progress report. Verh Orn Ges Bayern 23, 239–264 (1978).
Graaf, Der et al. Surfing on a green wave-how plant growth drives spring migration in the Barnacle Goose Branta leucopsis. Ardea Wageningen 94, 567–577 (2006).
Aikens, E. O. et al. The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecol. Lett. 20, 741–750 (2017).
Lendrum, P. E., Anderson, C. R., Monteith, K. L., Jenks, J. A. & Bowyer, R. T. Relating the movement of a rapidly migrating ungulate to spatiotemporal patterns of forage quality. Mamm. Biol. 79, 369–375 (2014).
Laforge, M. P., Bonar, M. & Wal, E. V. Tracking snowmelt to jump the green wave: phenological drivers of migration in a northern ungulate. Ecology 102, e03268 (2021).
Rivrud, I. M. et al. Leave before it’s too late: anthropogenic and environmental triggers of autumn migration in a hunted ungulate population. Ecology 97, 1058–1068 (2016).
Jönsson, K. I. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66 (1997).
Aikens, E. O. et al. Migration distance and maternal resource allocation determine timing of birth in a large herbivore. Ecology 102, e03334 (2021).
Lowrey, B. et al. Individual variation creates diverse migratory portfolios in native populations of a mountain ungulate. Ecol. Appl. 30, e2106 (2020).
Xu, W. et al. The plasticity of ungulate migration in a changing world. Ecology https://doi.org/10.1002/ecy.3293 (2021).
Middleton, A. D. et al. Green-wave surfing increases fat gain in a migratory ungulate. Oikos 127, 1060–1068 (2018).
Middleton, A. D. et al. Animal migration amid shifting patterns of phenology and predation: lessons from a Yellowstone elk herd. Ecology 94, 1245–1256 (2013).
Barker, K. J., Mitchell, M. S. & Proffitt, K. M. Native forage mediates influence of irrigated agriculture on migratory behaviour of elk. J. Anim. Ecol. 88, 1100–1110 (2019).
Hebblewhite, M., Merrill, E. & McDermid, G. A multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecol. Monogr. 78, 141–166 (2008).
Bolger, D. T., Newmark, W. D., Morrison, T. A. & Doak, D. F. The need for integrative approaches to understand and conserve migratory ungulates. Ecol. Lett. 11, 63–77 (2008).
Wilcove, D. S. & Wikelski, M. Going, going, gone: Is animal migration disappearing?. PLoS Biol. 6, 1361–1364 (2008).
Peet, R. K. Latitudinal variation in southern Rocky Mountain forests. J. Biogeogr. 5, 275–289 (1978).
Du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 18, 1–12 (2020).
Bunnefeld, N. et al. A model-driven approach to quantify migration patterns: Individual, regional and yearly differences. J. Anim. Ecol. 80, 466–476 (2011).
Spitz, D. B., Hebblewhite, M. & Stephenson, T. R. ‘MigrateR’: extending model-driven methods for classifying and quantifying animal movement behavior. Ecography 40, 788–799 (2017).
Calabrese, J. M., Fleming, C. H. & Gurarie, E. ctmm: an r package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 7, 1124–1132 (2016).
Murtaugh, P. A. Simplicity and complexity in ecological data analysis. Ecology 88, 56–62 (2007).
Burnham, K. P. & Anderson, D. R. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304 (2004).
Dormann, C. F. et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46 (2013).
Scrucca, L., Fop, M., Murphy, T. B. & Raftery, A. E. mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R J. 8, 289–317 (2016).
Ortega, et al. Migrating mule deer compensate en route for phenological mismatches. Nat. Commun. 14, 2008 (2023).
Gurarie, et al. Tactical departures and strategic arrivals: Divergent effects of climate and weather on caribou spring migrations. Ecosphere 10, e02971 (2019).
Jones, J. D. et al. Supplemental feeding alters migration of a temperature ungulate. Ecol. Appl. 24, 1769–1779 (2014).
Peters, W. et al. Migration in geographic and ecological space by a large herbivore. Ecol. Monogr. 87, 297–320 (2017).
Monteith, K. L. et al. Functional attributes of ungulate migration: landscape features facilitate movement and access to forage. Ecol. Appl. 28, 2153–2164 (2018).
Festa-Bianchet, M. Birthdate and survival in bighorn lambs (Ovis canadensis). J. Zool. 214, 653–661 (1988).
Post, E., Bøving, P. S., Pedersen, C. & MacArthur, M. A. Synchrony between caribou calving and plant phenology in depredated and non-depredated populations. Can. J. Zool. 81, 1709–1714 (2003).
Sawyer, H., Merkle, J. A., Middleton, A. D., Dwinnell, S. P. H. & Monteith, K. L. Migratory plasticity is not ubiquitous among large herbivores. J. Anim. Ecol. 88, 450–460 (2019).
Owen-Smith, N., Fryxell, J. M. & Merrill, E. H. Foraging theory upscaled: the behavioural ecology of herbivore movement. Philos. Trans. R. Soc. B Biol. Sci. 365, 2267–2278 (2010).
Mueller, T. et al. How landscape dynamics link individual- to population-level movement patterns: A multispecies comparison of ungulate relocation data. Glob. Ecol. Biogeogr. 20, 683–694 (2011).
Kauffman, M. J. et al. Mapping out a future for ungulate migrations. Science 372, 566–569 (2021).
Cremonese, E. et al. Heat wave hinders green wave: The impact of climate extreme on the phenology of a mountain grassland. Agric. For. Meteorol. 247, 320–330 (2017).
Aikens, E. O. et al. Wave-like patterns of plant phenology determine ungulate movement tactics. Curr. Biol. 30, 3444–3449 (2020).
Rivrud, I. M., Meisingset, E. L., Loe, L. E. & Mysterud, A. Future suitability of habitat in a migratory ungulate under climate change. Proc. Biol. Sci. 286, 20190442 (2019).
Bond, M. L., Bradley, C. M., Kiffner, C., Morrison, T. A. & Lee, D. E. A multi-method approach to delineate and validate migratory corridors. Landsc. Ecol. 32, 1705–1721 (2017).
Acknowledgements
S. Crews was supported by the SIU W.D. Klimstra Reclamation Research Fellowship. Funding was provided by revenues from the sale of Colorado hunting and fishing licenses and matching United States Fish and Wildlife Service Federal Aid Research Grants to Colorado Parks and Wildlife (CPW), Auction and Raffle grants administered by CPW, the Rocky Mountain Elk Foundation, and Pitkin County Open Space and Trails. We appreciate the efforts of the many CPW staff who supported all aspects of this project. We thank the private landowners who provided access to their property for elk capture, monitoring, and birth site investigations. We thank J. Merkle for developing and sharing code to process NDVI data. We thank S. Frank, N. Jaffe, and C. Setash for helpful comments that improved this manuscript.
Author information
Authors and Affiliations
Contributions
SC, NDR, MWA, EJB, CRA, and GBR conceived the idea for the research. NDR, MWA, SC, and GBR collected and managed data used in the research. SC, NDR, MWA, EJB, CRA, and GBR developed the methodology and SC and GBR did the statistical analyses. SC, GBR, and NDR wrote the manuscript. All the authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Crews, S., Rayl, N.D., Alldredge, M.W. et al. Drivers of spring migration phenology in Rocky Mountain elk. Sci Rep 15, 7807 (2025). https://doi.org/10.1038/s41598-025-91947-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91947-4