Abstract
Hematopoietic stem cell transplantation (HSCT) emerged over sixty years ago as a groundbreaking and potentially curative treatment for patients with acute myeloid leukemia (AML) who were not responding to chemotherapy. In this study, we aimed to investigate prognostic factors for survival after allo-HSCT in AML patients. This retrospective cohort study was carried out using data from 742 adult AML patients underwent allo-HSCT. we analysis prognostic factors for survival after allo-HSCT with censored quantile regression model. The 5-year OS, DFS and GRFS rates were 58, 53, and 30%, respectively. OS for recipients older than 35 years was 0.95 and 1.12 years lower than that for recipients under 35 years in the 25th and 40th percentiles, respectively. Compared to patients in their CRІ, those with CRІІІ disease experienced a decrease in OS at the 25th and 40th percentiles by 1.72 and 3.72 years, respectively. Moreover, OS for ABO matched patients was 0.92 and 1.29 years longer than that of patients with an ABO major mismatch. This study could assist oncologists and hematologists in understanding the prognostic factors affecting patient survival across various survival ranges, thereby potentially extending patients’ lifespans.
Similar content being viewed by others
Introduction
Allogenic hematopoietic stem cell transplantation (allo-HSCT) emerged over sixty years ago as a groundbreaking and potentially curative treatment for patients with acute myeloid leukemia1 who were not responding to chemotherapy2,3. Further studies have established allo-HSCT as the preferred treatment approach for adults with AML in their first complete remission (CRІ), due to its effectiveness in lowering the risk of disease relapse by more than 60% compared to intensive chemotherapy alone4.
The utilization of novel medications and the availability of enhanced supportive care led to improvements in the outcomes of allo-HSCT5. Many analyses have shown that in the entire AML patient population, allo-HSCT is the most successful post remission management strategy to prevent relapse6.
Although AML recurrence occurred in approximately 50% of HSCT users in the registry context and indicated poor outcomes, allo-HSCT was associated with better overall survival (OS). Other therapies have not been able to equal HSCT’s record of disease-free survival (DFS) of 50% or greater7.
Numerous variables might influence the outcomes of HSCT. In addition to patient characteristics, donor factors may also influence transplant outcomes. Numerous studies have demonstrated that several factors, including age, sex, blood type ABO matching, donor and recipient sex, primary outcomes, achieving complete remission (disease status), transplant type, and occurrence of graft-versus-host disease (GVHD), can affect a patient’s prognosis following HSCT8,9,10,11,12,13,14,15.
The primary aim of the current study was to identify prognostic factors for OS, DFS, and GVHD-free relapse-free survival (GRFS) in AML patients undergoing allo-HSCT.
Methods
This retrospective cohort study was conducted by reviewing secondary data from 835 adult AML patients who underwent allo-HSCT. The information, categorized according to the WHO classification, was collected and registered by the Hematology-Oncology & Stem Cell Research Center (affiliated with Tehran University of Medical Sciences, Tehran, Iran). The participants included in this study were referred to the hematology clinic for allo-HSCT between 2008 and 2019, and their follow-up continued until 2021.
All AML patients in complete remission (CR) undergo transplantation, while those not in CR do not receive a transplant according to the center’s protocol. In most cases, patients received a myeloablative conditioning regimen consisting of two drugs, Busulfan and Cyclophosphamide (BuCy). In a few instances, however, a fludarabine-based regimen was administered. Eighty-three patients were excluded solely due to missing data, and ten patients with acute promyelocytic leukemia (APL) were also excluded. All patients included in the study were receiving their first transplant.
The separate events of interest in the study were the duration (years) from allo-HSCT to death (AML and transplant-related deaths) (OS), disease relapse or death (DFS), death or relapse or occurrence of cGVHD (GRFS).
The current study utilized various factors to assess time-to-event outcomes among patients, including recipient age at diagnosis (years), sex matching (whether the donor and recipient were of the same sex), time from diagnosis to transplant, and the disease status at the time of transplantation. Specifically, patients with favorable-risk disease were not considered for HCT in first complete remission (CRІ), while those in second complete remission (CRІІ) or third complete remission (CRІІІ) underwent allogeneic HSCT.
Additionally, donor type (categorized as Sibling, Other-related, and Unrelated) was analyzed. It is important to note that all cases classified as unrelated and other-related donor types were fully matched with their respective recipients based on HLA matching.
We also examined ABO matching (matched, minor mismatch, and major mismatch). Due to the limited number of bidirectional cases (37 patients) and their structural similarity to the major mismatch group, these cases were combined with the major mismatch category for statistical analysis. The definition of ABO mismatch is provided in Table 1.
The main event was death from AML patients, and all other deaths were regarded as right censored observations. Qualitative variables in more than two groups were defined as indicator variables.
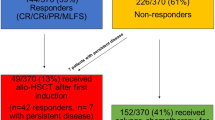
To ensure clarity and transparency, a flow diagram summarizing the study’s methodology has been provided (Fig. 1). The diagram details the steps from initial participant identification through the application of inclusion and exclusion criteria, culminating in the final cohort analyzed.
The median follow-up times (years) for OS, DFS, and GRFS in all patients were 2.7 (from 5 days to 13.2 years), 2.4 (from 5 days to 13.2 years), and 1.1 (from 5 days to 13.2 years), respectively. Additionally, in the case of censored patients during the study, the median follow-up time (years) for the three mentioned responses was 4.4 (from 21 days to -13.2 years), 4.5 (from 21 days to -13.2 years), and 4.1 (from 21 days to -13.2 years), respectively.
Statistical analysis
The mean (± standard error) and frequency (percentage) were used to indicate descriptive characteristics of the patients, including continuous and categorical variables, respectively.
Statistical methods for the survival data
In survival studies, time-to-event data pose unique challenges due to the presence of censored observations and skewed distributions with heavy tails.
Clinicians and medical researchers often seek to evaluate the impact of multiple risk factors on survival time in a multivariate framework. However, traditional methods like Kaplan-Meier are limited in their ability to address such complex relationships. Traditional survival models, such as the Cox proportional hazards model and the accelerated failure time (AFT) model, are widely used to analyze prognostic risk factors, but they have inherent limitations. The Cox proportional hazards model assumes proportional hazards, meaning the relative risk between groups remains constant over time. If this assumption is violated, it can lead to incorrect interpretations of the effects of covariates. On the other hand, the AFT model provides a direct interpretation of explanatory variables but assumes that covariate effects are uniform across the entire survival distribution. This assumption may not hold in many real-world scenarios, particularly when the effects of risk factors vary over time16,17.
Censored quantile regression (CQR) offers a robust alternative by estimating covariate effects across various quantiles of the survival distribution. Unlike traditional regression methods, CQR does not rely on strict assumptions such as proportional hazards, normality of error terms, or homoscedasticity (constant error variance). This flexibility makes CQR an effective and powerful tool for analyzing survival data with skewed distributions, censored observations, or heterogeneous covariate effects. By focusing on specific quantiles, CQR enables researchers to examine how prognostic factors influence short-term, medium-term, and long-term survival outcomes, offering a comprehensive and nuanced understanding of survival dynamics16,18.
Clinicians and medical researchers use a multivariate CQR model to evaluate the risk of events of interest over time. A model of the quantile function of time to event is accommodated by the CQR model to measure quantiles to indicate the level of the survival phase19,20.
In this study, the Laplace quantile regression model was used for survival data. They assumed that the error term follows an asymmetric Laplace distribution and considered the Laplace regression model as a method for modeling the conditional quantiles of survival time21,22.
Laplace regression model
Bottai and Zhang (2010), to estimate\(\:{\upbeta\:}\left(\text{p}\right)\), considered a regression model where the error term is assumed to follow an asymmetric Laplace distribution. They explored its use in the estimation of conditional quantiles of a continuous outcome variable given a set of covariates in the presence of random censoring.
They supposed that there exists a fixed r-dimensional parameter vector \(\:{\upbeta\:}\left(\text{p}\right)\) such that
where \(\:{{\upepsilon\:}}_{\text{i}}\) is an independent and identically distributed residual whose \(\:\text{p}\)th quantile equals zero (\(\:\text{P}\left({{\upepsilon\:}}_{\text{i}}\le\:0|{\text{x}}_{\text{i}}\right)=\text{p}).\)
Let \(\:{\text{T}}_{\text{i}}\) conditional on \(\:{\text{X}}_{\text{i}}\), follow a form of asymmetric Laplace distribution with a probability density function
In the presence of censored observations, the likelihood function is proportional to
The maximum likelihood estimators for the parameters are defined as the maximum of \(\:\text{l}\left({\text{T}}_{\text{i}}|{\upbeta\:}\left(\text{p}\right),{\upsigma\:}\left(\text{p}\right)\right)\). They used the algorithm proposed by Nelder and Mead (1965)23 to estimate parameters and inference on the parameters obtained by bootstrapping the point estimates for quantiles of interest21.
In all cases, analyses were performed using STATA version 14. A P value < 0.05 was considered significant in all statistical analyses.
Results
The mean (± standard deviation) age of the 742 participating patients was 36.10 (± 11.93) years. The median (confidence interval) DFS and GRFS times were 7.1 (4.9–10) and 1.4 (1.1–1.8) years, respectively.
During the follow-up period, 286 (38%) patients died, 326 (43%) patients died or relapsed (DFS), and 517 (70%) patients died or relapsed or experienced cGVHD (GRFS).
Table 2 gives the descriptive statistics of all the prognostic factors used in this study. Table 3 shows the OS, DFS, and GRFS rates after one, two, five, and ten years.
The causes of death of the patients under the study after HSCT were investigated during a follow-up period. Disease recurrence (46%), infection (22%), and the occurrence of cGVHD (20%) were the most common causes of death. The remaining causes of death included 12% of deaths.
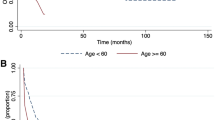
As shown in the Kaplan‒Meier plot in Fig. 2, considering that the OS, DFS, and GRFS rate of patients during follow-up is 0.55, 0.48 and 0.20, respectively, the maximum percentiles of OS, DFS, and GRFS that can be modeled were 45, 52, and 80, respectively. Thus, based on the percentage of patients who experienced the event, we took into account the 25th and 40th percentiles for OS, the 25th, 40th, and 50th percentiles for DFS, and the 25th, 40th, 50th, and 70th percentiles for GRFS based on the observed survival constraints.
Considering the results of the quantile regression models reported in Table 4 in the 25th and 40th percentiles, the times when 25 and 40% of patients have experienced death, recipient age, ABO matches, disease status, relapse and aGVHD occurrence were identified as significant prognostic factors affecting OS.
In Table 4, the effects of recipients aged over 35 years compared with those aged under 35 years on the 25th and 40th percentiles of OS were − 0.95 and − 1.12 years, respectively (P-value < 0.05). In the ABO match effect check, the effect of major mismatch compared with the patients with matched ABO were − 0.92 and − 1.29 years; also, the effect of CRIII compared to CRІ were − 1.38 and − 1.64 years and finally, patients who had relapses after HSCT have a shorter survival of 2.25 and 4.21 years, respectively. The effect of aGVHD was 1.30 years, on the 40th percentile of survival times (P-values < 0.05).
The plots of the estimated CQR coefficients of OS and their 95% confidence intervals and conditional quantile effects estimated by Cox model for \(\:\text{p}\)∈ (0.10, 0.20, 0.30, 0.40) are displayed in appendix 1. The Fig. 3 (for significant variables) and Fig. 4 (for not significant variables) show a clear upward or downward trend in the regression coefficients over time. As follow-up periods increased and the number of patients who experienced death increased, survival times for those over 35 years of age decreased compared to those under 35 years of age. However, survival times for CRІ patients improved compared to CRIII patients.
Based on Table 5, the times when 25, 40 and 50% of patients have experienced death or relapse, disease status, ABO match, and aGVHD occurrence were identified as significant factors affecting the DFS time. Additionally, recipient age and donor type in the 50th significantly affected the DFS time.
According to Table 5, the effect of recipients aged over 35 years compared with those under 35 years on the 50th percentile of DFS was − 1.26 years (P-value < 0.05). In the ABO match, the major mismatch compared with the patients with matched ABO on the 25th and 40th percentile of DFS were − 0.63 and − 1.92 years, respectively. In the examination of the disease status, the effect of CRП compared with CRІ was − 1.03 and − 1.86 years, the effect of CRIII compared with CRІ was − 1.49 and − 3.54 years, and finally, the effect of aGVHD was 1.17 and 1.87 years, respectively, on the mentioned percentile of survival times (P-value < 0.05). At the 50th percentile of DFS, survival in sibling donors is expected to be 2.06 years longer (P-value < 0.05).
The plots of the estimated CQR coefficients of DFS and conditional quantile effects estimated by Cox model for \(\:\text{p}\) ∈ (0.10, 0.20, 0.30 0.40, 0.50) are displayed in appendix 1. The Fig. 5 (significant variables) and Fig. 6 (not significant variables) show a clear upward or downward trend in the regression coefficients over time for DFS. The DFS of recipients aged over 35 years compared to those aged under 35 and those with major ABO mismatch compared to those with ABO matching decreased, and the DFS of patients with aGVHD occurrence compared to those without aGVHD and CRІ disease compared to those with CRIII disease increased over time.
According to Table 6, significant factors affecting GRFS in the 40th, 50th and 70th percentiles, the times when 25, 40,50 and 70% of patients have experienced death or relapse or occurrence of cGVHD, include recipient age, sex matching and disease status.
As seen in Table 6, the effect of recipient age over 35 years compared with under 35 years on the 50th and 70th percentiles of GRFS were − 0.59 and − 1.23 years, respectively (P-value < 0.05). Investigating the state of the disease, the effects of CRП compared with CRІ on the mentioned percentiles of GRFS were − 0.62 and − 1.91 years, respectively (P-value < 0.05). The GRFS of female recipients and male donors, at the 40th, 50th and 70th percentiles, compared to male recipients and donors were − 0.42, -0.25 and − 1.57 years, respectively (P value < 0.05).
The plots of the estimated CQR coefficients of GRFS and conditional quantile effects estimated by Cox model for \(\:\text{p}\) ∈ (0.10, 0.20, … 0.80) and are displayed in appendix 1. The Fig. 7 (significant variables) and Fig. 8 (not significant variables) show a clear upward or downward trend in the regression coefficients over time for GRFS. The GRFS of recipients aged over 35 years compared to those under 35 years, female recipients and male donors compared to male recipients and donors, and patients with CRП disease compared to those with CRІ disease decreased over time.
Additionally, the results of the Cox proportional hazards model, including the reported hazard ratios, are presented alongside the coefficient estimates for different quantiles in Tables 4, 5 and 6. This comparison provides a comprehensive view of the covariates’ effects across the Cox model and the quantile regression model. As can be seen, censored quantile regression examines the effects of prognostic factors on survival time at different quantiles of the survival distribution, rather than assuming a uniform hazard ratio for the entire population. The effects of the Cox model were almost the same in different quantiles while they changed in quantile regression models as the quantiles vary.
Discussion
The number of patients undergoing HSCT for acute myeloid leukemia1 is increasing.
HSCT is a suitable approach for patients suffering from hematologic disorders such as AML, but the outcome of this therapeutic procedure may be affected by various variables, including patient age, disease status, donor type, and diagnostic time to HSCT24.
In this study, we aimed to evaluate the impact of prognostic factors on patients’ survival with AML after allo-HSCT in Iran from 2008 to 2019, and quantile regression was chosen for assessments due to its benefits. This regression enables the determination of the number of patients at the end of the follow-up duration and all desired checkpoints. Additionally, to evaluate the covariate effects in survival analysis, this method can be used.
The 5-year OS was calculated to be 58% in our study, while other studies had similar rates after allo-HSCT (60%)25,26. Decreased transplant-related mortality, increased survival rates, and a larger number of patients who are suitable for transplants have been achieved as a result of advancements in supportive care, donor selection, and conditioning regimens27. Our results are consistent with other research showing considerable allo-HSCT advantages for DFS and OS15,28.
In this study, prognostic factors that are similar for OS, DFS and GRFS were identified and described as recipient age over 35 years, disease status, and occurrence of aGVHD. ABO major mismatch was a significant factor for OS and DFS but not for GRFS. Additionally, donor type was important for DFS and GRFS but not for OS. In addition, sex matching was important only in GRFS, and there was no relationship between OS and DFS with any type of sex matching.
However, diagnostic time to HSCT and minor ABO mismatch were not statistically significant prognostic factors for OS, DFS and GRFS.
Disease status was the first factor that revealed its important effect, showing a statistically significant difference between the OS of CRІ patients with CRIII patients in percentiles greater than the 25th percentile. At the 25th and 40th percentiles, CRIII patients are expected to live 1.38 and 1.64 years less than CRІ patients, respectively. The outcomes were similar for DFS and GRFS times. Similar studies have indicated that patients in the CRІ disease status have better OS and DFS, as well as our results29,30.
The effect of relapse after HSCT is significant for all percentile for OS. According to a related study, fewer patients who relapsed and survived than those who did not did so30. The survival rate of AML is greatly improved by allo-HSCT, but relapse is still one of the most important factors that influence survival of the AML patients.
The effect of recipient age was significant after the 25th percentile for OS, but for DFS and GRFS, the effects were significant only after the 50th and 70th percentiles, respectively. Patients aged less than 35 years are expected to live 0.72 and 1.26 years more than patients older than 35 years at the 25th and 40th percentiles, respectively. However, for DFS and GRFS time, this effect was seen only in the final percentiles. In the early days after allo-HSCT, age was not identified as a significant prognostic factor. This may be explained by the fact that older patients are subject to lower OS and DFS31,32,33,34,35 and that it took 7 years for our patients to reach the 40th percentile in DFS. In a study conducted in 2017, they showed that AML patients younger than 35 had a longer GRFS time36,37. In this regard, Middeke et al. reached similar results to our study, although age was not significantly affected by DFS time after HSCT38.
Donor types were significantly associated with GRFS and DFS time. For DFS time in the 50th percentile and GRFS time in the 70th percentile, matched sibling donors were expected to live 2.06 and years longer, respectively, then unrelated donors. In the study conducted by Schetelig (2008) on elderly patients with AML after allo-HMCT, donor type was not significantly associated with OS or DFS time, which may be due to the old age of the patients39. In similar study, the risk associated with the unrelated donor type was reported to be higher compared to that observed in the related donor types34,35.
The results from comparing male and female recipients from male donors showed that female recipients were expected to live 1.73 years less often than male recipients without aGVHD occurrence, relapse or death. in 70th percentiles. For OS and DFS time, a nonsignificant sex-matched relationship was found. Additionally, similar studies did not report a significant connection between the results and the gender of donor and recipients30.
In our study, a positive correlation was found between the occurrence of aGVHD and the OS and DFS of patients in different percentiles. As mentioned in the studies, aGVHD is one of the main problems of hematopoietic stem cell transplantation and of course one of the important factors affecting the outcome of transplantation40. In the study of Spring et al. (2015), the survival rate was higher in people who were not affected by aGVHD, similar to our results41. In similar studies, the comparison of survival has been performed mostly on the grades of aGVHD, which was statistically significant, but there was a nonsignificant relationship between the occurrence of aGVHD and survival30.
In the investigation of ABO blood group matching on the survival of AML patients, more survival was observed in ABO-matched patients than in major ABO-mismatched patients, and this difference increased over time. This relationship has become significant since 25% of patients have had an event. Patients who were ABO-matched were expected to live 0.98 and 1.89 years more than ABO major mismatch patients in the 25th and 0.40th percentiles, respectively. Ozkurt et al. (2009) showed that ABO matching resulted in better survival than ABO-Major mismatch. However, other studies37,42. in line with our research, did not find a significant relationship between ABO matching and the survival of AML patients. In most of these studies, the comparison of the survival between the two groups of ABO matching and nonmatching was carried out, and the differentiation of ABO nonmatching groups was ignored43.
The strength of this study is using a quantile regression model to analyze the data, as it could consider time-varying effects through the study, and it seems to be the proper choice for analyzing survival data. Additionally, using a large group of patients for this study reduced the possibility of random bias and irregular findings resulting from chance. A few limitations were applied while conducting this study. Assessing patients’ cytogenetic risks as well as having the initial and follow-up white blood cells (count) is crucial when evaluating the survival rate of AML patients after allo-HMCT. Future studies should evaluate these factors by using quantile regression analysis.
Additionally, the retrospective nature of the study introduced potential biases, such as incomplete or inconsistent data on follow-up care and patient adherence. Future studies should evaluate these factors more comprehensively by using quantile regression analysis, which would allow for a better understanding of the relationships between these variables and survival outcomes. Moreover, larger, multi-center studies with a more diverse patient population would help in generalizing the findings and minimizing selection bias.
In this study, 742 AML patients receiving allo-HCT were examined. After 5 years of follow-up, the OS, DFS, and GRFS rates were 58%, 53%, and 30%, respectively. Patients may live longer and have a higher quality of life in the case of receiving a transplant when their disease is still in its early status and they are still younger. This study can increase patient survival by helping oncologists and hematologists understand the prognostic factors of patient survival in different ranges of survival to increase patients’ lifetime.
Quantile regression offers a significant advantage by providing insights into relationships between variables beyond the mean, making it particularly valuable for analyzing outcomes with non-normal distributions and nonlinear associations with predictors. Through its application in this study, we were able to comprehensively identify the prognostic factors influencing OS, DFS, and GRFS across different points, offering a nuanced understanding of these outcomes throughout the follow-up period. Finally, conducting larger, multi-center studies with a more varied patient population would aid in generalizing the results and reducing selection bias.
Data availability
The data will be made available if requested by the researcher via email to the corresponding author of the article.
Abbreviations
- CQR:
-
Censored quantile regression
- AML:
-
Acute myeloid leukemia
- allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- GRFS:
-
GVHD-free relapse-free survival
- aGVHD:
-
Acute graft-versus-host disease
References
Koohi, F. et al. Leukemia in Iran: epidemiology and morphology trends. Asian Pac. J. Cancer Prev. 16 (17), 7759–7763 (2015).
Thomas, E. D., Lochte, H. L. Jr, Lu, W. C. & Ferrebee, J. W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 257 (11), 491–496 (1957).
Appelbaum, F. R. Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 357 (15), 1472 (2007).
Cornelissen, J. J. et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J. Clin. Oncol. 30 (17), 2140–2146 (2012).
Niederwieser, D. et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the worldwide network for blood and marrow transplantation group including the global survey. Bone Marrow Transplant. 51 (6), 778–785 (2016).
Döhner, H. et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood J. Am. Soc. Hematol. 115 (3), 453–474 (2010).
de Lima, M. et al. Proceedings from the National Cancer Institute’s second international workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol. Blood Marrow Transpl. 20 (1), 4–13. (2014).
Armand, P. et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood J. Am. Soc. Hematol. 120 (4), 905–913 (2012).
Kollman, C. et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood J. Am. Soc. Hematol. 127 (2), 260–267 (2016).
Lin, C-H. et al. Acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation: a retrospective study from a single institution. J. Int. Med. Res. 50 (2), 03000605221078466 (2022).
Takami, A. Hematopoietic stem cell transplantation for acute myeloid leukemia. Int. J. Hematol. 107 (5), 513–518 (2018).
Mehta, J. et al. Does donor–recipient ABO incompatibility protect against relapse after allogeneic bone marrow transplantation in first remission acute myeloid leukemia? Bone Marrow Transplant. 29 (10), 853–859 (2002).
Watz, E. et al. Analysis of donor and recipient ABO incompatibility and antibody-associated complications after allogeneic stem cell transplantation with reduced-intensity conditioning. Biol. Blood Marrow Transplant. 20 (2), 264–271 (2014).
Jang, J. E. et al. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann. Hematol. 94, 275–282 (2015).
Koreth, J. et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. Jama 301 (22), 2349–2361 (2009).
Klein, J. P. & Moeschberger, M. L. Survival Analysis: Techniques for Censored and Truncated Data (Springer, 2003).
Kalbfleisch, J. D. & Prentice, R. L. The Statistical Analysis of Failure time Data (Wiley, 2011).
Yazdani, A., Yaseri, M., Haghighat, S., Kaviani, A. & Zeraati, H. The comparison of censored quantile regression methods in prognosis factors of breast cancer survival. Sci. Rep. 11 (1), 18268 (2021).
Xue, X., Xie, X. & Strickler, H. D. A censored quantile regression approach for the analysis of time to event data. Stat. Methods Med. Res. 27 (3), 955–965 (2018).
Yazdani, A. & Haghighat, S. Determining prognostic factors of Disease-Free survival in breast Cancer using censored quantile regression. Breast Cancer Basic Clin. Res. 16, 11782234221108058 (2022).
Bottai, M. & Zhang, J. Laplace regression with censored data. Biom. J. 52 (4), 487–503 (2010).
Yazdani, A., Zeraati, H., Yaseri, M., Haghighat, S. & Kaviani, A. Laplace regression with clustered censored data. Comput. Stat. 1–28. (2022).
Singer, S. & Nelder, J. Nelder-mead algorithm. Scholarpedia 4 (7), 2928 (2009).
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354 (17), 1813–1826 (2006).
Bejanyan, N. et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplant. 49 (8), 1029–1035 (2014).
Döhner, H. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood J. Am. Soc. Hematol. 129 (4), 424–447 (2017).
Kassim, A. A. & Savani, B. N. Hematopoietic stem cell transplantation for acute myeloid leukemia: a review. Hematol. Oncol. Stem Cell Ther. 10 (4), 245–251 (2017).
Levi, I., Grotto, I., Yerushalmi, R., Ben-Bassat, I. & Shpilberg, O. Meta-analysis of autologous bone marrow transplantation versus chemotherapy in adult patients with acute myeloid leukemia in first remission. Leuk. Res. 28 (6), 605–612 (2004).
Warlick, E. D. et al. Allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia: similar outcomes regardless of donor type. Biol. Blood Marrow Transplant. 21 (2), 357–363 (2015).
Shokouhi, S. et al. Effects of aGVHD and cGVHD on survival rate in patients with acute myeloid leukemia after allogeneic stem cell transplantation. Int. J. Hematol. Oncol. Stem Cell. Res. 9 (3), 112 (2015).
Derolf, Å. R. et al. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood J. Am. Soc. Hematol. 113 (16), 3666-72. (2009).
Pulte, D., Gondos, A. & Brenner, H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica 93 (4), 594–600 (2008).
Sant, M. et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 15 (9), 931–942 (2014).
Yanada, M. et al. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia conducted in Japan during the past quarter century. Ann. Hematol. 99, 1351–1360 (2020).
Larue, M. et al. Long-term outcome of 2‐year survivors after allogeneic hematopoietic cell transplantation for acute leukemia. HemaSphere 8 (10), e70026 (2024).
Tan, J. et al. Prognostic factors on graft-versus-host disease-free and relapse-free survival after allogeneic hematopoietic stem cell transplantation for adults with acute leukemia. Leuk. Res. 59, 1–7 (2017).
Guru Murthy, G. S. et al. Association of ABO mismatch with the outcomes of allogeneic hematopoietic cell transplantation for acute leukemia. Am. J. Hematol. 98 (4), 608–619 (2023).
Middeke, J. M. et al. Outcome of patients with Abnl (17p) acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Blood J. Am. Soc. Hematol. 123 (19), 2960–2967 (2014).
Schetelig, J. et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German transplant study group. J. Clin. Oncol. 26 (32), 5183–5191 (2008).
Sayehmiri, K. et al. Prognostic factors of survival time after hematopoietic stem cell transplant in acute lymphoblastic leukemia patients: Cox proportional hazard versus accelerated failure time models. J. Exp. Clin. Cancer Res. 27 (1), 1–9 (2008).
Spring, L. et al. Risk factors for readmission after allogeneic hematopoietic stem cell transplantation and impact on overall survival. Biol. Blood Marrow Transplant. 21 (3), 509–516 (2015).
Ozkurt, Z. et al. (eds) Impact of ABO-Incompatible Donor on Early and Late Outcome of Hematopoietic Stem Cell Transplantation. Transplantation Proceedings (Elsevier, 2009).
Ciftciler, R. et al. Impact of ABO blood group incompatibility on the outcomes of allogeneic hematopoietic stem cell transplantation. Transfus. Apheres. Sci. 59 (1), 102597 (2020).
Author information
Authors and Affiliations
Contributions
M.Y. and H.Z. supervised the studies. M.Y., M.T. and A.K. designed the project, and MT. and AK. analyzed the results and generated figures and tables. M.T., I.M.O. and A.H.M. wrote the manuscript. AK and MY completed the statistical analysis. H.Z., M.Y., A.Y., M.T., M.M. and S.A.M. provided guidance on the design of this project and reviewed the manuscript. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the School of Public Health & Allied Medical Sciences-Tehran University of Medical Sciences (approval ID: IR.TUMS.SPH.REC.1400.091). Informed consent was provided according to the Declaration of Helsinki. Considering that we have used secondary data, none of the authors had direct access to the patients and this research was conducted in compliance with ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Appendix 1
See Figs. 3, 4, 5, 6, 7 and 8.
The effect of significant variables on the 10th, 20th, 30th, and 40th percentiles of OS times based on the Laplace regression model. The blue line indicates the difference in survival from the reference group, black dashed lines indicate the lower and upper limits of the 95% confidence interval for the estimated effect and red line indicate conditional quantile efects estimated by Cox model.
The effect of nonsignificant variables on the 10th, 20th, 30th, and 40th percentiles of OS times based on the Laplace regression model. The blue line indicates the difference in survival from the reference group, black dashed lines indicate the lower and upper limits of the 95% confidence interval for the estimated effect and red line indicate conditional quantile efects estimated by Cox model.
The effect of significant variables on the 10th, 20th, 30th, 40th and 50th percentiles of DFS times based on the Laplace regression model. The blue line indicates the difference in survival from the reference group, black dashed lines indicate the lower and upper limits of the 95% confidence interval for the estimated effect and red line indicate conditional quantile efects estimated by Cox model.
The effect of nonsignificant variables on the 10th, 20th, 30th, 40th, and 50th percentiles of DFS times based on the Laplace regression model. The blue line indicates the difference in survival from the reference group, black dashed lines indicate the lower and upper limits of the 95% confidence interval for the estimated effect, and red line indicate conditional quantile efects estimated by Cox model.
.
The effect of significant variables on the 20th, 40th, 60th, and 80th percentiles of GRFS times based on the Laplace regression model. The blue line indicates the difference in survival from the reference group, black dashed lines indicate the lower and upper limits of the 95% confidence interval for the estimated effect, and red line indicate conditional quantile efects estimated by Cox model.
The effect of nonsignificant variables on the 20th, 40th, 60th, and 80th percentiles of GRFS times based on the Laplace regression model. The blue line indicates the difference in survival from the reference group, black dashed lines indicate the lower and upper limits of the 95% confidence interval for the estimated effect, and red line indicate conditional quantile efects estimated by Cox model.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tatari, M., Kasaeian, A., Mousavian, AH. et al. Prognostic factors for survival after allogeneic transplantation in acute myeloid leukemia in Iran using censored quantile regression model. Sci Rep 15, 9055 (2025). https://doi.org/10.1038/s41598-025-92107-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92107-4