Abstract
Anxiety is an interactive disorder of the mind and body, characterized by excessive worry about uncertain future events and a dysfunction of the autonomic nervous system. Previous studies have shown that slow, deep breathing can reduce physical tension, and anxiety. Although we know that slow and deep breathing techniques can effectively regulate anxiety and other emotions, the psychological and neurophysiological mechanisms of slow breathing on anxiety have not been systematically explored. In the study, we combined the paced breathing task with the threat uncertainty task for the first time to investigate the role of slow breathing in regulating anxiety. Here we investigated this question, using Spectral analysis and Time-frequency domain of EEG to assess brain activity relating respiratory rate and the mechanism of respiratory rate impact on the anxious. Twenty-seven individuals participated in the experiment, which followed a 2 (respiratory rate: fast breathing, slow breathing) × 2 (certainty: certain, uncertain) within-subjects design. The results of showed that: (1) Slow breathing effectively reduced anxiety, the valence and arousal are lower under the slow breathing. (2) The EEG of fast and slow breathing showed different characteristics. There is an overall increase in power during slow breathing in the delta, theta, alpha and beta bands.(3) The interaction of respiratory rate and certainty were closely related to beta. In the uncertain, beta power decreased with slow breathing but increased with fast breathing.
Similar content being viewed by others
Introduction
Anxiety disorders are the most common mental illness in children and adolescents, with prevalence rates as high as 20%1. Anxiety disorders are typically characterized by persistent and excessive worry, often accompanied by physical and cognitive symptoms that can lead to dysfunction2. Anxiety in moderation is useful. However, when anxiety is severe, it can become chaotic and therefore counterproductive. Having information about a potential future event reduces the anxiety associated with it.However, there is often uncertainty as to whether the upcoming aversion event will actually happen, and how dangerous or negative it will be. Uncertainty about a possible future threat disrupts our ability to avoid it or to mitigate its negative impact and thus results in anxiety3, that has historical roots in animal studies of stress response and fear learning, as well as in previous influential models of anxiety pathology. Scholars provided evidence that anticipation about potential future threats induce anxiety in multiple levels, which range from affective and cognitive to behavioral manifestations3,4,5.Anticipatory anxiety is defined as the time between warning and stimulus6. In real life, it is frequently characterized by potential unfavorable clues that signify future threats, such that people with anticipatory anxiety remain in a state of stress, including physiological arousal, cortical activation, and cognitive bias5,7,8. Constant stress can take a toll on one’s body and mind. Uncertainty is a constant in everyday life, making it increasingly vital to address the negative expectations it brings.
New evidence has demonstrated that respiratory rhythms exert surprising, substantive influences on perception, emotion, and cognition9. Breathing is an integral component of interoceptive processing, that is, the state of the physiological condition of the body10. Respiration is unique compared with other systems (e.g. gastrointestinal) insofar as conscious regulation can immediately impact respiratory processes. Instructed breathing patterns are widely applied to treat a variety of complaints and conditions, e.g., pain, stress, post-traumatic stress disorder11,12,13. Most often, these patterns involve a voluntary reduction of breathing frequency, which is assumed to increase parasympathetic activity. Slow breathing at 4.5 to 6.5 breaths per minute (coherent or resonance breathing) has been shown to optimally balance sympatho-vagal stress response for most adults14,15,16,17.Heart rate variability(HRV), the beat-to-beat variation in either heart rate or the duration of the R-R interval–the period, has become a popular clinical and investigational tool. HRV is an index of cardiac autonomic activation as well as an outcome variable in breathing training or HRV biofeedback research18,19.
The relationship between breathing and electroencephalogram (EEG) activity was first mentioned by Hobson. The study found that with the change of frog’s state from rest to activity, EEG also changed from low frequency fast wave mode to high frequency slow wave mode, and the degree of synchronization of EEG was proportional to the respiratory frequency20. A sthdy described the oscillatory activity of the human EEG during the breathing cycle, and found that the amplitude of the EEG curve increased during inhalation, while the EEG curve showed the opposite trend during exhalation. A cyclic pattern of electrical activity in the brain can be observed during sleep as breathing changes21. Later, it was found that different modes of nasal breathing (including left nostril inhalation and right nostril exhalation, and vice versa) increased power in the beta and alpha bands22.The results of electroencephalogram (EEG) studies on breathing focused meditation activities indicate an increase in alpha and theta power in the posterior brain region and the frontal region23,24. An increase in frontal theta may indicate a need for cognitive control and call on other brain regions25. Recently, one study compared Heartbeat Evoked Potential (HEP)activity for heartbeats occurred during inhalation and exhalation at rest. the found higher HEP amplitude during exhalation, compared to inhalation, over fronto-centro-parietal areas. This suggests increased brain-heart interactions and improved cortical processing of the heartbeats during exhalation26.There is growing evidence that breathing directly effects on neural oscillations in various brain regions27. Previous studies have primarily focused on the characteristics of brain activity during exhalation and inspiration. However, given the benefits of slow breathing for emotional regulation, it is essential to also consider the characteristics of brain activity during slow breathing.
Anxiety are the psychological symptoms typically associated with autonomic overactivity, subjectively, the individual feels nervous and flushed, heart palpitations, shortness of breath, increased sweating28. Changes in breathing can be both the consequence of an increased level of anxiety as well as the source of threat experienced by the individual, which, in turn, leads to increased anxiety. Thus, assessing breathing might be a useful physiological marker of the level of anxiety but can also serve as an experimental tool to influence anxiety levels29. A review by Weng examined studies on the manipulation of breathing30. It suggests that slow breathing may activate cardiopulmonary pressure receptors, resulting in reduced reflexive sympathetic nerve activity and subsequently lower anxiety levels. However, the exact process remains unclear.
This study combined paced breathing with the threat uncertainty task31 to create a new experimental paradigm. The aim was to induce anticipatory anxiety and investigate whether slow breathing is beneficial in reducing responses (valence, arousal) associated with anticipatory anxiety. After participants performed breathing exercises, we investigated the genesis and development of biased estimates of emotional pictures under conditions of uncertainty. We continuously monitored participants’ self-reported scores of emotional pictures32and EEG measurements of neuronal activity during the experiment. On the basis of conceptualizing uncertainty as an anxiety state, we expected to observe biases related to uncertainty in post-experiment estimates, where uncertain cues would be overly associated with negative pictures. Across species, physiological responses to threats are heightened when there is uncertainty about their nature, probability, or timing33,34,35,36,37.Furthermore, aversive events that are not fully predictable have a greater negative impact on mood, state anxiety and physiological indices of reactivity than those that are fully predictable34,36,37. We hypothesized that: (1) Slow breathing has a significant regulating effect on anticipatory anxiety. Compared with fast breathing, individuals under slow breathing conditions exhibit lower valence and arousal in response to negative images; (2) Uncertainty can increase anticipatory anxiety, and individuals show more valence and arousal to pictures presented after uncertain cues; (3) The electrical activity of the brain varies significantly at different breathing rates, exhibiting distinct characteristics between fast and slow breathing patterns.
Materials and methods
Participants
Twenty-seven college students were recruited for this study, but two participants lacked complete EEG data. The final sample included 25 healthy female participants from Southwest University who took part in the study. The participants were 18–26 years old, with a mean age of 20.72 years (SD = 2.324). All individuals signed a written informed consent form. According to self-reports, all participants were right-handed, had no psychiatric or neurological diseases, and had normal or corrected-to-normal eyesight. This study was approved by the Ethics Committee of the Department of Psychology, Southwest University and all experiments were performed in accordance with relevant guidelines and regulations.
The paced breathing-threat uncertainty task and procedure
The experiment took place in a tranquil room with a suitable temperature. Upon their arrival at the laboratory, participants were given some time to rest. Following this, they received instructions regarding the tasks they were expected to perform and engaged in practice for the experimental procedures. Once they were comfortable with the tasks, the experiment was carried out in four blocks, each consisting of 15 trials. Participants had the opportunity to take a brief rest of 1–2 min after completing each block. Ultimately, all participants finished the trials by the conclusion of the study.
Twenty-seven individuals participated in the experiment, which followed a 2 (respiratory rate: fast breathing, slow breathing) × 2 (certainty: certain, uncertain) within-subjects design. In the paced breathing task, where they followed audio-guided breathing exercises. This audio was produced by an instructor with nine years of yoga teaching experience. The breathing conditions were categorized into fast breathing (both the exhalation time and the inhalation time were 2 s) and slow breathing(both the exhalation time and the inhalation time were 6 s), each breathing exercise lasted for 30 s.
During the threat uncertainty task, the task contained two different cue types: certain or uncertain. First, participants received a 3-second cue (“O” or “?”).To indicate the type of picture that will appear later. (certain: the “O” represented negative pictures, uncertain: “?” represented uncertainty about the type of picture to be presented, which could be neutral, positive, or negative (anticipatory anxiety: after “O” or “?”, before the picture.)) The images were shown after a 1 s blank screen, and viewing the picture for 3 s. Finally, participants evaluated its valence and arousal, with each rating allocated 5 s. participants rated the valence of the images on a scale from 0 (not negative at all) to 6 (very negative), and the arousal on a scale from 0 (not feeling at all) to 6 (very strong). In fact, in the “?”, only negative images were shown, and the subjects didn’t know it. This cycle continued until a total of 60 trials were completed. Through out the experiment, both electrocardiogram (ECG) and electroencephalography (EEG) data were collected simultaneously, and the entire procedure was managed using E-Prime 2.0 (see Fig. 1).
The 60 negative images from the International Affective Image System had a valence range of 1.26–3.07 (mean ± SD, 2.31 ± 0.41) and an arousal range of 2.50–6.89 (mean ± SD, 5.60 ± 0.63). The pictures were randomly assigned to each experimental condition. In order to ensure the equitable distribution of images, we conducted a 2 (respiratory rate: fast breathing, slow breathing) × 2 (certainty: certain, uncertain) repeated-measures ANOVA, with valence and arousal as dependent variables. The results show that whether valence or arousal is the dependent variable, the main effect of respiratory rate (p = 0.161) or (p = 0.121) was not significant, and the main effect of certainty (p = 0.500) or (p = 0.771) was not significant. The interaction between respiratory rate and determinism (p = 0.746) or (p = 0.941) was not significant. The findings show that the image distribution in each experimental condition does not influence the outcomes. Thus, the random assignment of images in this study is considered both reasonable and effective.
The experimental procedure of paced breathing-threat uncertainty task. After breathing exercises (the two breathing conditions were presented alternately, the fast breathing present in the first trail, the slow breathing will present in on the next trail), then, an anticipatory cue was presented, followed by the presentation of an emotionally negative stimulus, finally, the subjects rated the pictures.The gray figure indicates the blank screen.
Electrocardiogram and electroencephalography data acquisition, pre-processing and analysis
Acqknowledge 5.0 was used for Heart rate and heart rate variability (HRV) analysis; time and frequency domain parameters were calculated. The time domain indicator used was RMSSD (ms), namely the square root of the mean squared differences between successive RR intervals. The frequency domain index was HF (ms2), namely, the absolute power of the high-frequency band (0.15–0.4HZ). Specifically, RMSSD represents short-term variation in NN cycles and high-frequency oscillations caused by parasympathetic activity. HF is a marker of parasympathetic tone. In this study, to ensure the stability of each indicator in each experimental condition, we calculated the average value of each indicator under the same conditions for subsequent data analysis.
EEG data were collected with a 64-channel Brain Products system (Brain Products GmbH, Munich, Germany), according to the international 10–20 system. The midfrontal electrode (FCz) was used as the reference and the inion electrode (AFz) as the ground. Electrode impedance was kept below 10 kΩ for all channels. All signals were recorded with a sampling rate of 500 Hz; EEG data were pre-processed offline using EEGLAB (v2021.1;38) toolbox algorithms running on a MATLAB environment (R2021a, MathWorks Inc.). Two electrodes, HEO and VEO, were excluded. Then, the EEG signal was re-referenced to the average reference (i.e., calculated across all electrodes)39. An introduction to the event-related potential technique. MIT press.). Data were filtered by applying a high-pass filter of 0 Hz (forward-phase Butter-worth filter, 6-dB/octave roll-off) and a low-pass filter of 40 Hz (zero-phase Butterworth filter, 24-dB/octave roll-off) with an additional notch filter of 50 Hz (2 Hz width). The signal were visually inspected for the removal of artefacts and the detection of noisy channels. Bad segments were manually rejected. Noisy EEG channels were then removed and interpolated using their neighbouring channels40,41.
EEG data for the Spectral analysis employed the EEGLAB toolbox (41, http://sccn.ucsd.edu/eeglab). Spectral analysis of absolute power for 1–30 Hz was conducted using the multitaper spectral estimation with Hanning taper and 0.5 frequency resolution in EEGLAB.The spectral power was decomposed into the following spectral components: delta (0.5–3.5 Hz), theta (4.0–7.5 Hz), alpha (8.0–12.0 Hz) and beta (12.5–30.3 Hz)42. This spectral analysis was performed on data recorded during the breathing and emotional anticipation conditions in the whole brain.
EEG data for the Time-frequency analysis employed the EEGLAB toolbox. There are many approaches to time-frequency decomposition of EEG data, including the short-term Fourier transform (STFT), continuous or discrete wavelet transforms, Hilbert transform, and matching pursuits. In the study we used the continuous wavelet transforms (CWT) for time-frequency domain estimation. About the baseline, using a time period that is 10–20% of the overall epoch duration is recommended43, so the baseline time set as -500 to -200. The most common use of time-frequency analysis on EEG signals is to detect and analyze event-related synchronization / de-synchroni zation (ERS/ERD). (ERS/ERD) feature extraction uses visual detection of time-frequency regions that vary significantly from baseline. The region of interest (ROI) was selected after time-frequency analysis of EEG data. Each participant have a same ROI.The power value of the ROI is then calculated (the average of the power values at all time-frequency points within the ROI) and the calculated power value is used as the time-frequency feature of the ERS/ERD corresponding to the ROI.
Result
The result of self-report scores
To investigate the impact of and certainty on anxiety, we performed an ANOVA with respiratory rate (fast breathing or slow breathing) and certainty (certain or uncertain) as the independent variables, while using valence and arousal scores as dependent variables. Tukey’s correction was applied to the analysis. All statistical analyses were performed in jamovi (v2.2.2; The jamovi project, 2021). Descriptive statistics for self-report scores are shown in Table 1; Fig. 2.
The analysis revealed a significant main effect of respiratory rate on valence (F = 5.46, p = 0.028, η2 = 0.023), indicating lower valence scores during slow breathing compared to fast breathing. Certainty also had a significant main effect (F = 20.98,p = 0.001, η2 = 0.073) ,with valence scores being higher in uncertain situations than in certain ones. The interaction between respiratory rate and certainty approached significance (F = 4.16, p = 0.052, η2 = 0.013). Post hoc tests confirmed that the effect of respiratory rate was significant (p = 0.028), showing lower valence scores during slow breathing. Certainty’s effect was also significant (p = 0.001), with lower valence scores in certain situations.
For arousal, there was a significant main effect of respiratory rate (F = 5.473, p = 0.028, η2 = 0.018), indicating lower scores during slow breathing. The main effect of certainty was also significant (F = 5.940, p = 0.023, η2 = 0.017), with higher arousal scores in uncertain situations. However, The interaction between respiratory rate and certainty was not significant (F = 0.607, p = 0.443, η2 = 0.001). Overall, the findings suggest that certainty of success increases anxiety, while slow breathing helps to alleviate this anxiety.
The result of HR and HRV
To investigate the effect of breathing on heart activity, we performed the paired sample t-tests comparisons for different cardiorespiratory features of interest. Specifically, we measured the mean HR, RMSSD and HFlog power as cardiac features during respiration.Table 2 provides a descriptive statistical analysis and the t-test comparison for two different respiration rates. All statistical analyses were performed in jamovi (v2.2.2; The jamovi project, 2021). Our findings revealed a significant variation in heart rate (HR) between fast and slow respiration, with an increase in heart rate observed during fast respiration. These findings imply a strong connection between the rate of respiration and heart rate.
The result of spectral and Time-frequency analysis during in breathing phase
Spectral analysis was used to measure the absolute power of four frequency bands during breathing (see Table 3). We conducted a paired-sample t-test to explore the relationship between the EEG characteristics and breathing rate.All statistical analyses were performed in jamovi (v2.2.2; The jamovi project, 2021).
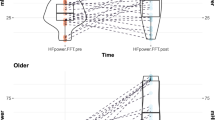
This test examined the total brain power during breathing, assessing the changes in the four frequency bands delta, theta, alpha, and beta during fast and slow breathing conditions (see Fig. 3). Within each frequency band, there was a significant effect by respiration rate (delta (p < 0.001), theta (p < 0.001), alpha (p < 0.001) and beta (p < 0.001)).There is an overall increase in power during slow breathing in the delta, theta, alpha and beta bands.
In order to explore the characteristics of EEG activity in the time-frequency domain at different respiratory rates, Cz was selected to analyze a respiratory cycle in the time domain (fast-breathing: 1–6 s, slow breathing: 1–13 s). Next, the region of interest (ROI) was chosen for statistical analysis (time: 0ms-500ms; frequency band: 10–15 Hz).The power value of the ROI was calculated by finding the average power value of all time-frequency points within the ROI, which was then used as the time-frequency feature of the ERS/ERD corresponding to this ROI. Each participant have a same ROI for this analysis.The average power value of the ROI under two different breathing rates was tested using a T-test and corrected by Tukey. The results indicated that there was no significant difference in time-frequency activity between the two respiratory rates. However, both respiratory rates exhibited an increase in power value in this region compared to the baseline condition, demonstrating synchronization characteristics.
The result of spectral and Time-frequency analysis during in anticipatory anxiety phase
Using the same method mentioned above, the absolute power of the four spectral bands during the emotional anticipation phase is obtained through spectral analysis (Table 4). Next, we performed ANOVA with the absolute power of delta, theta, alpha, and beta as the dependent variables, and respiration rate (fast breathing or slow breathing) and certainty (certain or uncertain) as the independent variables. The analysis was corrected by Tukey.All statistical analyses were performed in jamovi (v2.2.2; The jamovi project, 2021).
For the absolute power of delta (see Fig. 4), the ANOVA shows a main effect of respiratory rate (F = 4.358, p = 0.048, η2 = 0.034), the power under fast breathing is higher than that under slow breathing. The main effect of certainty was not significant. The interaction of respiration rate and certainty was not significant .For the absolute power of theta and alpha, the ANOVA shows that the main and interaction effects of respiration rate and certainty were not significant.
For the absolute power of beta, the ANOVA shows a main effect of respiratory rate (F = 9.95, p = 0.005, η2 = 0.013), the power under fast breathing is higher than that under slow breathing. The main effect of certainty was not significant. The interaction of respiration rate and certainty was significant (F = 4.65, p = 0.041, η2 = 0.008). To be specific, during fast breathing, the absolute power was higher under uncertain conditions than under certain conditions. Conversely, during slow breathing, the absolute power was higher under certain conditions than under uncertain conditions. Moreover, the absolute power of theta was highest when participants were engaged in fast breathing and faced uncertainty.
In summary, only beta, The interaction of respiration rate and certainty had a significant effect on brain activity of anticipatory anxiety. When breathing slowly, the absolute power of beta decreases when facing uncertain tasks. Conversely, when breathing quickly, the absolute power of beta increases in response to uncertain tasks.
EEG power for delta, theta, alpha and beta frequency bands (10*log10(\muV^2/HZ) (m = mean, σ = standard deviation) for anticipatory phase.
Spatial distribution map of anticipatory phase in each frequency band; The first column shows the uncertain condition of fast breathing, and the second column shows the certain condition of fast breathing. The third column is the uncertain condition of slow breathing, and the fourth column is the certain condition of slow breathing.
In order to explore the influence of respiratory rate on EEG activity characteristics in the time-frequency domain of anxiety, the Cz electrode was selected to conduct time-domain analysis of EEG data in the anticipated emotional stage. The regions of interest (ROI: time: 500ms-1000ms; frequency band: 12.5 –30.3 Hz) were visually chosen for statistical analysis to observe the time-frequency characteristics. The power value of the ROI was calculated by finding the average power value of all time-frequency points within the ROI, and this calculated power value was used as the time-frequency characteristics of the ROI corresponding to ERS/ERD. Each participant have a same ROI.An ANOVA was performed with respiratory rate (fast breathing or slow breathing) and certainty (certain or uncertain) as the independent variables, and the power value of the ROI as the dependent variable. However, we didn’t find any significant results.
Discussion
Respiratory rhythm is one of the main oscillating rhythms of the body, which is the main source of interoceptive information for the brain. Anxiety is a disease of physical and mental interaction, which is characterized by excessive worry about uncertain events in the future and disturbance of autonomic nervous system. Previous studies have explored the relationship between breathing and anxiety from low-level perceptual sensitivity and higher level metacognition44, but have not explored the regulating effect of interoception on anxiety at the regulatory level. This study explored the relationship between anxiety and interoception from the perspective of operable interoception-breathing, and explored the role and mechanism of slow breathing in regulating anxiety. This study combined paced breathing with the threat uncertainty task to create a new experimental paradigm for the first time. The self-reported results found that slow-paced breathing was associated with lower valence and arousal, which means that compared with fast-paced breathing rate, slow-paced breathing is a beneficial intervention response to uncertain threat information. Analysis of heart rate variability found that the heart rate was lower during slow breathing conditions. Some interesting results were found by the spectral analysis of EEG. In the breathing phase, the power of delta, theta, alpha and beta are increased during the slow-paced breathing in emotion anticipatory phase, only in beta, respiratory rate and certainty had a significant effect on the brain activity. When participants are faced with uncertain information, the beta power decreased during slow-paced breathing, however, the beta power increased during fast-paced breathing.
In the self-reported results, slow breathing was found to be more effective in reducing anxiety compared to fast breathing. We found higher levels of valence and arousal in the uncertain condition compared to the certain condition. Through slow breathing exercises, participants’ responses to the emotional stimuli of an impending uncertain threat were diminished. It may be that slow breathing prepares the individual physically and psychologically for future anxious events. Across disorders, uncertainty is thought to provoke anticipatory anxiety and to result in behaviors that are maladaptive attempts to reduce uncertainty, such as worry, reassurance seeking, checking, and hypervigilance45. In previous studies, uncertainty has been defined as a lack of information about an event and has been characterized as an aversive state that people are motivated to reduce. The researcher propose an uncertainty intensification hypothesis, whereby uncertainty during an emotional event makes unpleasant events more unpleasant46. This study supports the hypothesis. Why might online uncertainty amplify reactions to an ongoing event? One possibility is that uncertainty heightens people’s attention. That is, just as uncertainty keeps an event accessible after it occurs47, it might also maintain people’s attention on an ongoing event, intensifying their reactions to it. Another possibility is that uncertainty increases people’s curiosity about an emotional event, leading them to become more emotionally engaged with it. That is, people may pay equal attention to an emotional event, but those who are uncertain may be more curious about what is happening, which makes them more engaged in the event. In this study, it is confirmed that slow breathing can better regulate emotional experiences in anxious situations and expand the application of slow breathing as a convenient mode of regulation.
A higher HRV is an indicator of adequate adaptation to the new environment and effective functioning of the autonomic nervous system (ANS). The yoga practicing group showed a significant increase in HRV and a reduction in resting heart rate in a study48. The escalation in the heart rate is due to increased sympathetic and decreased parasympathetic activity. A significant improvement in HRV may be due to an increase in parasympathetic activity or a decrease in sympathetic activity. These factors indirectly contribute to reducing psychological parameters such as distress, anxiety, and depression in young healthy subjects49. In this study, the short, slow-paced breathing exercises reduced participants’ heart rate. The high spectral energy of the HRV power spectrum component reflects the activity of the parasympathetic nervous system. According to the results of Heart Rate Variability (HRV) analysis, we found that the RMSSD and HF values were higher during slow-paced breathing. This suggests that parasympathetic activity could be activated through slow breathing, effectively reducing participants’ negative emotional experiences. No significant effect of respiratory rate on RMSSD and HF was found, which may be attributed to the short duration of the breathing exercises. Previous studies have shown that the breathing exercises lasted longer than one minute. It is also worth exploring the influence of breathing on HRV from the perspective of time effect.
This study examines participants’ EEG activity during the breathing phase, revealing that slow breathing enhances neural oscillatory activity. We observed increased amplitudes in delta, theta, alpha, and beta bands during slow breathing. Other studies have also explored the effects of respiratory on EEG activity.The influence of the respiratory cycle on the EEG is also observed in the literature.During spontaneous breathing and bradypnea, there was an increase in delta power50. EEG studies on breathing-focused meditation show increased alpha and theta power in both the posterior and frontal regions of the brain23,24.The investigation of neural oscillations associated with varying respiratory frequencies during the phases of inhalation and exhalation presents a valuable area for research.Exploring the electrophysiological effects of slow breathing via EEG could shed light on the underlying neurophysiological mechanisms and aid in developing more effective strategies for managing anxiety and stress.
Over the past six years, a rapidly growing number of studies have shown that respiration exerts a significant influence on sensory, affective, and cognitive processes. At the same time, an increasing amount of experimental evidence indicates that this influence occurs via the modulation of neural oscillations and their synchronization between brain areas51.This study also examined the impact of slow breathing on the cognitive processing of anxiety-related cues at the neural activity level. During the anticipation anxiety phase, when participants are faced with uncertain information, the beta power increased during fast-paced breathing.High frequency oscillations of beta ranges could be considered as elementary signals of the brain, functionally related to diverse brain processes52 with higher power of these oscillations being a mark of higher cortical arousal. In this study, Higher breathing rates and uncertain information lead to higher levels of cortical arousal. At the same time, increased beta band activity in response to uncertain tasks may also be associated with mental effort level53. The elucidation of the physiological mechanisms and neural pathways regulating breathing can help to better understand how an emotional state emerges from the interaction between the body and the brain. Much work needs to be done to better delineate the direction of the relationship between breathing and anxiety, as well as to evaluate how brain systems respond to the modulation of breathing as a powerful intervention to reduce levels of anxiety.
The results of this study are encouraging. However, researchers must consider many potential shortcomings when interpreting these research results. Since it was difficult to find men to participate in this experiment, our subjects were only women, so the results cannot be generalized to men. Finally, this study only explores the regulation effect of slow breathing on anxiety, and other measures can be combined mindfulness, meditation to regulate anxiety in the future.
Conclusion
In conclusion, the present study we combined the paced breathing task with the threat uncertainty task for the first time to investigate the role of slow breathing in regulating anxiety, and using Spectral analysis and Time-frequency domain of EEG to assess brain activity relating respiratory rate and he mechanism of respiratory rate impact on the anxious. In the study, including self- report scores, heart rate, and EEG provided evidence for the mood-regulating effects of slow breathing. These findings provide direct insight into slow breathing between anxiety, providing direct evidence that slow breathing reduces anxious.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Costello, E., Jane, H. L., Egger, W., Copeland, A. & Erkanli and Adrian Angold. Thedevelopmental Epidemiology of Anxiety Disorders: Phenomenology, Prevalence, and Comorbidity. In Anxiety Disorders in Children and Adolescents, edited by Wendy K. Silverman and Andy P. Field, 2nd ed., 56–75. Cambridge University Press. (2011). https://doi.org/10.1017/CBO9780511994920.004
Connolly, S. D. & Bernstein, G. A. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J. Am. Acad. Child. Adolesc. Psychiatry. 46 (2), 267–283. https://doi.org/10.1097/01.chi.0000246070.23695.06 (2007).
Grupe, D. W. & Nitschke, J. B. Uncertainty and anticipation in anxiety: an integrated Neurobiological and psychological perspective. Nat. Rev. Neurosci. 14 (7), 488–501. https://doi.org/10.1038/nrn3524 (2013).
Mobini, S., Reynolds, S. & Bundy Mackintosh Clinical implications of cognitive Bias modification for interpretative biases in social anxiety: an integrative literature review. Cogn. Therapy Res. 37 (1), 173–182. https://doi.org/10.1007/s10608-012-9445-8 (2013).
Acheson, D. T. et al. Effects of anxiolytic treatment on potentiated startle during aversive image anticipation. Hum. Psychopharmacology: Clin. Experimental. 27 (4), 419–427. https://doi.org/10.1002/hup.2243 (2012).
Masaoka, Y. & Ikuo Homma The effect of anticipatory anxiety on breathing and metabolism in humans. Respir. Physiol. 128 (2), 171–177. https://doi.org/10.1016/S0034-5687(01)00278-X (2001).
Carlson, J. M., Greenberg, T., Rubin, D., Lilianne, R. & Mujica-Parodi Feeling anxious: anticipatory Amygdalo-Insular response predicts the feeling of anxious anticipation. Soc. Cognit. Affect. Neurosci. 6 (1), 74–81. https://doi.org/10.1093/scan/nsq017 (2011).
Onoda, K. et al. Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia 46 (1), 102–110. https://doi.org/10.1016/j.neuropsychologia.2007.08.006 (2008).
Allen, M., Varga, S. & Detlef Heck Respiratory rhythms of the predictive Mind. Psychol. Rev. 130(August) https://doi.org/10.1037/rev0000391 (2022).
Craig, A. D. How do you feel?? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3 (8), 655–666. https://doi.org/10.1038/nrn894 (2002).
Zautra, A. J., Fasman, R., Davis, M. C. & Arthur, D. (Bud) Craig. 2010. The effects of slow breathing on affective responses to pain stimuli: an experimental study. Pain 149 (1): 12–18. https://doi.org/10.1016/j.pain.2009.10.001
Kim, S. et al. PTSD symptom reduction with Mindfulness-Based stretching and deep breathing exercise: randomized controlled clinical trial of efficacy. J. Clin. Endocrinol. Metabolism. 98 (7), 2984–2992. https://doi.org/10.1210/jc.2012-3742 (2013).
Laborde, S., Allen, M. S., Göhring, N. & Dosseville, F. The effect of Slow-Paced breathing on stress management in adolescents with intellectual disability: Slow-Paced breathing. J. Intellect. Disabil. Res. 61 (6), 560–567. https://doi.org/10.1111/jir.12350 (2017).
Bernardi, L., Porta, C., Gabutti, A., Spicuzza, L. & Sleight, P. Modulatory effects of respiration. Auton. Neurosci. 90 (1–2), 47–56. https://doi.org/10.1016/S1566-0702(01)00267-3 (2001).
Lehrer, P. et al. Voluntarily produced increases in heart rate variability modulate autonomic effects of endotoxin induced systemic inflammation: an exploratory study. Appl. Psychophysiol. Biofeedback. 35 (4), 303–315. https://doi.org/10.1007/s10484-010-9139-5 (2010).
Song, H. S. & Paul, M. L. The effects of specific respiratory rates on heart rate and heart rate variability. Appl. Psychophysiol. Biofeedback (2003).
Vaschillo, E. G., Vaschillo, B. & Lehrer, P. M. Characteristics of resonance in heart rate variability stimulated by biofeedback. Appl. Psychophysiol. Biofeedback. 31 (2), 129–142. https://doi.org/10.1007/s10484-006-9009-3 (2006).
Del Pozo, Jessica, M., Richard, N., Gevirtz, B., Scher & Erminia Guarneri Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am. Heart J. 147 (3), 545. https://doi.org/10.1016/j.ahj.2003.08.013 (2004).
Sakakibara, M., Hayano, J., Oikawa, L. O., Katsamanis, M. & Paul Lehrer Heart rate variability biofeedback improves cardiorespiratory resting function during sleep. Appl. Psychophysiol. Biofeedback. 38 (4), 265–271. https://doi.org/10.1007/s10484-013-9232-7 (2013).
Allan Hobson, J. Electrographic correlates of behavior in the frog with special reference to sleep. Electroencephalogr. Clin. Neurophysiol. 22 (2), 113–121. https://doi.org/10.1016/0013-4694(67)90150-2 (1967).
Evans, B. M. Periodic activity in cerebral arousal mechanisms — the relationship to sleep and brain damage. Electroencephalogr. Clin. Neurophysiol. 83 (2), 130–137. https://doi.org/10.1016/0013-4694(92)90026-e (1992).
Stančák, A. & Michal Kuna EEG changes during forced alternate nostril breathing. Int. J. Psychophysiol. 18 (1), 75–79. https://doi.org/10.1016/0167-8760(84)90017-5 (1994).
Tang, Y. Y. The neuroscience of mindfulness meditation. (2017). https://doi.org/10.1007/978-3-319-46322-3
Tang, Y. Y., Tang, R., Rothbart, M. K. & Posner, M. I. Frontal theta activity and white matter plasticity following mindfulness meditation. Curr. Opin. Psychol. https://doi.org/10.1016/j.copsyc.2019.04.004 (2019).
Cavanagh, J. F. & Michael J. Frank Frontal Theta as a mechanism for cognitive control. Trends Cogn. Sci. 18 (8), 414–421. https://doi.org/10.1016/j.tics.2014.04.012 (2014).
Zaccaro, A., Perrucci, M. G., Parrotta, E., Costantini, M. & Francesca Ferri Brain-Heart interactions are modulated across the respiratory cycle via interoceptive attention. NeuroImage 262 (November), 119548. https://doi.org/10.1016/j.neuroimage.2022.119548 (2022).
Kluger, D. S. and Joachim Gross. Tidal volume and respiration phase modulate Cortico-Muscular communication. (2020). https://doi.org/10.1101/2020.01.13.904524
Goddard, A. & Dennis Charney Toward an integrated neurobiology of panic disorder. J. Clin. Psychiatry. 58 (Suppl 2 (February), 4–11 (1997). discussion 11.
Paulus, M. P. THE BREATHING CONUNDRUM-INTEROCEPTIVE SENSITIVITY AND ANXIETY: review: the breathing conundrum. Depress. Anxiety. 30 (4), 315–320. https://doi.org/10.1002/da.22076 (2013).
Weng, H. Y. et al. Interventions and manipulations of interoception. Trends Neurosci. 44 (1), 52–62. https://doi.org/10.1016/j.tins.2020.09.010 (2021).
Morriss, J. What Do I Do Now? Intolerance of Uncertainty Is Associated with Discrete Patterns of Anticipatory Physiological Responding to Different Contexts. Psychophysiology, May, e13396. (2019). https://doi.org/10.1111/psyp.13396
de Jong, P. J. & Harald Merckelbach, and Arnoud Arntz Covariation Bias in phobic women: the relationship between a priori expectancy, on-Line expectancy, autonomic responding, and a posteriori contingency judgment. J. Abnorm. Psychol. 104 (1), 55–62. https://doi.org/10.1037/0021-843X.104.1.55 (1995).
Shankman, S. A., Jenna Robison-Andrew, E., Nelson, B. D., Altman, S. E. & Campbell, M. L. Effects of predictability of shock timing and intensity on aversive responses. Int. J. Psychophysiol. 80 (2), 112–118. https://doi.org/10.1016/j.ijpsycho.2011.02.008 (2011).
Sarinopoulos, I. et al. Uncertainty during anticipation modulates neural responses to aversion in human Insula and amygdala. Cereb. Cortex. 20 (4), 929–940. https://doi.org/10.1093/cercor/bhp155 (2010).
Grillon, C., Baas, J. P., Lissek, S., Smith, K. & Jean Milstein Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 118 (5), 916–924. https://doi.org/10.1037/0735-7044.118.5.916 (2004).
Dunsmoor, J. E., Peter, A., Bandettini & David C. Knight Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage 40 (2), 811–817. https://doi.org/10.1016/j.neuroimage.2007.11.042 (2008).
Grupe, D. W. & Nitschke, J. B. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion 11 (2), 413–424. https://doi.org/10.1037/a0022583 (2011).
Delorme, A. & Scott Makeig EEGLAB: an open source toolbox for analysis of Single-Trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 134 (1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009 (2004).
Dien, J. Issues in the application of the average reference: review, critiques, and recommendations. Behav. Res. Methods Instruments Computers. 30 (1), 34–43. https://doi.org/10.3758/BF03209414 (1998).
Al, E., Iliopoulos, F., Nikulin, V. V. & Arno Villringer Heartbeat and somatosensory perception. NeuroImage 238 (September), 118247. https://doi.org/10.1016/j.neuroimage.2021.118247 (2021).
Junghöfer, M., Elbert, T., Tucker, D. & Rockstroh, B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology 37 (August), 523–532. https://doi.org/10.1111/1469-8986.3740523 (2000).
Motamedi-Fakhr, S. et al. Respiratory cycle related EEG changes: modified respiratory cycle segmentation. Biomed. Signal Process. Control. 8 (6), 838–844. https://doi.org/10.1016/j.bspc.2013.08.001 (2013).
Luck, S. J. An Introduction To the Event-Related Potential Technique (MIT Press, 2014).
Harrison, O. K. et al. Interoception of breathing and its relationship with anxiety. Neuron 109 (24), 4080–4093e8. https://doi.org/10.1016/j.neuron.2021.09.045 (2021).
Meisler, A. Anxiety and its disorders: the nature and treatment of anxiety and panic. Clin. Psychol. Rev. 26 (1), 105–106. https://doi.org/10.1016/j.cpr.2005.10.001 (2006).
Bar-Anan, Y., Wilson, T. D. & Daniel T. Gilbert The feeling of uncertainty intensifies affective reactions. Emotion 9 (1), 123–127. https://doi.org/10.1037/a0014607 (2009).
Wilson, T. D., Centerbar, D. B., Kermer, D. A. & Daniel T. Gilbert The pleasures of uncertainty: prolonging positive moods in ways people do not anticipate. J. Personal. Soc. Psychol. 88 (1), 5–21. https://doi.org/10.1037/0022-3514.88.1.5 (2005).
Iii, J. J. et al. Comparison of the ratio of the standard deviation of the R-R interval and the root mean squared successive differences (SD/rMSSD) to the low frequency-to-High frequency (LF/HF) ratio in a patient population and normal healthy controls. (2007).
Gaur, S., Panjwani, U. & Kumar, B. EEG brain wave dynamics: A systematic review and Meta analysis on effect of yoga on Mind relaxation. J. Biomedical Res. Environ. Sci. 1 (7), 353–362. https://doi.org/10.37871/jbres1166 (2020).
Bušek, P. & D Kemlink The influence of the respiratory cycle on the EEG. Physiol. Res. 327–333. https://doi.org/10.33549/physiolres.930551 (2005).
Heck, D. H., Correia, B. L., Mia, B., Fox, Y., Liu, M. A. & Somogy Varga Recent insights into respiratory modulation of brain activity offer new perspectives on cognition and emotion. Biol. Psychol. 170 (April), 108316. https://doi.org/10.1016/j.biopsycho.2022.108316 (2022).
Schurmann, M., Demiralp, T., Basar-Eroglu, C. & Basar, E. Selectively distributed gamma-band responses studied in cortex, reticular formation,hippocampus, and cerebellum. In: (ed Basar, E.) Brain Function and Oscillations. II. Integrative Brain Function. Neurophysiology and Cognitive Processes. Springer, Berlin, Heidelberg, 61–67 (1999).
Smit, A. S. et al. Mental and physical effort affect vigilance differently. Int. J. Psychophysiol. 57 (3), 211–217. https://doi.org/10.1016/j.ijpsycho.2005.02.001 (2005).
Funding
This research was supported by the National Natural Science Foundation of China (31771231, 32071070), Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0520, cstc2020jcyj-msxmX0299), 111 program (B21036), the Research Program Funds of the Collaborative Innovation Center of Assessment toward Basic Education Quality at Beijing Normal University, the planned project of Chongqing humanities and Social Sciences (2018PY80, 2019PY51), Fundamental Research Funds for the Central Universities (SWU119007), Chang Jiang Scholars Program, National Outstanding Young People Plan and Chongqing Talent Program.
Author information
Authors and Affiliations
Contributions
Qian Luo: analysis; interpretation of data; have drafted the work. Xianrui Li analysis. Jia Zhao: analysis. Qiu Jiang: conception or design of the work; substantively revised. Dongtao Wei: conception or design of the work; substantively revised; Every author have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study); Every author have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Q., Li, X., Zhao, J. et al. The effect of slow breathing in regulating anxiety. Sci Rep 15, 8417 (2025). https://doi.org/10.1038/s41598-025-92017-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92017-5