Abstract
To reveal the evolution law of the mechanical failure of the root-soil composite and identify the main control factors and their coupling and mutual feeding relationship, this paper takes the most common naturally growing plants in Yan ‘an area as the research object and studies the evolution process of the mechanical deformation and failure of the root-soil composite by applying the methods of in-situ pull-out test, indoor direct shear test of the root-soil composite, numerical simulation, and theoretical analysis. The mechanical characteristics of root-soil interaction were analyzed, and the mechanism of root-soil fixation was explained. The results show that: (1) the root-soil composite’s mechanical deformation and failure characteristics have obvious regularity and stages and are affected by plant growth state, root morphology, soil physical and mechanical properties, and other factors. (2) There are obvious evolutionary stages in the deformation and failure process of the root-soil composite, that is, the coordinated deformation stage of the root-soil, the stress redistribution stage, the secondary root break stage, the main root break stage and the complete failure stage, which correspond to the linear deformation section, the acceleration section, the shock rise section, the steep fall section and the residual deformation section of the F-S curve (Force-displacement curve)obtained by the in-situ pull out test. (3) In the in-situ pull-out test, the final failure body of the root-soil composite was inverted cone shape. The root fracture interface was basically near the boundary of the final inverted cone failure body, in which the stress state of the root system was directly affected by the stress-strain state of the microelement and the characteristics of the root material. (4) The plant roots showed obvious oblique deformation and axial tensile stress with the soil shear dislocation on the fracture surface, which verified the rationality of the “oblique root” hypothesis based on the transformation of shear stress to tensile stress.
Similar content being viewed by others
Introduction
The Loess Plateau, located in the central part of the Yellow River basin, is characterized by the highest level of soil erosion, the most delicate and vulnerable ecological environment, and the most severe geological disasters in China. Soil erosion covers 71% of the area, and 90% of the Yellow River sediment comes from the Loess Plateau1. Yan’an, located in the central part of the Loess Plateau, is the region’s most densely populated area. It experiences the highest conflicts between humans and land and the most intense human engineering activities in this area2. The problem of geologic hazards in the area directly threatens residents’ lives and property, exacerbating the area’s already severe problems with soil erosion and the ecological environment. In turn, soil erosion will further affect the fragile ecological environment, thus inducing new geological disasters3. It can be stated that the long-term development of the ecology of the Loess Plateau in the middle reaches of the Yellow River basin must be balanced between protective management and ecological restoration, which directly maintains the Yellow River basin’s healthy operation, so ecological management is vital4,5.
From the perspective of controlling soil erosion, plant coverage can reduce runoff damage caused by precipitation and can effectively prevent water and soil loss6,7. However, plant growth is limited by the type of substrate, for example on steep slopes or in areas covered by poor soils8. The study of plant slope protection in loess areas has a long history, and the existing research results mainly focus on the effect of root system soil consolidation and its influencing factors9.
Nevertheless, root-soil composites’ deformation and failure process under natural conditions is highly complex, resulting in variable and location-specific influencing factors. Consequently, the existing research on this topic is not sufficiently comprehensive, making it challenging to establish a unified perspective on the division of the evolutionary stage10. Furthermore, the degradation process of loess slopes is intricate and influenced by multiple factors, including climate, geomorphology, geotechnical properties, vegetation, and others. It has been discovered that vegetation can partially mitigate the increase in slope shear strain and overall displacement11. The root system plays a crucial role in enhancing the stability of the top layer of slopes, preventing slope erosion and soil erosion. The stems and leaves of vascular plants in the upper soil can act as a hydrophobic layer, and their roots can also consolidate the soil11,12,13. However, the interconnection and reciprocal influence between the root system and the soil and their mechanical interaction characteristics still require further clarification14,15.
Currently, conventional indoor mechanical tests, numerical modeling and theoretical analyses, and other mainstream research methods have achieved a lot of results. Chia-Cheng Fan16 uses In-situ large shear tests, to investigate the spatial distribution of root forces in root-permeated soils that are subject to shear. E. Comino17realized in situ tests to quantify the contribution of the root system to the soil shear strength, and laboratory tests estimated the root tensile strength of the different species. Gerrit J. Meijer18 created a newly developed model and the beam-type model yields predictions for the evolution of root-reinforced shear strength as a function of increasing shear displacements.
However, it is difficult to reflect the environmental conditions and evolutionary characteristics of root-soil composite force deformation and failure. For instance, a statistical analysis of the pertinent experimental research literature indicates that the experimental research primarily employs artificial soil modification to prepare the sample. This practice significantly enhances the applicability of the test data but unavoidably diminishes the dependability of the test outcomes.
The emphasis in the study of plant root tensile properties is on indoor single-root tensile tests, with fewer studies on in situ pulling tests in the field due to the inability to respond to geographic variability, which leads to poor applicability of the test results. In the small number of in-situ pulling tests, few experiments were conducted that did not disrupt the native state of the root-soil composite. In the experiment of Norris J E19. , roots were recessed by conducting in situ root pull-out experiments on a London Clay cutting in the southeast.
In-situ mechanical testing is costly, time-consuming, and requires customized design and special manufacturing of test instruments and protocols20,21. However, due to the geographical variability and environmental specificity of northern Shaanxi, in situ mechanical testing remains the most direct and effective research method for studying the force failure evolution process of root-soil composites under special environmental conditions, and it serves as the foundation for both theoretical and indoor experimental research.
Given this, to reveal the evolutionary law of root-soil composite force failure and to clarify the main controlling factors and their coupling and mutual feedback relationships, this paper investigates the prevalent Indigenous plant species in the Yan‘an region by conducting in-situ pullout tests and analyzing the results of original condition Root-soil composite indoor direct shear test, numerical simulations, and theoretical analyses were synthesized. Through the analysis of the root-soil composite force deformation and failure process at the macroscopic level, as well as the analysis of the mechanical characteristics of root-soil interaction at the fine level, the mechanical mechanism of root-soil consolidation was finally elaborated from the stress-strain state of the root system at the microscopic level. It lays the foundation for quantitatively evaluating ecological plant management’s effects and analyzing root consolidation’s mechanical mechanisms.
Research methodology
Overview of the study area
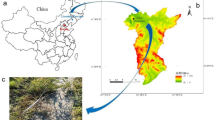
The Loess Plateau, located in the central and western regions of China along the Yellow River, is characterized by a significant lack of economic and social progress, resulting in widespread poverty. This region holds great importance in eliminating poverty in central and western China. Qing‘Jian, Wu‘bao, Zi‘Cang, An’Sai, Yan‘Chuan, and Yi‘Chuan counties around the core of the Yan‘an part of China’s human-land coupling contradictions are the most prominent, the most fragile ecological environment and one of the most serious geologic hazards of the region. Given this, this paper selects the experimental study area in Yan‘an City and its surrounding areas (Fig. 1).
The study area has a typical mesothermal continental semi-arid monsoon climate. The average annual precipitation is relatively low. However, during the summer months of July and August, there is a high intensity of precipitation, often characterized by severe and short-term heavy rainfall. (Fig. 2) The study area is located at the southern edge of the Ordos Plateau and has a simple geological structure; the main strata in the area are Upper Pleistocene Malan loess, Middle Pleistocene Lishi loess, Upper Neogene Sanzuma red clay and sandy mudstone of Zaoyuan section of Yan‘an Formation of Jurassic. The typical climatic conditions in the Yan‘an area, combined with the porous and permeable characteristics of loess, result in soil water scarcity on the slopes during the dry season, leading to a low rate of plant survival, And in the heavy rainfall season, erosion is intense, grass seeds and seedlings are difficult to attach, and the soil matrix, water, nutrient loss is too rapid. Therefore, the study area has a high degree of soil erosion, which is highly representative of the Yellow River Basin.
Test subjects
Ecological management should be guided by adapting to local conditions and constructing a vegetation structure that is scientifically determined to be the best fit for the actual ecological environment5,6. Considering this, this study initially examined the connections between plant growth characteristics and geomorphological habitats in the area and identified the features of the natural vegetation structure communities. Then, we will carefully choose pioneer tree species and their combinations from the most appropriate local plants that naturally grow in the area. These selected plants will be used for the initial ecological restoration and protection. Subsequently, we will systematically conduct a thorough study of the impacts of these plants on ecological restoration and soil consolidation. Furthermore, due to the potential ecological hazards such as species invasion and the ongoing economic development in northern Shaanxi, it is not suitable to implement artificial slope protection plants routinely employed in other regions directly.
The group working on this project has undertaken a comprehensive study of newly created loess slopes in rural Yan‘an from 2014 to now, focusing on slopes that are less than three years old. The survey aims to identify the most suitable pioneer tree species and their combinations for these slopes’ first ecological restoration and protection. An analysis of the vegetation structure of 323 natural slopes revealed the presence of 33 dominant plant species, primarily consisting of cold and drought-tolerant varieties that thrive in barren conditions. The number of species was relatively low, resulting in a more uniform vegetation community structure. The frequency statistics for each species can be found in Fig. 3.
Figure 3 shows that among the more common plants in the study area, the number of herbaceous and woody plant types is about the same. On the other hand, the number of surveys of herbaceous plants hit 74%. Artemisia, the most common genus in this group, showed up 23% of the time. The significant edge over other herbaceous genera is also shown by the numbers in Fig. 4, which show the structure of the plant community. Thus, Artemisia populations were mostly chosen for this investigation, alongside common non-Artemisia herbaceous plants and low shrubs, which were also selected as test samples for the comparative study.
Design of test apparatus
Figure 5 illustrates the project team’s implementation of focused design and specialized manufacturing of the test apparatus and program. This is done to accommodate the study of the failure process of the root-soil composite under unique environmental conditions in the northern region of Shaanxi Province, specifically with force and deformation. The test system consists of an upper fixture, three hollow retractable aluminum alloy leg braces, a pulley structure, and a force gauge, among other components.
The internal part of the fixing device is provided with grooves, the surface is provided with connecting holes, and the surface of the connecting holes is penetrated with fixing bolts. The three leg structures are provided with connection holes in the upper, middle, and lower parts, which are hinged to the upper rigid fixture using fixing bolts, and the middle connection holes and the inner layer of the aluminum alloy movable bracket are fixed by movable pins; Each of the three toe sections is provided with a connection hole and is hinged to the toe section utilizing a fixing bolt; The pulley structure can be flexibly arranged on the leg support as required, and the force gauge is pulled by the wire rope inside the pulley structure to perform the test under the cooperation of two sets of mechanical gears.
The experimental design of the pull-out test equipment is equipped with an independent power supply in the field through an electro-mechanical winch (which can also be manually loaded in case the field power supply runs out of power). The output precision of the motor can reach 0.01 mm/s after the calibration test before the test, and the precision meets the test demand. The electric motor transmits the pulling force to the fixed pulley for the pull-out test. During the motor test, the tensile force meter records real-time mechanical data of the pulling process, while the high-precision laser displacement meter records real-time deformation data of the pulling process through the bracket on top. All the data is transmitted and recorded in real-time on a laptop, resulting in a comprehensive pulling force deformation curve.
Testing program
In the in-situ pulling-out test of plant roots, the force and deformation curves of the whole process of the pulling test are mainly recorded, as well as the basic information of the test samples, the growth environment, and other influencing factors. The main process of the test work is shown in Fig. 6. The steps of the in-situ pull-out test are as follows:
(1) Information Recording.
The basic information to be recorded before the test includes:
① Geographic information. For example, latitude, longitude, altitude, slope direction, and inclination.
② Geological environment information. For example, soil type, density and humidity, slope erosion, slope vegetation cover, and plant community structure. Based on the discussions and principles of the relevant norms and manuals, such as the " Code for investigation of geotechnical engineering” (GB50021-2001 (2009 edition)), certain parameters were recorded in the field grading of the original records. For instance, the moisture content of the soil body was categorized into three grades: dry (ω < 10%), slightly wet (10% ≤ ω < 20%), and wet (20% ≤ ω ≤ 30%). The soil compactness is categorized into three grades based on n (porosity): slightly dense (n > 0.474), moderately dense (0.429 ≤ n ≤ 0.474), and dense (n < 0.429). In other words, e (porosity ratio): is classified into three corresponding grades slightly dense (e > 0.9), moderately dense (0.75 ≤ e ≤ 0.9), and dense (e < 0.75).
Simultaneously, soil sample and interior physical testing were performed at the test site to ensure the correctness of the field site records.
③ Sample information. For example, plant species name, plant growth, etc. (Fig. 6(a)).
(2) Test Procedure and Data Acquisition.
① The labeled samples were selected in sequence. Before the pulling test, the test equipment was set up directly above the test plant, and the main stem was fixed at 1/3 of the plant height using a fixer. To prevent relative slippage between the fixator and the trunk from affecting the test results, paper towels can be wrapped between the fixator and the trunk. Pre-tension the winch, ensure the tensiometer and the test specimen are in the same vertical line, connect the test system to the computer, and when everything is ready, the pull-out test can begin (Fig. 6(b)).
② Turn on the motor mechanical winch constantly to keep the stranded wire tightened at 5 cm/min and gradually pull out the plant. The test was deemed successful when the entire plant’s root system was lifted from the earth, indicating the completion of the test. At the end of the test, the effective root length, average root diameter, maximum root diameter, root distribution status (Fig. 6(e)), and force versus displacement (F-S) curves during the test (Fig. 6(d)) were recorded, and the obtained data were transferred to a computer.
③ At the end of the test, the pulled root samples were put into special preservation bags. In contrast, soil samples of the root-soil composite in its original state were taken at the test site (Fig. 6(c)), sealed in bags, and brought back to the laboratory for geotechnical tests.
④ Preparation of data processing program software using MATLAB to process the experimental data and analyze the correlations (Fig. 6(f), (g), (h)).
Characteristics of deformation and failure of root-soil composite
Force-displacement (F-S) curve characteristics
168 plants were sampled for this pull-out test, and the vast majority obtained morphologically complete force-displacement (F-S) curves. To study the effect of plant growth state on the pulling and deformation failure of root-soil composite, the maximum root diameter of the test samples was used as the standard grouping, i.e., three grades of Class I (6 > d), Class II (9 > ≥ 6), and Class III (d ≥ 9) (unit: mm). For example, the force-displacement (F-S) curves of Artemisia carvifolia Buch.-Ham. Ex Roxb and Artemisia capillaris are shown in Fig. 7.
As seen from Fig. 7, the root-soil composite force deformation failure characteristics in the in situ pullout test have a certain regularity. Since the root systems of plants of the same family and genus have similar characteristics, the overall morphological trends of similar plants’ force-displacement (F-S) curves show some similarity. Secondly, the state of plant growth has a significant effect on the mechanical impact of root consolidation, as can be seen from the statistics of the average maximum pulling force of different root levels of Artemisia as an example in Fig. 7(a): Primary root diameter (56.27 N) > secondary root diameter (28.48 N) > tertiary root diameter (22.81 N), which indicates that as the maximum root diameter of the test samples increased, the root system became more developed, and the maximum pulling force measured in the test increased accordingly, and the magnitude of the increase showed an accelerated expansion trend.
Root morphology
According to botanical classification, plant root morphology can be broadly classified into two main categories: taproot and fibrous root systems. Still, there is often some crossover in the actual root morphology of plants. Taking Artemisia populations as an example, Artemisia populations in the Yan‘an area are characterized by a long, thick, woody root system with many lateral roots (Fig. 8). The primary concentration of the root system of Artemisia plants is in the vertical direction, specifically in the soil layer of 0–100 cm (with the deepest roots reaching up to 200 cm in some cases). In the horizontal direction, the root system is mainly concentrated within a 0–40 cm range from the Artemisia plants. This distribution range is significantly larger than other herbaceous plants’ root systems.
For the porous, loose, and permeable loess within the depth range of 1 m below the slope surface, the shallow root reinforcement and deep root anchorage of Artemisia spp. are significant. Therefore, Artemisia populations belong to the herbaceous group of plants. Still, their root systems are more characteristic of woody taproots with woody, robust, and deeply embedded primary roots. However, the high number of roots of herbaceous fibrous-rooted plants and their large horizontal extension are retained in their young growth stages and within shallow soils. Overall, the root distribution characteristics of Artemisia populations are the same as those of shrubs such as Amorpha fruticosa (shrubs). Hence, the curve patterns of the test results are similar, as can be seen from Fig. 6(h), Fig. 8(a) and Fig. 9(a).
To further illustrate the effect of root morphology, samples 1 (Artemisia carvifolia), 64 (Artemisia carvifolia), and 56 (Artemisia carvifolia) were used for comparative analyses, as shown in Figs. 8, 9 and 10.
Figures 8, 9 and 10 demonstrate that Sample No. 1, Sample No. 64, and Sample No. 56 are all Artemisia carvifolia, the developmental characteristics and morphology of their root systems will vary to some degree due to the different environmental conditions in which they grow. This leads to the variability in the expression of the force-displacement (F-S) curves of the root-soil composite in the process of force deformation and failure, as well as the differences in the presentation characteristics of the microscopic evolutionary process of tensile deformation and failure of the root-soil composite.
As can be seen from Fig. 8(b), the development of the primary root of sample No. 1 (Artemisia carvifolia) was significantly stronger than that of the other secondary root systems, reflecting the characteristic of the dominant primary root. Therefore, it can be seen in Fig. 8(a) that the main root has a great tensile capacity at the D point, making FD 208.1 N, the maximum pullout force of the test. After the main root breaks off at the E point, making FE 39.3 N. The drop of DE in the steeply falling section of the force-displacement (F-S) curve reaches 168.8 N due to the sudden release of the pull-out force carried by the main root. As can be seen from Fig. 9(b), the dominance of the primary root of sample No. 64 is average, with a decrease of 34.6 N in the steeply declining section DE of the force-displacement (F-S) curve in Fig. 9(a), which is only 20.5% of that of sample No. 1 (Artemisia carvifolia). The d in Fig. 9(a) is an interruption of the drop in pulling force at the time of the main root fracture, and the dE phase represents the small oscillations of the root fiber tissue at the time of fracture.
During the group’s field investigations and experiments, we found that there is also a small number of Artemisia carvifolia in some special growth environments. Samples that do not have obvious primary root dominance development. As shown in Fig. 10(b), the root system of sample 56 (Artemisia carvifolia), did not have an obvious primary root development, instead, multiple lateral roots developed together. The root statistics show that about 5–7 or so lateral roots are better developed, but there is no relative dominance in relation to each other. Therefore, in Fig. 10(a), it can be seen that the force-displacement (F-S) curve does not have a steeply falling section of the curve caused by the breakage of the main root, but rather a multistage, small, steep fall formed with the successive breakage of these 5–7 or so lateral roots. Moreover, these lateral roots carried the main pull-out loads during the later stages of test loading of sample 56 (Artemisia carvifolia); therefore, the successive disconnection of these lateral roots is the most important cause of the ultimate destruction of the root-soil composite, so this multi-stage small-amplitude steep drop occurs in the falling stage of the curve except once in the process of curve rise.
In general, the root system of sample 56 (Artemisia carvifolia), with multiple lateral roots (no obvious main root), is more similar to the characteristics of bearded plants, which is also evidenced by the results of the herbaceous bearded plant " Echinochloa crucial (Linn.) Beauv. " Shown in Fig. 11(a). Although there is a slight difference in the shape of the ascending segment of the two force-displacement (F-S) curves, the overall trends are relatively similar. It should be noted that most herbaceous fibrous-rooted plants, such as Echinochloa crucial (Linn.) Beauv. Sample No. 141 tares shown in Fig. 11(b), grow in a face-covered distribution on slopes, unlike Artemisia, which grows as a single, independent plant. Therefore, the pulling test for tares is conducted in clusters or bundles as unit samples, not in plants as in the case of Artemisia.
Physical properties of the soil
In the root-soil composite, the soil, as the object of the root system, has physical properties that affect the mechanical effects of plant root consolidation, including the more obvious impacts of soil compactness and moisture.
RAR(Root Area Ratio)is defined as the fraction of the soil cross-sectional area occupied by roots per unit area. In this paper, the maximum pulling force of Artemisia spp. Samples were used to comprehensively characterize the mechanical impact of root consolidation and the root area ratio (RAR) of Artemisia spp. Samples were used to reflect the plant growth status, with a sampling depth of 45 cm and a radius of 30 cm measured with the plant’s root system as the center of the circle.
\(RAR=\mathop \sum \limits_{{i=1}}^{N} \frac{{\pi {\text{d}}_{i}^{2}}}{{4\,A}}\)
Where: RAR is the root area ratio (%), N is the number of roots, di is the diameter of the ith root, and A is the area of the soil profile. The number of roots and the soil profile area were measured in the field, and the root diameter was derived from pixel measurements.
The effects of soil compactness and soil moisture on the force deformation and failure characteristics of the root-soil composite were finally analyzed and obtained, as shown in Fig. 12(a) and Fig. 12(b), respectively.
As shown in Fig. 12(a), the maximum pullout force of the in situ pullout test increases with the enhancement of soil compactness, and the statistical results of the two also show a linear positive correlation. On this basis, it is worth noting that the extent to which soil compactness affects the maximum root pullout force increases with the increase in the root surface area ratio. For example, near the location of root surface area ratio of 0.15%, the average values of maximum root pullout force corresponding to slightly dense, moderately dense, and dense soils were 88 N, 79.6 N, and 122.4 N, respectively, with an increase of 8.4 N and 34.4 N. Near the root surface area ratio of 0.025% position, the corresponding growth was 6.8 N vs. 15.3 N, respectively. Analyzing the reasons, it is believed that in the early stage of root growth, the root surface area is relatively small, the development of fibrous roots and main roots is not yet formed, and the maximum pulling force is less affected by external factors. The maximum pulling force is less affected by external factors. The change in compactness condition is not the dominant factor affecting the maximum pullout force, so the increase is small. At the late stage of root growth, with the growth of fibrous roots and main roots, the root area ratio increases, and the root system consolidates the soil body’s ability to enhance the soil compactness, begins to become one of the dominant factors affecting the strength of the root-soil composite.
Soil moisture also significantly affects the strength of the root-soil composite, as shown in Fig. 12(b). As the soil moisture increases, the statistical value of the maximum pullout force of the root in-situ pullout test is highest at slightly wetter soil grades, i.e., the soil is too dry or saturated to be favorable for the strength of the root-soil composite. With the increase of humidity in the soil, the soil and the root system from the original relatively loose contact state, due to the increase of water in the soil and the formation of soil-cement film produced by the water cementation effect of the root-soil composite, becomes more and more solid. This explains the test result: the maximum pullout force increases first as the humidity increases. As the humidity continues to increase, the cement film between the soils becomes thicker, and the water cementation formed by the combination of soils and moisture is weakened, which decreases the maximum static friction at the root-soil interface. At this point, the excess moisture enhances the lubrication of the root-soil interface, reducing the maximum pullout force by the greater soil moisture. Second, according to the compaction test statistics, the optimum moisture content of the pulverized soil is about 16–22%, which is close to the grade of “Slightly Wet,” so there is a roughly corresponding relationship between the moisture content of the soil and the compactness of the soil, as shown in Fig. 12, the data points of “Slightly Wet” and “Compact” grades overlap relatively frequently.
Mechanical mechanisms of root consolidation
Evolution of deformation and failure in root-soil composites
The in-situ pull-out test revealed the fundamental laws and trends governing the deformation and failure process of the root-soil composite under stress, as well as the significant effects of plant growth status, root distribution characteristics, soil physical and mechanical properties, and other factors on the deformation and failure process. This paper integrates an in situ pulling test, a direct shear test, and a numerical model to analyze the coupling and mutual feedforward relationship between root-soil interaction and composite deformation and failure evolution process.
As can be seen from Fig. 7, the overall trend of the force-displacement (F-S) curve of the plant-root-soil composite during the in-situ pullout test still shows some regularity. To analyze the pulling deformation failure evolution process of plant root-soil composite, the pulling force-displacement (F-S) curve of sample No. 64 in Fig. 9 is extracted separately in this paper, as shown in Fig. 13.
As shown in Fig. 13(a), the pullout force-displacement (F-S) curve of sample No. 64 (Artemisia annua) varied continuously with the loading process, showing five stages in general:
(1) Linear deformation section (AB): In the initial loading stage, the pull-out force is small, and the curve rises in a straight line from the origin;
(2) Curve acceleration section (BC): after the initial linear deformation, with point B as the demarcation point, the slope of the force-displacement (F-S) curve gradually increases from a straight line to a curve rising;
(3) Oscillating rising section (CD): from point C, the curve still displays an ascending trend, but the slope of the curve gradually increases from BC to CD, and the curve appears to oscillate up and down, and the magnitude of the oscillation is plainly increased with the load of the test.
(4) Steep decline section (DE): at the end point D of the oscillatory rise section, the pull-out force reaches its maximum value; after that, the curve falls almost vertically in a very small range of displacements from point D, showing the sudden destruction of the root-soil composite;
(5) Residual deformation section (EF): Following the steep fall section, the root-soil composite regained some of the residual force state at the end point E of the steep fall section, and the force-displacement (F-S) curve after the E point began to accelerate the decline in an irregular curve state until the root system was completely pulled out at the F point, and the root-soil composite was destroyed.
Mechanical characterization of root-soil interaction
From the analysis of Fig. 13(a), it can be seen that the pulling force-displacement (F-S) curves of Artemisia spp. in the in situ pulling test generally showed five phases: Linear deformation section, curved acceleration section, oscillatory rise section, steep fall section, and residual deformation section, which indicates that the tensile deformation failure of root-soil composite has an obvious evolutionary stage. From the record of the deformation process of the root-soil composite during the in situ pulling test and the analysis of the actual damage of the root system, there is also a certain regularity, as shown in Fig. 13(b).
Figure 13(b) shows that the final failure body of the root-soil composite is an inverted cone shape. The residual soil on the failure body is mostly loose and fragmented, indicating that the soil body has long been destroyed. The root fracture interface is nearly identical to the boundary of the final inverted cone-shaped failure body. Second, in addition to a portion of the root system fractured along the destructive body’s interface, a significant portion of the root system is entirely exposed and in a state of pull-out. As a result, soil tensile cracking damage, root fracture, root pull-out, and root-soil interactions all influence the overall tensile deformation failure of the root-soil composite.
In summary, the five stages of the pullout force-displacement (F-S) curves of the pullout tests can correspond to the five stages of fine-scale evolution of the root-soil composite damaged by tensile deformation:
(1) Root-soil coordinated deformation phase.
The initial pull-out force loading is low, the root-soil composite body is in a coordinated deformation state, the majority of the soil body is in elastic deformation and local plastic deformation, and the soil body has not yet reached the obvious tensile limit damage. Due to the root system’s relatively high tensile strength, it is in a state of elastic deformation, and the tensile force is conducted downward from the ground through the root system in the root-soil composite, causing the root-soil composite, which is stressed, to expand further.
At this stage, due to the coordinated deformation of the root-soil, the contact state between the root-soil is mainly bonded, and the root-soil composite maintains good integrity.
(2) Stress redistribution stage.
In the root-soil composite, due to the different tensile ultimate strengths of the two materials, soil and root system, after the coordinated deformation of the root-soil in the early stage of loading, the soil of the root-soil composite in the middle and upper part of the root-soil composite begins to enter the limit state earlier with the increase of the tensile force, This, in turn, causes tensile plastic degradation in parts of the soil, resulting in pullout forces that are initially sustained primarily by the root system in these places, which then transmits the newly enhanced pullout forces to the root-soil composite in the lower and surrounding areas. As shown in Fig. 13(a), the curve at point B suddenly fluctuates downwards due to the local soil damage, but with the force of the root system near the damaged surface, the curve then recovers the original morphological characteristics and begins to change into an accelerated curve rise. As more and more of the soil body enters the limit state from the top down and the center out, the proportion of the pullout force borne by the root system in these areas increases accordingly.
At this stage, the root system and the surrounding soil still maintain a viscous friction contact state; that is, the root-soil composite body of the upper part of the root-soil material due to the deformation capacity of the root-soil material by force is different, part of the root-soil material out of the bonding state, the root-soil separation into the friction contact state, and with the increase of the pulling load, through the upper part of the root system down the enter the friction contact state.
In addition, the root-soil composite is a special composite material, and in the initial stage of its deformation (Root-soil coordinated deformation phase and Stress redistribution stage), the stiffening effect also leads to a relatively smooth rise in the curve segments considering the large displacement and rotation of the test material, such as the AC segment in Fig. 13(a).
(3) Subordinate root detachment phase.
With increasing pull loading, the stress redistribution between the two materials, root and soil, leads to an increasing proportion of the pull load being applied to the root system, and the portion of the root system with lower tensile load carrying capacity of the secondary roots begins to fracture. The fracture process evolves from top to bottom in the root-soil composite with the conduction of tensile force; in terms of root type, the fracture process begins to expand from low-strength fibrous root fracture to high-strength lateral root fracture. Secondary root breaking causes a constant redistribution of tensile and pullout loads in the root system, and the primary root, which has the highest tensile capacity, bears a significantly increased tensile load.
As illustrated in section CD of Fig. 13(a), the process of root pullout stress redistribution caused by the secondary root’s dislocation leads the pullout force-displacement (F-S) curve of the pullout test to oscillate, which gradually increases.
To investigate the cause of the oscillations, numerical simulation studies are carried out on the root-soil composite pullout experiments to establish a numerical simulation model that is consistent with the field experiments. The material property parameters required for the simulation can be obtained through indoor soil-related property tests as shown in Table 1.
The current model chooses elastic materials, treating the plant root material as a linear-elastic material. To more accurately depict the stress-strain properties of the soil under varied loading scenarios, the Mohr-Coulomb model is employed. Surface-to-surface contact is selected between different materials.
The numerical simulation model for the in-situ pull-out test is illustrated in Fig. 14. The dimensions of the soil model are 20 cm x 20 cm x 40 cm, the length of the single root is 20 cm, and the multi-root system model contains two lateral roots.
A single root pull-out experiment was used as a calibration experiment for the properties of plant root material. The average tensile strength of 17.52 MPa was selected as the strength of the root material.
The root-soil composite under stress and deformation damage to the 5-phase model shown can be observed not only from the in-situ pull-out test process but also verified from the finite element numerical simulation shown in Fig. 14. Firstly, the curve patterns of numerical simulation and in-situ tests are the same. Secondly, as the pulling action of the numerical model continues, when a single root in the root system reaches its ultimate strength and exits the normal working state, a corresponding oscillation in the pulling force-displacement (F-S) curve will occur, except that the oscillatory process (CD section) can still be recovered after the sub root breaks off, but the main root breaks off and then there will be a sharp and steep drop (DE section) until the root-soil composite is destroyed. Moreover, the numerical simulation results also show that the more complex the root system morphology and the more complete the development of primary and secondary roots, the closer the pull-out force-displacement (F-S) curve of the root-soil composite under stress and deformation damage is to the 5-phase model shown in Fig. 15(a).
(4) Primary root break-off phase.
① Primary root breakage stage.
The process of dislodgment of the secondary roots leads to a continuous redistribution of tensile stresses in the root system, and the tensile load borne by the primary root, which has the highest tensile capacity, increases dramatically. When the tensile load borne by the main root reaches the ultimate damage value, the main root appears to fracture abruptly, followed by the quick release of the main tensile strain borne on the main root, and the root-soil composite is nearly destroyed.
As shown in Fig. 13(a) with segment DE in Fig. 15, this sudden release of pullout force due to the fracture of the main root causes the pullout force-displacement (F-S) curve of the pullout test to show the first vertical steep drop (segment Dd).
② Stump pull-out phase.
After the main root breaks, the sudden release of the pulling load cannot be immediately carried by the redistribution of the other root systems, and the residual roots of the main root after the break also pull off quickly, causing the main root to lose contact with the soil. Also, the sudden release of the pull-out load led to the sudden destruction of the other secondary roots.
The second rapid release of pullout force induced by this stub pullout resulted in a second vertical steepening of the pullout test’s Force-displacement (F-S) curve. After an extremely brief oscillation, the curve at the d-point position begins a new steep drop, indicating that the root-soil composite, faced with the sudden release of a large load from the fracture of the main root, rapidly fails in its attempts to find a new equilibrium of forces that will allow the root-soil composite to return to a stable state of stress distribution.
According to the statistics of the experimental results, the process and performance of the main root break-off stage are more complex. For example, the force-displacement (F-S) curves for the primary root breakage phase of some of the trials showed only one steep drop (Fig. 8), suggesting that primary root breakage and stump pull-out in these samples were completed almost simultaneously.
(5) Complete destruction phase.
After the main root breaks off, the structure of the root-soil composite is essentially damaged, and the destroyed root-soil composite can still perform some residual deformation until the pulling test entirely removes the root system. These five stages of destructive evolution are distinct in their own right and strongly related to one another, and numerous stages of microscopic evolution may occur concurrently over time. Furthermore, because different plant samples have different root morphology and growth environments, the performance characteristics of the pull-out force-displacement (F-S) curves and the stage of tensile deformation and failure microscopic evolution of the root-soil composite will differ.
Stress-strain state of the root system
Combining the macroscopic analyses of the root-soil composite force deformation and damage process and the microscopic analyses of the mechanical characteristics of the root-soil interaction described in the previous section. The paper concludes with a description of the mechanical mechanism of root consolidation in terms of the stress-strain state of the root system at the microscopic level. As shown in Fig. 13(b), the final failure body of the root-soil composite is in an inverted cone shape. The root fracture interface is basically near the boundary of the final inverted cone-shaped failure body, so the analysis should be carried out for the micro metamorphic body’s stress-strain state at the fracture interface’s location, as shown in Fig. 16.
As shown in Fig. 16, the microelements at different locations on the rupture surface of the root-soil composite were not subjected to the same state of force during the pullout test. Microelement ① is mainly damaged in tension, Microelement ② is mostly in tension and shear simultaneously, and Microelement ③ is primarily in shear damage. The difference between the stress-strain states of the microelements is illustrated by the angle α between the rupture surface of the microelements and the vertical direction (the direction of pullout loading). It can be seen that α takes the value of the interval of [0°,90°], α is 90 ° when the microelement is for the tensile damage state, α is 0 ° when the microelement is for the shear damage state, α for other angles when the microelement for the simultaneous tensile shear damage state. The root-soil composite rupture surface is in a tensile-shear damage state at most locations, and the percentage of the microelement subjected to tensile action increases as α increases.
The stress-strain state of the microelement will directly affect the root system in which the force state, for example, microelement ① of the main root by the action of pulling and pulling in the overall tensile state, microelement ③ with a certain lateral stiffness of the lateral root will be due to the microelement of the vertical shear misalignment and withstand a certain amount of shear force.
Secondly, the material properties of the root system also influence the root stress state. In the root-soil composite, the root system has more obvious flexible-plastic material characteristics than the soil material, so even if shear deformation of the microelement occurs, resulting in shear damage to the soil body, the root system in it will also exhibit flexible tensile deformation characteristics. The vertical shear misalignment of the ③ microelement can cause shear force, even on lateral roots with lateral shear stiffness and partly woody features. However, the deformation exhibited predominantly evident oblique tensile deformation and axial tensile stress-strain patterns. In contrast, the other fine fibrous roots, which were nearly entirely bendable, virtually always displayed tensile stress-strain states. Despite its strong lateral shear stiffness, the primary root of microelement ① remains in a tensile stress-strain state due to tension-related damage.
The size ratio between the numerical simulation model and the indoor direct shear experimental model depicted in Fig. 17 is 1:1; specifically, the dimensions of the ring cutter in the model are 61.8 mm in diameter, 20 mm in height, and 10 mm in root length. To streamline the testing procedure and enhance model compatibility, vertical pressure at all levels was simulated using a fixed-area load, while the displacement boundary condition replaced the shear box constraint.
When the direct shear test loading increases until the soil is completely shear damaged and through the shear surface, the soil body, in addition to the residual interfacial friction on the shear rupture surface of the basic exit from the state of force, the external shear load is borne entirely by the root system. In the opposite direction of shear loading, a local tensile de-hollowing region was formed between the back side of the root system and the soil body. In contrast, in the positive direction of shear loading, the front side of the root system squeezed the soil body, resulting in local extrusion deformation and stress increase. Similarly, to the force state of a full-length bonded flexible connection, the root system is subjected to the greatest tensile force in the region adjacent to the soil’s shear rupture surface under the operation of the root-soil bond. As a result, if the experimental applied shear load continues to rise until the tensile force on the root system in the region of the area exceeds its tensile capacity, the relatively flexible root system will break off after undergoing significant tensile deformation. And cause stress redistribution as more and more pulled-off root systems leave the functioning state, resulting in a chain reaction of consecutive tensile fractures or root-soil pull-outs of following root systems.
The numerical simulation data of the single root in Fig. 17(c) is extracted and analyzed to obtain the stress-strain analysis results as shown in Fig. 18. This study demonstrates that the root system is closer to a flexible-plastic material than to earth material, with a more evident characterization of the tensile stress-strain state. Even lateral roots with some lateral shear stiffness and partial woody features will experience some shear force due to vertical shear misalignment of the microelements. Still, the deformation is primarily oblique tensile deformation and an axial tensile stress-strain state. This also verifies the validity of the simplified model of root-soil composite strength represented by the Wu-Waldron model11, which is based on the assumption of a “slanting root” that transforms tangential to tensile stresses.
Conclusion
(1) Root-soil composite force deformation damage characteristics have clear regularity and stage and are determined by the plant growth state, root shape, soil physical and mechanical properties, and other elements;
(2) The root-soil composite force deformation and damage process have obvious evolutionary stages, i.e., root-soil coordinated deformation stage, stress redistribution stage, secondary root breakage stage, primary root breakage stage, and complete destruction stage, which correspond to the linear deformation section, accelerated section, oscillating rising section, steeply descending section, and residual deformation section of the force-displacement (F-S) curves.
(3) Each stage of the root-soil composite body force deformation damage evolution process has its own set of visible characteristics. Still, also mutual limitations and effects, its key control variables, and their coupling and mutual feedback relationship are more complex.
(4) The final damage body of the root-soil composite of the in-situ pullout test was inverted cone shape, and the root fracture interface was essentially near the boundary of the final inverted cone shape damage body, in which the root force state was directly affected by the stress-strain state of the microelement body and the material properties of the root system;
(5) Compared to the soil material, the root system is closer to the flexible-plastic material, presenting more obvious characteristics of the tensile stress-strain state, even if the lateral roots with certain lateral shear stiffness of partial ligneous characteristics will be subjected to a certain amount of shear due to the microelement body of the vertical shear misalignment, but the deformation is also primarily presenting obvious oblique tensile deformation and axial tens.
Data availability
The data are owned by the research laboratory and cannot be disclosed for copyright reasons.Raw data were generated at Rui Xu. Derived data supporting the findings of this study are available from the corresponding author, Rui Xu, on request.
References
Jianbing, P. E. N. G. et al. Scientific research framework of livable yellow river. J. Eng. Geol. 28 (2), 189–201 (2020).
Xu, R. et al. Use of local plants for ecological restoration and slope stability: a possible application in Yan’an, loess plateau. Geomatics Nat. Hazards Risk. 10 (1), 2106–2128 (2019).
Xu, R. et al. Research on the evaluation model of ecological environment change and its internal driving mechanisms. Geomat. Nat. Hazards Risk 15(1) (2024).
Rui, X. U. et al. Research on the growth characteristics and slope protection of Artemisia Ordosica in Yan‘an area. J. Railway Eng. Soc. 32 (09), 19–24 (2015).
Rui, X. U. et al. Research on adaptability of slope-protecting plants in North of Shaanxi loess area. J. Chang’an Univ. (Natural Sci. Edition). 36 (04), 26–32 (2016).
Durán Zuazo, V. H. et al. Soil-erosion and runoff prevention by plant covers in a mountainous area (se spain): Implications for sustainable agriculture. Environmentalist 26, 309–319 (2006).
Durán Zuazo, V. H., Rodríguez Pleguezuelo, C. R., Francia Martínez, J. R., Cárceles Rodríguez, B. & Martínez Raya, A. Pérez Galindo. Harvest intensity of aromatic shrubs vs. soil erosion: an equilibrium for sustainable agriculture (SE Spain). Catena 73 (1), 107–116 (2008).
Lázaro, R. et al. The influence of competition between lichen colonization and erosion on the evolution of soil surfaces in the Tabernas Badlands (SE Spain) and its landscape effects. Geomorphology 102 (2), 252–266 (2008).
Alejandro Gonzalez-Ollauri & Mickovski, S. B. Plant-soil reinforcement response under different soil hydrological regimes. Geoderma 285, 141–150 (2017).
Sanandam Bordoloi, Charles Wang Wai Ng. The effects of vegetation traits and their stability functions in bio-engineered slopes: A perspective review. Eng. Geol. 275, 105742 (2020).
Waldron, L. J. The shear resistance of root-permeated homogeneous and stratified soil. Soil Sci. Soc. Am. J. 41, 843–849 (1977).
Kazutoki Abe and Robert R. Ziemer. Effect of tree roots on a shear zone: modeling reinforced shear stress. Can. J. For. Res. 21 (7), 1012–1019 (1991).
Franck Bourrier, F., Kneib, B., Chareyre, T. & Fourcaud Discrete modeling of granular soils reinforcement by plant roots. Ecol. Eng. 61, 646–657 (2013).
Operstein, V. & Frydman, S. The influence of vegetation on soil strength. Ground Improv. 4 (2), 81–89 (2000).
Tien, H. & Wu Alex WatsonIn situ shear tests of soil blocks with roots. Can. Geotech. J. 35 (4), 579–590 (1998).
Tsai, C. C. F. M. H. Spatial distribution of plant root forces in root-permeated soils subject to shear. Soil Tillage. Res. 156, 1–15 (2016).
Comino, E., Marengo, P. & Rolli, V. Root reinforcement effect of different grass species: A comparison between experimental and models results. Soil Tillage. Res. 110, 60–68 (2010).
Meijer, G. J., Wood, M., Knappett, D., Bengough, J. A., Liang, T. & A. G., & Root reinforcement: continuum framework for constitutive modelling. Geotechnique 73 (7), 1751–7656 (2023).
Norris, J. E. Root reinforcement by Hawthorn and oak roots on a highway Cut-Slope in Southern England. Plant. Soil. 278, 43–53 (2005).
Gray, D. H. & Ohashi, H. Mechanics of Fiber reinforcement in sand. J. Geotech. Eng. 109, 335–353 (1983).
Nasrin Saadati, M. R., Mosaddeghi, M. R. & Sabzalian Mehrnoosh Jafari. Soil mechanical reinforcement by the fibrous roots of selected rangeland plants using a large soil-root shear apparatus. Soil Tillage. Res 234, 001–013 (2023).
Funding
This work is financially supported by the National Natural Science Foundation of China (Grant No. 41572261), the Basic Research Program of Natural Sciences of Shaanxi Province (Grants No. 2022JM-280), China Jikan Research Institute of Engineering Investigations and Design, Co., Ltd 2022 scientific research fund (Grants No. 11740903120220021), the University student innovation and entrepreneurship training programs (Grants No. X202210710391, X202210710510), the China National Petroleum Corporation Changqing Oilfield Branch 2023 Annual Science and Technology Project (No. 2023DJ0701), PowerChina Northwest Engineering, Co., Ltd 2024 Key science and technology projects (Grants No. XBY-KJ-2024-55). All supports are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
R Xu was responsible for conceptualization, methodology and investigation; Y H and G W wrote the main manuscript text; H C and J D prepared formal fnalysis and funding acquisition; Huan WEI prepared data curation; X L and W Y reviewed and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, R., He, Y., Cheng, H. et al. Mechanical mechanism of soil consolidation by plant roots in loess area of northern Shaanxi. Sci Rep 15, 8014 (2025). https://doi.org/10.1038/s41598-025-92179-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92179-2