Abstract
Oncogenic human papillomavirus (HPV) infection is a major cause of anogenital cancers in both females and males. Nevertheless, only a limited number of studies have explored the epidemiology of HPV infection across genders within the same population. This retrospective study analyzed 299,089 samples from Hangzhou, China, collected between 2017 and 2023, including 283,556 females and 15,533 males. The overall HPV infection rate was 23.8%, with a prevalence of 22.85% in females and 41.13% in males. Gender differences were observed in the prevalence of high-risk HPV. In females, the most common high-risk HPV type was HPV52 (5.58%), followed by HPV58 (2.84%) and HPV16 (2.64%). In males, HPV52 (5.28%) was also the most prevalent, followed by HPV16 (5.08%) and HPV51 (3.57%). The age-related HPV infection rate shows a bimodal pattern, peaking in individuals under 20 years old and those aged 61–65. During the COVID-19 pandemic, the overall HPV infection rate declined by approximately 3% across all age groups. This study provides valuable insights into the dynamics of HPV infection in the context of the pandemic and emphasizes the need for HPV vaccination for adolescent males.
Similar content being viewed by others
Background
Epidemiological studies have consistently linked Human Papillomavirus (HPV) with various human diseases, including anogenital cancers and anogenital warts1,2. Cervical cancer, ranking as the fourth most common cancer among women, stands as a significant burden of HPV-related disease, with the International Agency for Research on Cancer (IARC) estimating approximately 660,000 new cases and 350,000 deaths globally in 20223.
Human Papillomavirus (HPV) is a small, non-enveloped, circular double-stranded DNA virus, measuring approximately 8 kb in size4. It primarily spreads through sexual contact, encompassing vaginal, anal, and oral intercourse. These viruses are categorized into two distinct groups: high-risk (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and low-risk (HPV6, 11, 42, 43, 53, 81) types based on their carcinogenic potential. HPV 16 and HPV 18 are chiefly implicated in the development of various cancers, including cervical, anal, penile, and oropharyngeal cancers5, whereas HPV 6 and 11 are accountable for nearly all cases of anogenital warts.
The rates of HPV infection exhibit similarity between genders (3.5-45% vs. 2-44%)2, as do the incidence rates of infection transmission5. Recent HPV-related research has emphasized that HPV is not only a leading cause of cervical cancer in women but also influences sperm status, potentially leading to infertility and cancers in men6. However, epidemiological studies have primarily focused on women, and data on HPV in men, particularly studies exploring differences and similarities between HPV-infected men and women, remain scarce.
HPV vaccination is the most effective method for preventing cervical cancer. However, vaccination rates in China remain low, with first-dose coverage at just 10.15% and third-dose coverage at only 6.21% as of 2022. These rates are significantly lower than the global coverage rates of 20% for the first dose and 15% for the full series among females in 20197.
Being the largest developing country, China demonstrates notable variations across different periods and regions, especially regarding the characterization of HPV epidemiological features during the COVID-19 pandemic. In this analysis, we integrated retrospective data from three hospitals in Hangzhou city spanning the period before, during, and after COVID-19 from 2017 to 2023. Our study delineates the prevalence of HPV infection, genotype distribution, and the distribution of HPV infection across different genders, periods, and age groups to provide useful references for the prevention and control of HPV infection in Hangzhou City.
Methods
Study subjects
In this study, we incorporated a total of 306,480 records of HPV tests conducted between January 1, 2017, and December 31, 2023. For patients with multiple HPV tests, only the results from their first test were included (6,004 tests were excluded). Any samples with incomplete information, such as missing age or gender data (933 individuals were excluded), were excluded from the study. Additionally, we excluded 454 patients who had undergone a hysterectomy or cone biopsy, as well as those with other sexually transmitted infections like HIV-1 or HSV, as documented in their medical records at the time of their initial HPV test. In final, these data comprised 283,556 tests from female subjects and 15,533 from male subjects, sourced from three distinct hospital facilities: Hangzhou Xixi Hospital, the Second Affiliated Hospital Zhejiang University School of Medicine, and The Affiliated Hospital of Hangzhou Normal University.
HPV clinic
In Hangzhou, HPV screening is a routine examination for women. In this study, most women underwent screening as part of regular health check-ups, while a smaller proportion sought testing due to gynecological symptoms. Men, on the other hand, are rarely tested unless their sexual partner tests positive for HPV or they exhibit symptoms related to the urinary system. Regarding referral cases, patients with positive HPV screenings are generally not referred, as most hospitals are equipped to manage such cases. This retrospective study analyzed HPV clinical test results from the hospital over three distinct periods: three years before the COVID-19 pandemic (2017–2019), three years during the pandemic (2020–2022), and one year post-pandemic (2023).
HPV detection and genotyping
The subjects were asked to refrain from sexual activity and avoid washing their genital area for 48 h prior to sample collection. Cervical samples were obtained by using a cervical brush, and male urethral secretion swabs were placed in a preservative buffer solution. The samples were stored at 2–8 °C for no more than 7 days. The Human Papillomavirus (HPV) Nucleic Acid Detection Kit (Bohui-24 version, Biochip Method, BOHUI Biotechnology, China) is designed with specific primers and probes targeting the L1 region of the HPV genome8. It employs a combination of PCR amplification in vitro and DNA reverse dot blot hybridization, a DNA chip technology that effectively provides qualitative detection and genotyping of HPV. It is capable of accurately identifying 14 high-risk and 6 low-risk HPV types. All experimental protocols adhere to the manufacturer’s manual. A Ct value of ≤ 39 indicates a positive HPV result, while a Ct value > 39 is interpreted as negative. The ABI7500 instrument was used in this study, and quality controls were included in all the experiments, including DNA amplification and genotyping, with positive and negative controls included in the PCR assays.
Statistical analysis
The collected data underwent meticulous filtering and refinement within Microsoft Excel 2016 before statistical analyses were conducted using SPSS 22.0 software (IBM, USA). Descriptive statistics were employed to present the data, with continuous variables expressed as mean ± standard deviation and categorical variables as frequency and percentage. Significance testing between groups was performed utilizing the Chi-square test, with p-values denoted by asterisks (* for p < 0.05 and ** for p < 0.01) to indicate statistical significance. Additionally, graphical representations were meticulously crafted using GraphPad Prism 9.0.0 software.
Ethics statement
All participants willingly opted to undergo an HPV test and actively participated in this research. Before their involvement, participants or their guardians were provided with comprehensive information about the study. The studies involving human participants were reviewed and approved by the Ethics Committees of The Affiliated Hospital of Hangzhou Normal University.
Results
Baseline profile of study participants
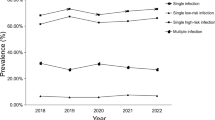
This study examined 20 HPV genotypes (14 h-HPV and 6 LR-HPV) among a cohort of 299,089 patient cases in the Hangzhou region spanning from 2017 to 2023. Among these cases, 283,556 (94.81%) were females, with an average age of 40.2 years, while 15,533 (5.19%) were males, with an average age of 31.9 years (Table 1). As indicated in Table 1, the total HPV infection rate stood at 23.80% (female: 22.85%, male: 41.13%). Within 71,187 positive cases, 16.6% (female: 16.19%, male: 24.20%) were caused by a single HPV type, especially one high-risk (HR) type (total: 11.83%, female: 12.00%, male: 8.37%). Consequently, single infection emerged as the predominant pattern of HPV infection in both female and male patients. Regarding the 7.20% (female: 6.31%, male: 16.93%) of multiple infections, 4.84% (female: 4.57%, male: 9.68%) comprised double infections, 1.51% (female: 1.37%, male: 3.99%) were triple infections, and 0.85% (female: 0.72%, male: 3.26%) were quadruple infections or more (Fig. 1A-B and Table 1). This distribution pattern was observed in both male and female groups and was consistent with previous reports from Guangzhou regions in China9. Additionally, our analysis of pairwise combinations among the 20 HPV genotypes in multiple HPV infections (resulting in 190 combinations) revealed that the top 10 combinations were all co-infected with HR52, and the most common combinations were with HR58 (1091 times), HR16 (1076 times), HR51 (936 times), LR81 (926 times), LR53 (820 times), and LR42 (779 times) (Figure S1).
Prevalence of HPV infection in different age groups
Subsequently, we delved into a thorough investigation of HPV infections, both single and multiple, across different age groups. Our result revealed a marked bimodal pattern (Fig. 2A). The first peak occurs in the group under the age of 20 (50.74% for overall prevalence, 21.13% for single positivity, and 29.6% for multiplicity rate). With increasing age, there is a brief decline in HPV infection rates, reaching a nadir in the 41–45 age group. Subsequently, rates rise again, culminating in a second peak in the 61–65 age group (28.01% for overall prevalence, 19.00% for single positivity, and 9.01% for multiplicity rate). Additionally, we observed similar trends in HPV (only HR/only LR/HR + LR) infection across different age groups from both female and male groups (Table S1). It’s worth noting a slight anomaly in the male group, where there was an unusual fluctuation observed in the > 66 age groups (Table S1).
(A) prevalence rate of HPV infection grouped by age (“positive” includes one or more HPV genotype infections; “single positive” indicates the rate of infection with a single HPV genotype; “multi positive” represents the rate of infection with two or more HPV genotypes). (B) heatmap illustrates the correlation between different age groups and the infection rates of various HPV genotypes. (C) Bar charts depict the infection rates among different gender groups stratified by high-risk or low-risk HPV genotypes (“Only LR” signifies exclusive low-risk HPV infection, “Only HR” denotes exclusive high-risk HPV infection, and “LR + HR” represents a co-infection of high-risk and low-risk HPV genotypes), Using chi-square test to assess the significance of gender differences in various infection rates (only LR, only HR, HR + LR)( p-value < 0.05 indicates statistical significance) (D) Sorting the infection rates of high-risk or low-risk HPV based on the overall sample, showcasing gender disparities in the prevalence of different HPV genotypes.
Furthermore, we employed heatmap analysis to visualize the contribution rates of different HPV genotypes across various age groups. Our findings revealed that HPV52 was the predominant genotype across all age groups, followed by HR58 and HR16. Particularly noteworthy, LR53 and LR81 exhibited significance in the age group of 41–65(Fig. 2B).
Prevalence of HPV infection in various gender groups
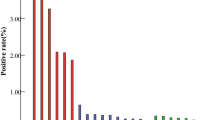
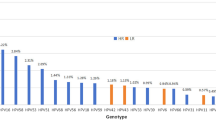
We next investigated the HPV prevalence characteristics among males and females. Significant disparities exist in the prevalence of various HPV risk types (only HR/only LR/HR + LR infections) between genders (p < 0.001) (Fig. 2C). Intriguingly, a further examination of the gender-based discrepancies in the 20 HPV genotypes revealed that only HR31 (p < 0.712), HR39 (p < 0.256), and HR52 (p < 0.118) exhibited no significant differences (Table 2). Among females, HR52 (5.58%), HR58 (2.84%), HR16 (2.64%), HR51 (2.19%), and LR53 (2.18%) emerge as the top five prevalent genotypes, aligning with numerous prior studies9,10,11. Conversely, among males, LR6 (13.98%), LR11 (9.66%), HR52 (5.28%), HR16 (5.08%), and LR42 (3.62%) stand out as the top five prevalent genotypes (Fig. 2D).
Prevalence features of HPV before, during, and after the COVID-19 pandemic
During the seven-year period from 2017 to 2023, the overall average HPV infection rate was 24.07% ± 1.52, with high-risk (HR) HPV alone averaging 14.78% ± 0.69 and low-risk (LR) HPV alone averaging 5.27% ± 0.35. Co-infection rates with both HR and LR HPV averaged 4.02% ± 0.69. As illustrated in Fig. 3A, there was a slight decline in HPV infection rates observed from 2020 to 2023 compared to the initial three years of the study, with a potential uptick noted in 2023 (Fig. 3A). Notably, HPV infection rates exhibited a seasonal trend, declining from spring to autumn and experiencing a resurgence from autumn to winter. However, the chi-square test revealed no significant difference between summer and winter or between autumn and winter, highlighting the absence of significant disparities in these comparisons, unlike the others (Fig. 3B). These findings contrast with a previous study conducted within the same province12.
(A) The prevalence of HPV (only HR、only LR、HR + LR) infections among total samples from 2017 to 2023. (B) presenting the changes in the overall HPV infection rate from 2017 to 2023 based on seasonal classifications in China (spring represents January to March, summer represents April to June, autumn represents July to September, and winter represents October to December). (C) the distribution of HPV across different age groups before, during, and after the COVID-19 pandemic (pre-COVID-19 refers to January 1, 2017, to December 31, 2019; mid-COVID-19 refers to January 1, 2020, to December 31, 2022; post-COVID-19 refers to January 1, 2023, to December 31, 2023). (D) scatter plot of the distribution of 20 HPV genotypes before, during, and after the COVID-19 pandemic. The Chi-square test indicates the significance levels between each pair. (E) displaying the changes in HPV infection rates before, during, and after the COVID-19 pandemic based on the sorted infection rates of different genotypes in the overall sample.
The COVID-19 pandemic broke out at the end of 2019, prompting China to swiftly conduct scientific assessments and implement effective epidemic prevention policies. These measures effectively curbed the spread of the novel coronavirus and reduced mortality rates, achieving remarkable results. However, there is limited research on the prevalence of HPV before, during, and after the COVID-19 pandemic. To address this gap, we categorized data from 2017 to 2019 as the pre-COVID-19 period, data from 2020 to 2022 as the mid-COVID-19 period, and data from 2023 as the post-COVID-19 period. We found that during the COVID-19 period, the overall HPV infection rate decreased by approximately 3%, with declines observed in both single and multiple infections (Table S2). This trend was also evident across different age groups (Fig. 3C and Figure S2). Further analysis revealed a unique phenomenon of fluctuating HPV infection rates in the “Only LR” group, particularly pronounced in males (Figure S2 and Table S2-3). The results indicated that compared to the pre-COVID-19 period, some genotypes experienced significant suppression, while others showed no significant suppression, and a few exhibited slight increases (Fig. 3D-E).
To accurately assess the changes in each HPV genotype in males and females before and after the COVID-19 pandemic, we further delineate all types of changes. In females, HR66 showed a pattern of initial increase followed by decline (UD), while HR59 and LR42 displayed a consistent upward trend (UU). On the other hand, HR45, LR81, and LR53 exhibited a pattern of initial decline followed by an increase (DU), with the latter two showing more pronounced changes (Fig. 4A and Table S4). In contrast, for the male individuals, five genotypes exhibited a pattern of initial decline followed by an increase (Fig. 4B and Table S4).
(A-B) The left figure displays four types of changes in the infection rates of 20 HPV genotypes (“DD” indicates continuous decrease, “DU” indicates decrease followed by increase, “UU” indicates continuous increase, “UD” indicates increase followed by decrease). The right figure illustrates the trend of HPV genotype infection changes for the latter three types of variations. (A)Female; (B)Male.
Discussion
HPV infection can lead to a multitude of serious disorders, including cervical cancer, anal cancer, penile cancer, and genital warts, posing a significant threat to human health and well-being. While the prevalence and genotype distribution of HPV infection are known to vary significantly among different regions and populations. Analyzing the prevalence of type-specific HPV and its distribution within specific areas is of great significance for developing effective prevention and control strategies. Our study presents a detailed analysis of HPV genotypes in a large cohort of 299,089 patient cases from the Hangzhou region between 2017 and 2023. The overall prevalence of HPV infection in our study was similar to previously reported from Chengdu (22.51%)13, Guangdong (21.06%)14, Taizhou area (22.8%)15, higher than in Shanxi (8.92%)16, Beijing (8.22%)17, Guizhou (10.33%)18, Shanghai (18.81%)19, Guangxi (18.1%)20, Chongqing (18.59%)18, but lower than in Jilin (34.4%)21, Shandong (28.4%)22 and Jiangsu (26.92%)23.
HPV is primarily transmitted through sexual intercourse. An understanding of the prevalence of HPV among men is critical to preventing male genital cancers and subsequent HPV infections in women, thereby reducing the incidence of cervical cancer. However, numerous previous studies have mainly concentrated on HPV infection among the extensive female population, merely a restricted number in males. In recent investigations, it has been astonishingly revealed that the positive rate of HPV among men is strikingly high. The prevalence of male genital HPV infection in a multitude of countries across the globe amounts to 31%2. The positive rate of HPV among men in Henan, China, is approximately 30%24. In our study, the positive rate of HPV within the male subgroup reaches 41.13%, which is comparable to the 42% positive rate of HPV in men detected in the sexually transmitted disease clinics from Shandong25. The role of men in the transmission chain of HPV cannot be overlooked. Their unawareness of potential infections and the lack of routine testing and vaccination measures pose significant risks not only to their health but also to the overall control and prevention of HPV-related diseases. Comprehensive detection and vaccination strategies for men are crucial steps in breaking the cycle of HPV transmission and reducing the burden of HPV-related disorders in the population.
Our study shows that the most common HPV type in women is HPV52 (5.58%), followed by HPV58 (2.84%) and HPV16 (2.64%). The results are consistent in all age groups. This is consistent with the results we previously observed in Hangzhou26. In the northern Guangdong region and Yunnan Province of China, HPV52 is also the most common genotype27,28. We also found significant gender differences in HPV infection. In men, LR6 (13.98%) and LR11 (9.66%) are the most common HPV types, but for high-risk HPV, the most common is HPV52 (5.28%), followed by HPV16 (5.08%). Epidemiological data from multiple regions show that HPV16 is the most common genotype in females and males2,29,30,31. The discrepancy observed in our study may be attributed to regional variations or the impact of HPV vaccination programs, which have begun to reduce the prevalence of vaccine-targeted genotypes, such as HPV16 and HPV18. Three HPV vaccine formulations on the market can effectively control HPV16 infection: bivalent (HPV types 16 and 18), quadrivalent (HPV types 6, 11, 16, and 18), and nine-valent (HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58). Based on the low incidence of HPV16/18 observed in our study, it is unlikely that the bivalent or quadrivalent vaccines alone would be sufficient to control HPV infections in the regions we studied. Notably, we observed that HPV16 is the second most common high-risk infection among men. One of the possible explanations is differences in transmission routes in males. Recent studies demonstrate a growing population of men who have sex with men (MSM) in China, where HPV16 prevalence is significantly higher within the MSM population32,33. These findings provide valuable insights for guiding the selection of appropriate vaccines and underscore the importance of tailoring vaccination strategies to the specific epidemiological context.
We analyzed the changes in the prevalence of HPV before and after the COVID-19 pandemic. We found that during the COVID-19 pandemic, the overall HPV infection rate decreased by 3%. This might be attributed to the strict epidemic control measures during the pandemic. These measures also controlled other seasonal respiratory virus infections and sexually transmitted infections34,35,36. Additionally, during the COVID-19 pandemic, the medical system was overwhelmed, and cervical cancer screenings and HPV tests were postponed or canceled, which might have led to delays in the detection and management of HPV infections and related diseases. However, as the pandemic subsided and social activities gradually resumed, along with the reduced vaccination rate during this period, the HPV infection rate might surge37. But, our results show that there was no significant difference in the HPV infection rate during and after the COVID-19 pandemic, which might be because our post-pandemic data needs to be further collected. After the COVID-19 pandemic, timely attention should be paid to strengthening HPV screening and vaccination to mitigate the potential surge in HPV transmission.
Our study has several limitations. First, we did not examine the relationship between the cytological status of cervical samples and HPV genotypes due to the lack of relevant data. Second, we were unable to assess the HPV vaccination rate in Hangzhou and its correlation with the prevalence of HPV genotypes. Third, while we analyzed the prevalence and distribution of HPV genotypes, we did not evaluate the HPV clearance rate within our study cohort. Since clearance rates can influence the distribution and infection rates of HPV genotypes. Lastly, as highlighted in previous studies, the interpretation of p-values in very large sample sizes requires caution, as they tend to approach zero rapidly, potentially limiting the insights gained from statistical analysis38,39. Addressing these limitations will be a key focus for future research.
Conclusion
In conclusion, our comprehensive analysis of the HPV infection rate and genotype distribution among the two genders in the large-scale study has obtained several results worthy of attention. We observed a relatively high HPV infection rate, accounting for 23.8% of the entire study population, especially with the HPV infection rate reaching as high as 41.13% among men.
There were significant differences in HPV infections between male and female populations, but HR52 and HR16 were common high-risk genotypes in both types. The age-related HPV prevalence trend presented a bimodal pattern, and the impact of the COVID-19 epidemic on the prevalence of HPV was relatively obvious. Our study has provided valuable insights for our understanding of HPV epidemiology and emphasized the importance of conducting epidemiological studies in the male population to monitor the prevalence trend among men. Additionally, it highlights the need to consider implementing vaccination programs for males.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Kombe Kombe, A. J. et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front. Public. Health. 8, 552028 (2021).
Bruni, L. et al. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob Health. 11, e1345–e1362 (2023).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249 (2021).
Farooq, Q. et al. Inferring Virus-Host relationship between HPV and its host Homo sapiens using protein interaction network. Sci. Rep. 10, 8719 (2020).
Zheng, L. et al. Human papillomavirus prevalence and genotype distribution in Liaocheng men between 2016 and 2022. J. Med. Virol. 96, e29360 (2024).
Olesen, T. B. et al. Prevalence of human papillomavirus DNA and p16INK4a in penile cancer and penile intraepithelial neoplasia: A systematic review and meta-analysis. Lancet Oncol. 20, 145–158 (2019).
Chen, J. et al. Estimated human papillomavirus vaccine coverage among females 9–45 years of Age — China, 2017–2022. China CDC Wkly. 6, 413–417 (2024).
Yin, J. et al. Head-to-head comparison of 7 high-sensitive human papillomavirus nucleic acid detection technologies with the SPF10 LiPA-25 system. J. Natl. Cancer Cent. 2, 148–154 (2022).
Yang, X. et al. Cervical HPV infection in Guangzhou, China: An epidemiological study of 198,111 women from 2015 to 2021. Emerg. Microbes Infect. 12, e2176009 (2023).
Yin, X. et al. HPV prevalence and distribution characteristics in postmenopausal women from Nanjing, China. BMC Womens Health. 24, 68 (2024).
Zhang, J., Zha, T., Wang, X. & He, W. Prevalence and genotype distribution of HPV infections among women in Chengdu,China. Virol. J. 21, 52 (2024).
Yan, X. et al. Prevalence, characteristics, and distribution of HPV genotypes in women from Zhejiang Province, 2016–2020. Virol. J. 18, 208 (2021).
Wang, Q. et al. Prevalence characteristics of cervical human papillomavirus infection in Chengdu and Aba District, Sichuan Province, China. PLoS One. 19, e0304760 (2024).
Luo, G. et al. Cervical human papillomavirus among women in Guangdong, China 2008–2017: Implication for screening and vaccination. J. Med. Virol. 91, 1856–1865 (2019).
Xu, H. H. et al. Prevalence characteristics of cervical human papillomavirus (HPV) genotypes in the Taizhou area, China: A cross-sectional study of 37 967 women from the general population. BMJ Open. 7, e014135 (2017).
Yang, J. et al. Prevalence, genotype distribution and risk factors of cervical HPV infection in Yangqu, China: A population-based survey of 10086 women. Hum. Vaccines Immunother. 16, 1645–1652 (2020).
Yang, H. et al. Factors affecting HPV infection in U.S. And Beijing females: A modeling study. Front. Public. Health. 10, 1052210 (2022).
Chen, L. et al. The genomic distribution map of human papillomavirus in Western China. Epidemiol. Infect. 149, e135 (2021).
Li, X. et al. Prevalence of cervicovaginal human papillomavirus infection and genotype distribution in Shanghai, China. Virology Journal. 19, 146 (2022).
Wei, L., Ma, L., Qin, L. & Huang, Z. The prevalence and genotype distribution of human papillomavirus among women in Guangxi, Southern China. Infect. Agent Cancer. 17, 19 (2022).
Hao, S., Wang, C., Liu, S., He, J. & Jiang, Y. HPV genotypic spectrum in Jilin Province, China, where non-vaccine-covered HPV53 and 51 are prevalent, exhibits a bimodal age-specific pattern. PloS One. 15, e0230640 (2020).
Jiang, L. et al. HPV prevalence and genotype distribution among women in Shandong Province, China: Analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PloS One. 14, e0210311 (2019).
Zhang, C., Cheng, W., Liu, Q., Guan, Q. & Zhang, Q. Distribution of human papillomavirus infection: a population-based study of cervical samples from Jiangsu Province. Virology Journal. 16, 67 (2019).
Wang, H. et al. The prevalence and genotype distribution of human papillomaviruses among men in Henan Province of China. Front. Med. 8, 676401 (2021).
Ma, D-M. et al. Distribution of high-risk human papillomavirus genotypes in male attendees at a clinic for sexually transmitted infections in Northern China. Eur. Rev. Med. Pharmacol. Sci. 23, 9714–9720 (2019).
Wang, J. et al. Prevalence and genotype distribution of HPV infection from Hangzhou of Zhejiang Province pre- and during COVID-19 pandemic. Front. Public. Health. 12, 1357311 (2024).
Huang, W. et al. The prevalence of human papillomavirus among women in Northern Guangdong Province of China. Sci. Rep. 12, 13353 (2022).
Li, Z. et al. Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, southwest China. Sci. Rep. 6, 21039 (2016).
de Sanjosé, S. et al. Burden of human papillomavirus (HPV)-Related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2, pky045 (2019).
Dai, M. et al. Human papillomavirus infection in Shanxi Province, People’s Republic of China: A population-based study. Br. J. Cancer. 95, 96–101 (2006).
Lin, X. et al. Age-specific prevalence and genotype distribution of human papillomavirus in women from Northwest China. Cancer Med. 11, 4366–4373 (2022).
Li, Y. et al. Prevalence of human papillomavirus and genotype distribution in Chinese men: A systematic review and Meta-Analysis. Cancer Med. 14, e70686 (2025).
Zhang, D-Y. et al. HPV infections among MSM in Shenzhen, China. PloS One. 9, e96364 (2014).
Batiha, O., Al-Deeb, T., Al-Zoubi, E. & Alsharu, E. Impact of COVID-19 and other viruses on reproductive health. Andrologia 52, e13791 (2020).
Ogunbodede, O. T., Zablotska-Manos, I. & Lewis, D. A. Potential and demonstrated impacts of the COVID-19 pandemic on sexually transmissible infections. Curr. Opin. Infect. Dis. 34, 56–61 (2021).
Tao, J. et al. Impact of the COVID-19 pandemic on sexually transmitted infection clinic visits. Sex. Transm Dis. 48, e5–7 (2021).
Liu, H., Yao, Q., Li, D., Zhao, Z. & Li, Y. Impact of COVID-19 outbreak on the gynecological outpatients HPV infection rate in Wuhan, China: A retrospective observational study. Front. Med. 9, 799736 (2022).
Gómez-de-Mariscal, E. et al. Use of the p-values as a size-dependent function to address practical differences when analyzing large datasets. Sci. Rep. 11, 20942 (2021).
Lin, M., Lucas, H. C. & Shmueli, G. Research Commentary—Too big to fail: Large samples and the p-Value problem. Inf. Syst. Res. 24, 906–917 (2013).
Acknowledgements
We thank Weijuan Wang for secretarial assistance.
Funding
This work is supported by the Hangzhou Youth Innovation Team Project (TD2023020).
Author information
Authors and Affiliations
Contributions
Q.K., K.Z., and Y.M. performed the data analysis, Q.K., Y.J., and X.P. collected samples from the clinic, and L.Z. and X.S. supervised data analysis and wrote the paper. All the authors read and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the data has been obtained from the Ethics Committee in the Affiliated Hospital of Hangzhou Normal University, Hangzhou Xixi Hospital, and the Second Affiliated Hospital of Zhejiang University School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Informed written consent was obtained from each participant, who was ensured that data would remain confidential and used for research purposes only. All methods were carried out following the Chinese Statistical Law to ensure that participants’ personal information was kept confidential. All experimental protocols were approved by the institutional review board of the Affiliated Hospital of Hangzhou Normal University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ke, Q., Zhao, K., Jin, Y. et al. Multicenter study on the distribution and prevalence of human papillomavirus types in Hangzhou, Zhejiang from 2017 to 2023. Sci Rep 15, 9374 (2025). https://doi.org/10.1038/s41598-025-92102-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92102-9
Keywords
This article is cited by
-
Age distribution of high-risk HPV infection and cervical lesions in an unvaccinated adult Brazilian population within an organized screening program
Scientific Reports (2026)
-
COVID-19 pandemic disruptions drive decreases in HPV prevalence and shift genotype distribution in Western China
BMC Infectious Diseases (2025)
-
Trends in the molecular epidemiology of human papillomavirus in males from the plateau region of Southwest China: an 11-year retrospective analysis (2014–2024)
Virology Journal (2025)