Abstract
Sepsis, a life-threatening organ dysfunction caused by a dysregulated host response to infection, has an approximately 25% in-hospital mortality rate. Identifying early biomarkers of pediatric sepsis is crucial for improving outcomes. This study explored the differential expression of peptides in patients with sepsis compared to healthy controls and those with common infections using plasma peptidomic analysis. Blood samples were collected from 10 pediatric patients with sepsis admitted to Hunan Children’s Hospital in 2021, along with 20 age- and sex-matched healthy controls and five children with common infections. Differential peptide precursor proteins underwent gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses and protein–protein interaction analysis using the STRING database. Intotal, 3149 endogenous peptides corresponding to 480 precursor proteins were identified. Compared to the healthy group, the sepsis group exhibited 1113 differentially expressed peptides, with 880 upregulated and 233 downregulated. Compared with the common infection group, the sepsis group showed 181 upregulated and 86 downregulated peptides. These differences were primarily in the humoral immune response and complement and coagulation cascades. This study identified specific alterations in peptide expression in the plasma of patients with sepsis, most notably in peptides related to SAA1, complement C3, hemoglobin, and haptoglobin. These peptides are involved in the acute inflammatory response, complement system, and free hemoglobin pathways, indicating their crucial roles in sepsis pathology. These findings provide new insights into the mechanisms of sepsis and suggest potential applications for these peptides in sepsis diagnosis and treatment, to enhance early diagnosis and therapeutic outcomes.
Similar content being viewed by others
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection1, with an in-hospital mortality rate of approximately 25%2,3. Children, particularly those under the age of five, are among the most susceptible to sepsis, accounting for approximately40% of global sepsis cases in this age group4. Data from Germany indicate that sepsis is responsible for 11.5% of pediatric in-hospital deaths5. Research has shown that every hour of delay in medication administration negatively affects the length of hospital stay, secondary organ damage, and mortality rate6. Therefore, early identification and timely treatment of sepsis are crucial for improving prognosis and reducing mortality. Owing to its unique physiological characteristics, the symptoms and pathological features of sepsis in children differ from those in adults. Pediatric sepsis often presents with atypical symptoms, such as poor appetite, lethargy, fever, or hypothermia, making early recognition more challenging. The immature immune system of children may respond differently to infections than that of adults, thereby affecting disease progression and prognosis. Hence, identifying the specific characteristics and early biomarkers of pediatric sepsis is vital for improving the outcomes.
In recent years, the significance of serum peptides as early disease biomarkers has become increasingly prominent. Endogenous peptides are widely distributed across various organs, tissues, cells, and body fluids, typically ranging from 3 to 50 amino acids in length with a molecular weight of less than 10 kDa. These peptides play key roles in physiological and pathological processes such as immune regulation, cell differentiation, neurotransmitter modulation, and tumorigenesis7,8. Studies have shown that changes in the type and quantity of proteins and peptides often occur before the appearance of obvious symptoms or pathological changes, thus presenting broad clinical diagnostic applications.
Numerous studies have confirmed that procalcitonin has significant clinical value in the diagnosis, monitoring, and guiding the use of antibiotics in infectious diseases9,10. The peptide model constructed by Meng et al. achieved an accuracy and sensitivity of 82.20% and 82.50%, respectively, for diagnosing patients with type 2 diabetes11. Other studies have shown that ASRPS(a small regulatory peptide of STAT3) can inhibit angiogenesis in triple-negative breast cancer (TNBC), providing a new target for TNBC treatment12. The peptide COL-4A1 can disrupt endothelial cells through the TGF-β/PI3K/AKT pathway, increasing blood pressure in preeclampsia (PE) model rats, reducing the weight of the placenta and fetus, and exacerbating pathological changes in the kidneys and placenta, thus offering a new target for PE treatment13. These findings underscore the significant potential of peptidomics for the early diagnosis and treatment of diseases.
The advantages of serum peptides as a focus of disease research are primarily reflected in the following aspects7,14,15: First, changes in the types and quantities of peptides can often be observed before the appearance of obvious symptoms or pathological changes. Second, as ideal target molecules, peptides have strong specificity and low immunogenicity compared to chemical or protein-based drugs. Therefore, this study aimed to analyze the plasma peptidomics in pediatric patients with sepsis, explore the peptidomic characteristics of pediatric sepsis to provide new insights and strategies for early diagnosis and treatment.
Methods
Patients and sample collection
Blood samples were collected from 10 pediatric patients with sepsis admitted to Hunan Children’s Hospital in 2021. Additionally, blood samples from 20 age- and sex-matched healthy children undergoing routine checkups were collected as the control group, along with samples from five children with common infections as the common infection group. Sample collection occurring in January and from May to August. Clinical data were extracted from the electronic medical records. Diagnostic criteria for sepsis were based on the “International Consensus Criteria for Pediatric Sepsis and Septic Shock.”1 This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee of Hunan Children’s Hospital (HCHLL-2020-93). All blood samples were collected after informed consent was obtained.
LC-MS/MS analysis
In this study, the collected blood samples (EDTA tube) were processed within 24 h. They were centrifuged at 2700×g for 10 min to collect the supernatant (plasma), which was stored at − 80 °C. One aliquot of each sample was pooled equally together to form one quality control (QC) sample, this QC divided into 9 parts and each part could be used as a QC for this study and repeat 9 times. For the rest samples, each sample (100 µL) was mixed with 300µL of 0.5% TFA solution, vortexed, and centrifuged at 4 °C and 17,000 g for 10 min to collect the supernatant. Desalting involved column activation with 0.1% TFA and 80% acetonitrile, followed by equilibration with 0.1% TFA and 1% acetonitrile, centrifuged at 1000 g. After washing, peptides were added into the column, and washed with 0.1%TFA and 1% acetonitrile, centrifuged at 1000 g. Then they were eluted with 0.1% TFA and 80% acetonitrile and freeze-dried.
Samples were separated on an EASY-nLC 1000 nano-LC system, with solvent A as 0.1% formic acid in water and solvent B as 0.1% formic acid in acetonitrile. Samples dissolved in 10µL of solvent A were injected at 500nL/min. The gradient included: 0–45 min, B solvent from 2 to 32%; 45–55 min, 32–80%; 55–56 min, 80–100%, with 100% maintained for 4 min.
Mass spectrometry was conducted on an Orbitrap Fusion, with a spray voltage of 2.4 kV and a capillary temperature of 320 °C. Data-dependent acquisition ranged from m/z 350–1600, MS1 resolution 120,000. MS/MS was performed on 2–5 charge ions, with a 10-second collection per ion and a 21-second exclusion. For each sample there is only one technical replicate.
Proteome Discoverer database search Vendor’s raw MS files were processed using Proteome Discoverer (PD) software (Version 2.4.0.305) and the built in Sequest HT search engine. MS spectra lists were searched against their specieslevel UniProt FASTA databases (Uniprot-SwissProt Homo sapiens, 2021-08-18, reviewed entries 20396.fasta), Oxidation (M) and Acetyl (Protein Nterm) as variable modifications. No Enzyme (Unspecific) was used in this study. False positive rate (FDR) for spectra, peptides and proteins ≤ 1%; proteins match at least 1 unique peptide; peptides contain at least 6 amino acids and a fragment mass deviation of 0.02Da. Unique peptide and Razor peptide were used for protein quantification and total peptide amount for normalization. All the other parameters were reserved as default.
Bioinformatics analysis
Protein annotation methods
The raw mass spectrometry files were analyzed using the Sequest HT search engine in the Proteome Discoverer software (version 2.4.0.305). Differentially expressed peptides were annotated using gene ontology (GO) analysis based on the Uniprot-SwissProt database (species Homo sapiens, 2021-03-18; reviewed entry 20396).
Protein functional enrichment
Functional enrichment analysis was performed using the clusterProfiler, org.Hs.eg.db, and enrichplot packages in R. Protein functional enrichment was categorized using GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses. KEGG pathway enrichment was conducted to identify significantly enriched biological pathways16,17,18. The GO annotations were divided into biological processes, cellular components, and molecular functions. The KEGG annotations were categorized into organismal systems, human diseases, cellular processes, metabolism, and environmental information processing. The InterPro database was used to analyze the enrichment of the functional domains of differentially expressed proteins.
Protein-protein interaction network analysis
Differential protein database identifiers or sequences from different comparison groups were compared using the STRING protein interaction network database (version 12.0). Interactions were extracted with a confidence score of > 0.7, and the interaction network was visualized using Cytoscape software (version 3.8+).
Statistical analysis
Peptide expression levels were compared between groups using the rank-sum test in Python’s scipy.stats library, with group differences represented as log-fold change (FC). For comparisons of mean values across multiple groups, an analysis of variance was performed using SPSS version 25. The Benjamini-Hochberg method, implemented through statsmodels.stats.multitest.multipletests with method=‘fdr_bh‘, was applied to adjust the P-values for differences in peptide expression between groups. Statistical significance was set at an adjusted P-value < 0.05. GO and KEGG pathway enrichment analyses were performed on the precursor proteins of the differentially expressed peptides, and a protein–protein interaction network was constructed.
Results
Clinical characteristics of study participants
This study included 35 participants aged from 1.8 months to 13.5 years. The healthy control group comprised 20 participants with a median age (P25, P75) of 3.26 (0.94, 8.56) years. The common infection group includedfive participantswith a median age of 3.32 (1.23, 3.44), and the sepsis group included 10 participants with a median age of 2.80 (0.94, 9.36) years. White blood cell levels in the sepsis group (18.54 ± 12.58) were significantly higher than those in the common infection (9.01 ± 4.14) and healthy control (8.42 ± 3.08) groups(P < 0.05). Platelet levels decreased sequentially across the healthy control, general infection, and sepsis groups (346.59 ± 90.94, 254.00 ± 113.68, and 246.40 ± 188.77, respectively), as did hemoglobin (Hb) levels (121.88 ± 12.59, 115.80 ± 14.79, and 105.50 ± 18.18, respectively). Additionally, aspartate aminotransferase (AST) levels showed a progressive increase across groups, from 28.33 ± 12.36 in the healthy controls to 68.14 ± 35.21 in the infection group, reaching 1616.81 ± 3137.17 in the sepsis group. Alanine aminotransferase (ALT) levels followed a similar trend (12.72 ± 4.61, 115.60 ± 151.20, and 475.56 ± 771.97, respectively) (Supplementary Table 1). Patients in the general infection group had no comorbidities. Regarding the initial symptoms in the sepsis group, two cases presented with respiratory system symptoms, four with digestive system symptoms, and four with nervous system symptoms (Table 1).
Analysis of differential plasma peptides
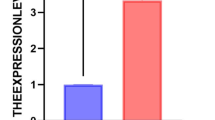
In total of 3149 endogenous peptide segments and 480 related precursor proteins were identified. By comparing the sepsis group with the healthy control group, 1113 differentially expressed peptides and 157 precursor proteins were identified. Among these, 880 peptides (121 precursor proteins) were upregulated and 233 peptides (64 precursor proteins) were downregulated (Fig. 1). When comparing the sepsis and general infection groups, 181 peptides (44 precursor proteins) were upregulated and 86 peptides (37 precursor proteins) were downregulated. According to the logFC ranking between the groups, the peptide segment of the precursor protein serum amyloid A1(SAA1) had the highest differential fold-change in both the sepsis vs. healthy and sepsis vs. common infection groups, with logFC values of 9.434 and 5.580, respectively (Table 2).
GO functional enrichment analysis of differentially expressed peptide-related precursor proteins
GO functional enrichment analysis was conducted on the precursor proteins of differentially expressed peptides. In the sepsis vs. healthy group, the biological process classification revealed that these precursor proteins were mainly involved in processes such as the humoral immune response, negative regulation of catalytic activity, and wound healing. In the cellular component classification, these precursor proteins were mainly located in the blood microparticles, collagen-containing extracellular matrix (ECM), and endoplasmic reticulum lumen. In the molecular function classification, these precursor proteins exhibited enzyme inhibitor, peptidase regulator, and peptidase inhibitor activities(Fig. 2A).
GO, KEGG, and protein-protein interaction network analysis of precursor proteins of differential peptides between sepsis group and healthy group, and between sepsis group and common infection group. (A) GO analysis of precursor proteins of differential peptides between the sepsis and healthy groups. (B) GO analysis of precursor proteins of differential peptides between the sepsis and common infection groups. (C) KEGG analysis of precursor proteins of differential peptides between the sepsis and healthy groups. (D) KEGG analysis of precursor proteins of differential peptides between the sepsis and common infection groups. (E) Protein-protein interaction network analysis of precursor proteins of differential peptides between the sepsis and healthy groups. (F) Protein-protein interaction network analysis of precursor proteins of differential peptides between the sepsis and common infection groups.
In the sepsis vs. general infection group, the biological process classification showed that these precursor proteins were mainly involved in processes such as the humoral immune response, negative regulation of catalytic activity, and wound healing. In the cellular component classification, these precursor proteins were mainly located in the blood microparticles, collagen-containing extracellular matrix, and vesicle lumen. In the molecular function classification, these precursor proteins exhibited enzyme inhibitory, peptidase regulatory, and endopeptidase regulatory activities (Fig. 2B).
KEGG pathway enrichment analysis of differentially expressed peptide-related precursor proteins
KEGG pathway enrichment analysis of the sepsis vs. healthy groups revealed that the precursor proteins of the differentially expressed peptides were primarily involved in several key pathways. In the organismal system category, they participated in the complement and coagulation cascades, neutrophil extracellular trap formation, and platelet activation pathways. In the human disease category, they were involved in pathways related to coronavirus disease 2019 (COVID-19), Staphylococcus aureus infection, and pertussis. The phagosome pathway was prominent in the cellular process category. In the metabolism category, they were involved in glycolysis/gluconeogenesis pathways. Finally, in the environmental information processing category, they participated in the ECM-receptor interaction pathway(Fig. 2C).
KEGG pathway enrichment analysis comparing the sepsis and common infection groups showed that the precursor proteins of the differential peptides were primarily involved in the complement and coagulation cascades, neutrophil extracellular trap formation, and platelet activation pathways in the organismal system category. In the human disease category, they were mainly involved in pathways related to COVID-19 and S. aureus infection (Fig. 2D).
Protein–protein interaction network of differentially expressed precursor proteins
Protein-protein interaction analysis of the differentially expressed proteins between the sepsis and healthy groups identified the top five nodes with the highest degrees as ALB (36), AHSG (28), APOA1 (28), APOB (27), and A2M (25) (Fig. 2E). Similarly, analysis of the sepsis and general infection groups identified the top five nodes as APOA1 (23), ALB (22), AHSG (19), A2M (18), and APOE (15) (Fig. 2F).
Comparative analysis of differentially expressed peptides between groups
An analysis of peptides with logFC > 2 or logFC < -2 revealed 705 peptides meeting the criteria in the comparison between the sepsis and healthy groups, and 128 peptides in the comparison between the sepsis and common infection groups. A total of 66 overlapping peptides were identified between the two comparisons (Fig. 3A). The comparative expression of these overlapping peptides, ranked by logFC in the sepsis vs. common infection groups, is shown in Fig. 3B. A comparison of inter-group expression levels of the top 10 peptide segments for common sepsis vs. infection is shown in Fig. 3C. Notably, the SAA1-related peptide RSFFSFLGEA shows significant upregulation in the sepsis group, exhibiting the highest logFC when compared to both the healthy group (logFC = 6.828) and the general infection group (logFC = 5.580) (Table 3).
Comparison of differential peptides between sepsis group and healthy group, and between sepsis group and common infection group. (A) Venn diagram of differential peptides between the sepsis and healthy groups, and between the sepsis and common infection groups. (B) Heatmap of normalized expression of overlapping differential peptides between the sepsis and healthy groups, and between the sepsis and common infection groups. (C) Comparison of peptide expression levels between the groups.
Discussion
In this study, we identified significant differential peptide expression among patients with sepsis, healthy individuals, and individuals with general infections using plasma peptidomic analysis. These differences were primarily observed in processes such as the humoral immune response and complement and coagulation cascades. Among these, peptides related to SAA1 exhibited the most notable changes.
The serum amyloid A (SAA) gene family comprises four distinct loci: SAA1 and SAA2 encode acute-phase SAAs (A-SAAs), whereas SAA3 and SAA4 encode other types of SAA proteins. A-SAA is a crucial acute-phase reactant that plays a significant role in the immune response, including induction of cytokine production, promotion of leukocyte migration and adhesion, and activation of the complement system19,20. A-SAA can activate the nuclear factor-kappa B (NF-κB) signaling pathway by binding to Toll-like receptors (TLR) 2 and TLR4, thereby inducing cytokine production and participating in the inflammatory response21.
Studies have shown that combining SAA1 with other inflammatory markers can enhance the diagnostic accuracy of sepsis. For example, a predictive model combining SAA1, C-reactive protein (CRP), and procalcitonin (PCT) achieved an area under the receiver operating characteristic curve of 0.89 (95% confidence interval 0.82–0.95] for diagnosing sepsis, significantly outperforming the individual predictive values of CRP, PCT, and interleukin-6 (IL-6)22. SAA has also demonstrated good diagnostic and monitoring value for neonatal sepsis23. Moreover, SAA1 can directly bind to pathogens, such as gram-negative bacteria, thereby enhancing the phagocytic and bactericidal activities of macrophages and neutrophils21. Research has also shownthat SAA plays a protective role in lipopolysaccharide (LPS)-induced inflammation and tissue damage by binding to LPS and promoting its clearance24.
SAA1 is an importantmarker that reflects the degree and activity of inflammation. The SAA1 level increases rapidly upon infection or injury and decreases quickly after antigen clearance, making it valuable for early diagnosis, risk assessment, treatment monitoring, and prognostic evaluation in clinical practice. Our study found that SAA1-related peptides were significantly elevated in children with sepsis, distinguishing them from healthy controls and common infected patients, with the peptide RSFFSFLGEA showing the greatest significant difference. These findings further support the diagnostic value of SAA1 in pediatric sepsis.
The complement system is part of the immune system that initiates inflammatory responses by recognizing pathogens and damaged cells. Activation of the complement system can lead to cell lysis, the release of inflammatory mediators, and the recruitment of immune cells. Complement component 3 (C3) is a key protein in the complement system that is involved in pathogen marking and clearance, as well as in regulating the inflammatory response25. In this study, we found that peptides related to C3 and complement factor B were significantly elevated in children with sepsis, with the peptides IITPNILRLESEE and IEGVDAEDGHGPGEQQ showing the most notable differences. Activation of the complement system not only enhances the immune response but may also exacerbate inflammation, leading to more severe pathological states.
Studies haves revealed that the carboxypeptidase B1 (Cpb1)-C3-C3a receptor (C3aR) pathway significantly affects the severity of endotoxemia. C3aR deficiency or the use of C3aR inhibitors can reduce the expression of inflammatory mediators and weight loss in mice with endotoxemia, thereby improving their survival rates. C3aR plays an important role in primary human macrophages by enhancing the inflammatory response, and its expression is associated with increased caspase-5 expression in the peripheral blood mononuclear cells of patients with sepsis. The complement pathway amplifies caspase-11-dependent cell death through the Cpb1-C3-C3aR signaling pathway, which is crucial for the severity of gram-negative bacterial infections and endotoxemia. Thus, C3aR may serve as an early therapeutic target for sepsis, and C3aR and caspase-5 transcripts may serve as potential biomarkers for sepsis diagnosis26.
In addition, this study found that the levels of (Hb)-related peptides, specifically those related to Hb subunit beta and Hb subunit gamma-2, were significantly elevated in pediatric patients with sepsis. Among these, the peptides WGKVNVDEVGGEALGRLL and GHFTEEDKATITSL exhibited the most notable differences. Erythrocyte destruction induced by infection may be an important early marker of sepsis.
Erythrocyte destruction leads to elevated levels of free-Hb. Using Raman spectroscopy, Verma et al. observed free-Hb in an LPS induced endotoxin shock model, but not in a thioglycolate-induced sterile peritonitis model. This study suggests that hemolysis is a marker of systemic rather than local inflammation27. The onset of the systemic inflammatory response is an early event in sepsis; hence, hemolysis-related markers induced by infection have potential value in the early diagnosis of sepsis. Free-Hb also plays an important role in sepsis progression. Elevated free-Hb levels increase mortality and lung injury in animals with septic shock. The mechanism of action of free-Hb may be related to the generation of a cytokine storm and its nutritional role in bacteria. Free-Hb can significantly elevate cytokine and chemokine levels, thereby promoting a cytokine storm. Free-Hb may also provide iron, which promotes bacterial growth, further exacerbating the condition28,29.
Notably, our previous study identified haptoglobin (HP), another important protein involved in hemolysis-related injury, as an independent risk factor for mortality in pediatric sepsis30. HP can specifically bind to free-Hb, facilitating its transfer to the liver for catabolism, thus mitigating the toxic effects of free-Hb and inhibiting bacterial growth29,31. HP also binds to high-mobility group box 1 protein and mediates the production of IL-10 and heme oxygenase-1 through the CD163 signaling pathway, thereby reducing inflammation and oxidative stress32. Consequently, HP is a natural scavenger of hemolytic toxic effects within the body. In the present study, we found that HP-related peptides were significantly elevated in sepsis, with the peptide YVGKKQLVE showing the most significant difference. This finding further supports the evaluative value of HP for sepsis severity.
To our knowledge, this is the first study to describe the peptidomic characteristics of pediatric sepsis. However, the sample size was relatively small, which limits the generalizability of the findings. Further large-sample validation studies are required to confirm the clinical relevance of the identified candidate biomarkers and their potential application in the diagnosis and treatment of sepsis, with the aim of improving early detection and treatment outcomes.
Conclusion
In summary, this study revealed specific changes in peptide expression in the plasma of patients with sepsis using peptidomic analysis. In particular, significant changes were noted in peptides related to SAA1(RSFFSFLGEA), C3 (IITPNILRLESEE), Hb (GHFTEEDKATITSL and WGKVNVDEVGGEALGRLL), and HP (YVGKKQLVE). These peptides are associated with the acute inflammatory response processes, complement system, free-Hb, and other pathways, suggesting their important roles in the pathology of sepsis. These findings provide novel insights into the pathological mechanisms underlying sepsis and highlight the potential of these peptides as biomarkers for the disease.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Schlapbach, L. J. et al. International consensus criteria for pediatric Sepsis and septic shock. Jama 331 https://doi.org/10.1001/jama.2024.0179 (2024).
Xiao, C. et al. Epidemiology of pediatric severe Sepsis in main PICU centers in Southwest China*. Pediatr. Crit. Care Med. 20, 1118–1125. https://doi.org/10.1097/pcc.0000000000002079 (2019).
Humoodi, M. O. et al. Epidemiology of pediatric sepsis in the pediatric intensive care unit of King Abdulaziz medical city, Jeddah, Saudi Arabia. BMC Pediatr. 21 https://doi.org/10.1186/s12887-021-02686-0 (2021).
World Health Organization. Sepsis. World Health Organization, 3 May 2024. www.who.int/news-room/fact-sheets/detail/sepsis
Born, S. et al. Epidemiology of Sepsis among children and neonates in Germany: results from an observational study based on nationwide Diagnosis-Related groups data between 2010 and 2016*. Crit. Care Med. 49, 1049–1057. https://doi.org/10.1097/ccm.0000000000004919 (2021).
Ko, B. S. et al. Time to antibiotics and the outcome of patients with septic shock: A propensity score analysis. Am. J. Med. 133, 485–491e484. https://doi.org/10.1016/j.amjmed.2019.09.012 (2020).
Sen, A., Nigam, A. & Vachher, M. Role of polypeptide inflammatory biomarkers in the diagnosis and monitoring of COVID-19. Int. J. Pept. Res. Ther. 28 https://doi.org/10.1007/s10989-022-10366-5 (2022).
Hayakawa, E. et al. Improving the identification rate of endogenous peptides using Electron transfer dissociation and Collision-Induced dissociation. J. Proteome Res. 12, 5410–5421. https://doi.org/10.1021/pr400446z (2013).
Downes, K. J., Fitzgerald, J. C., Weiss, S. L. & Kraft, C. S. Utility of procalcitonin as a biomarker for Sepsis in children. J. Clin. Microbiol. 58 https://doi.org/10.1128/jcm.01851-19 (2020).
Schuetz, P. How to best use procalcitonin to diagnose infections and manage antibiotic treatment. Clin. Chem. Lab. Med. (CCLM). 61, 822–828. https://doi.org/10.1515/cclm-2022-1072 (2023).
Meng, Q. et al. Screening for potential serum-based proteomic biomarkers for human type 2 diabetes mellitus using MALDI‐TOF MS. Proteom. – Clin. Appl. 11 https://doi.org/10.1002/prca.201600079 (2016).
Wang, Y. et al. LncRNA-encoded polypeptide ASRPS inhibits triple-negative breast cancer angiogenesis. J. Exp. Med. 217 https://doi.org/10.1084/jem.20190950 (2020).
Li, T. et al. The COL-4A1 polypeptide destroy endothelial cells through the TGF-beta/PI3K/AKT pathway. Sci. Rep. 11, 15761. https://doi.org/10.1038/s41598-021-94801-5 (2021).
Vu, N. Q., DeLaney, K., Li, L. & Neuropeptidomics Improvements in mass spectrometry imaging analysis and recent advancements. Curr. Protein Pept. Sci. 22, 158–169. https://doi.org/10.2174/1389203721666201116115708 (2021).
Hulme, H. et al. Simultaneous mass spectrometry imaging of multiple neuropeptides in the brain and alterations induced by experimental parkinsonism and L-DOPA therapy. Neurobiol. Dis. 137 https://doi.org/10.1016/j.nbd.2020.104738 (2020).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Sack, G. H. Serum amyloid A – a review. Mol. Med. 24 https://doi.org/10.1186/s10020-018-0047-0 (2018).
Sun, L. & Ye, R. D. Serum amyloid A1: structure, function and gene polymorphism. Gene 583, 48–57. https://doi.org/10.1016/j.gene.2016.02.044 (2016).
Zhang, Y., Zhang, J., Sheng, H., Li, H. & Wang, R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv. Clin. Chem. 90, 25–80. https://doi.org/10.1016/bs.acc.2019.01.002 (2019).
Li, M. et al. A biomarker panel of C-reactive protein, procalcitonin and serum amyloid A is a predictor of sepsis in severe trauma patients. Sci. Rep. 14 https://doi.org/10.1038/s41598-024-51414-y (2024).
Bourika, V. et al. Clinical value of serum Amyloid-A protein, High-density lipoprotein cholesterol and Apolipoprotein-A1 in the diagnosis and Follow-up of neonatal Sepsis. Pediatr. Infect. Disease J. 39, 749–755. https://doi.org/10.1097/inf.0000000000002682 (2020).
Cheng, N., Liang, Y., Du, X. & Ye, R. D. Serum amyloid A promotes LPS clearance and suppresses LPS -induced inflammation and tissue injury. EMBO Rep. 19 https://doi.org/10.15252/embr.201745517 (2018).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I - Molecular mechanisms of activation and regulation. Front. Immunol. 6, 262. https://doi.org/10.3389/fimmu.2015.00262 (2015).
Napier, B. A. et al. Complement pathway amplifies caspase-11–dependent cell death and endotoxin-induced sepsis severity. J. Exp. Med. 213, 2365–2382. https://doi.org/10.1084/jem.20160027 (2016).
Verma, T. et al. Cell-free hemoglobin is a marker of systemic inflammation in mouse models of sepsis: a Raman spectroscopic study. Analyst 146, 4022–4032. https://doi.org/10.1039/d1an00066g (2021).
Wang, J. et al. Mechanistic insights into cell-free hemoglobin-induced injury during septic shock. Am. J. Physiol. Heart Circ. Physiol. 320, H2385–H2400. https://doi.org/10.1152/ajpheart.00092.2021 (2021).
Sakamoto, K. et al. IL-22 controls Iron-Dependent nutritional immunity against systemic bacterial infections. Sci. Immunol. 2 https://doi.org/10.1126/sciimmunol.aai8371 (2017).
Luo, T. et al. Proteomic analysis identified potential age-associated prognostic biomarkers in pneumonia-derived paediatric sepsis. Proteomics. Clin. Appl. 16, e2100036. https://doi.org/10.1002/prca.202100036 (2022).
Buehler, P. W., Humar, R. & Schaer, D. J. Haptoglobin therapeutics and compartmentalization of cell-free hemoglobin toxicity. Trends Mol. Med. 26, 683–697. https://doi.org/10.1016/j.molmed.2020.02.004 (2020).
Schaer, D. J., Buehler, P. W., Alayash, A. I., Belcher, J. D. & Vercellotti, G. M. Hemolysis and free hemoglobin revisited: exploring hemoglobin and Hemin scavengers as a novel class of therapeutic proteins. Blood 121, 1276–1284. https://doi.org/10.1182/blood-2012-11-451229 (2013).
Funding
This study was supported by the Hunan Provincial Natural Science Foundation of China (No. 2022JJ40203, grant to HY; 2023JJ40351, grant to LX; 2023JJ30324, grant to XLu), the National Natural Science Foundation of China (Young Scientists Fund, No. 82102285, grant to XLi), the Hunan Provincial Science and Technology Department Project (2020SK1014-3 and 2020SK2114, grant to XLu), the Hunan Provincial Key Laboratory of Emergency Medicine for Children (No. 2018TP1028, grant to ZX), and the Scientific Research Project of Hunan Provincial Health Commission (No. 202217012704, grant to HY; B202217018341, grant to LX). The study sponsors have no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
HY contributed to the study’s conception, analysed and interpreted the data, and wrote the manuscript. ZX and XLu contributed to the study design, interpreted the data, and revised the manuscript. XunLi, XiaoLi, XHZ, TL, LX, XW, YY, XZ, JH, PL, LL, JC, and HZ performed chart reviews, interpreted the data, and revised the manuscript. ZX and XLu designed the study, interpreted the data, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The study protocol was reviewed and approved by the Medical Ethics Committee of the Hunan Children’s Hospital (HCHLL-2020-93). Informed consent was waved because of the retrospective design. The authors had no access to information that could identify individual participants during and after data collection.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.